Circadian rhythms are 24-hour physiological oscillations found at all levels of organization from gene expression to behavior. They have been described in organisms across the tree of life, from bacteria to humans. Photosynthesis in plants and sleep/wake cycles in animals, are 2 examples of circadian rhythms.
What parameters characterize a circadian rhythm?
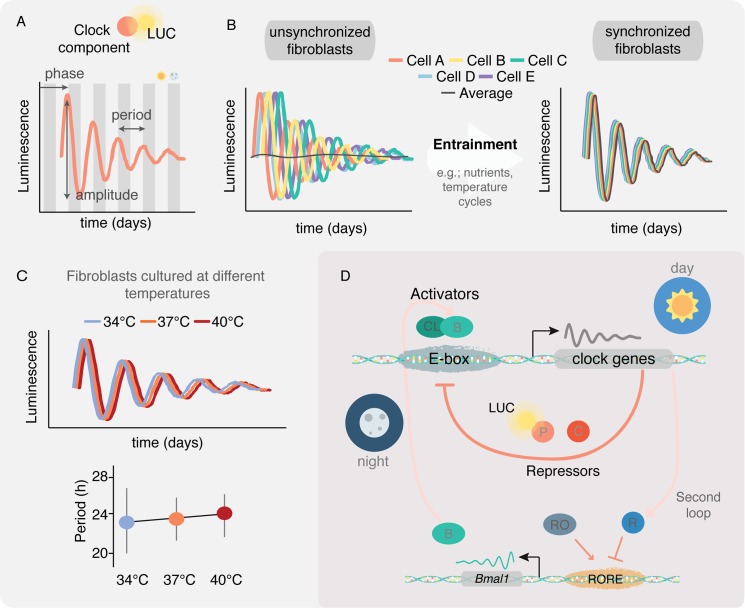
Rhythms are self-sustained 24-hour oscillations. In such oscillations, we can measure phase, amplitude, and period (Fig 1A). Mathematical algorithms help us estimate such values from high-throughput data [1, 2].
Fig 1. Properties of circadian clocks.
A. Bioluminescence rhythms of a tissue explant, measured by light emission of Luciferase (LUC) fused with the clock component PERIOD2. Period, phase, and amplitude can be estimated from such rhythms. Gray shading represents the projected subjective day/night cycles (based on the pattern that the animal was exposed to prior to the start of the explant culture) given that cultures are maintained in constant conditions. B. When cultured without any external stimuli for many days, individual fibroblasts retain oscillations but they become asynchronous. To obtain a synchronized population of fibroblasts, entrainment cues need to be given. Once synchronized, the population of fibroblasts will cycle coherently even in the absence of any stimuli for approximately 1 week (depending on the entrainment signals). C. Bioluminescence at 3 different temperatures. The period of the circadian clock is maintained, highlighting that circadian rhythms are temperature compensated. Plot adapted from the experiments of Bieler & Cannavo [7]. D. Core molecular components of the mammalian circadian clock. CL represents CLOCK, B represents BMAL1, C represents CRYPTOCHROMEs, and P represents PERIODs proteins. A common circadian reporter consists of Luciferase (LUC) fused to PERIOD2 protein, one of the clock repressors. In the second loop involving the nuclear receptors, R represents REV-ERBs (α, β) and RO represents RAR-related orphan receptors RORs (α, β, γ). RORE represents receptor-related orphan receptor response elements.
What defines a circadian rhythm?
Not all daily rhythms are circadian rhythms. The earth’s rotation exposes organisms to rhythmic changes in the environment, such as light/dark cycles. These environmental changes impose rhythms in organisms; however, many of these rhythms may not persist when in constant conditions. The 24-hour rhythms that do persist in these constant conditions are circadian rhythms. For a process to be defined as a circadian rhythm, it must fulfill 3 properties: i) have a periodicity of around 24 hours (± 2 hours), even in the absence of environmental cues (free-running period); ii) be able to readjust its timing (phase and period) in response to environmental cues (entrainment); iii) and maintain an approximately 24 hour period at different temperatures within the organism’s physiological range (temperature compensation of the period) (Fig 1A–1C).
What is entrainment? What are free-running conditions? and what is temperature compensation?
To answer these questions, we will refer to studies with fibroblasts as an example. Entrainment is the process by which circadian rhythms become synchronized to a cyclic environmental cue. Because each individual cell has a slightly different period, rhythms of fibroblast cultures become asynchronous [3] when cultured in constant (free-running) conditions for many days. However, when environmental cues, such as nutrients (serum, medium change), hormones (glucocorticoids), or temperature cycles are given periodically, the circadian rhythms of the population synchronize (entrain) [4–6] (Fig 1B). These rhythms maintain their period when at constant 34°C, 37°C, and 40°C, which shows the temperature compensation of circadian rhythms [6, 7] (Fig 1C).
What drives the circadian rhythms?
In mammals, circadian rhythms are regulated by intricate loops that at their core involve 2 transcription factors: CLOCK and BMAL1. These transcription factors activate the transcription of their own repressors, PERIOD (PER) and CRYPTOCHROME (CRY) proteins, among many other downstream genes involved in several physiological pathways (reviewed in [8]) (Fig 1D). A second loop involves the nuclear receptors REV-ERBs (α, β, also known as nuclear receptor subfamily 1, group D, members 1 and 2) and RAR-related orphan receptors RORs (α, β, γ, Retinoic Acid-Related Orphan Receptors). REV-ERBs transcription is driven by CLOCK:BMAL1. These nuclear receptors translocate back into the nucleus and bind to receptor-related orphan receptor response elements (ROREs) in the promoter of Bmal1, repressing its expression. RORs, on the contrary, activate Bmal1 transcription through binding to the same elements on its promoter (Fig 1D). These same nuclear receptors modulate the expression of the nuclear factor interleukin (Nfil3), which in turn regulates RORs expression, forming a third loop. The second and third loops play important roles in immune response [9–11], regulating, for example, the timing of proinflammatory Th17 cell differentiation [12].
How can we detect circadian rhythms at the molecular level?
Fibroblast cultures have been widely studied to investigate the properties of circadian rhythms. These cells, as do virtually all cells in the animal body, have cell-autonomous circadian rhythms that can be monitored using luciferase reporters [13, 14] (Fig 1A and 1D) or fluorescent proteins [4] driven by cycling clock genes.
At the population level, it is also possible to detect circadian rhythms using genome-wide technologies: RNA-sequencing identified approximately 20% of genes oscillating in the mouse liver; ChIP-seq revealed rhythms in the epigenetic and transcriptional patterns [15]; Metabolomic analysis revealed oscillations of many metabolites [16]; and Proteomics and Phosphoproteomics also identified circadian oscillations in protein abundance and their phosphorylation status [17–19].
Have daily rhythms been detected in parasites?
Many parasitic infections show rhythmic daily patterns. Malaria blood-stage parasites have a synchronous asexual cycle, with a coordinated cycle from the moment of invasion of the red blood cells until their bursting. This cycle lasts 24 hours or multiples of 24 hours, depending on the species [20], and is associated with recurrent fevers in the host. The human infectious stage of the Schistosoma mansoni parasite (known as cercariae forms) emerges from snails and swims in fresh water to infect humans by penetrating through the skin. Interestingly, the emergence of this infectious stage is rhythmic and matches the behavior of its final host: occurring during the daytime in parasites that infect humans and in the early evening in parasites that infect nocturnal rats [21, 22]. For filarial parasites, the appearance of the transmissible form in the blood is also rhythmic, with their higher numbers matching the vector feeding pattern [23]. A rhythmic number of parasites in the blood is common to many other parasite species. The number of Trypanosoma rotatorium in the blood of the frog [24] and Trypanosoma congolense and Trypanosoma lewisi in the blood of rodents [25, 26] varies throughout the day.
Despite these and many other examples of rhythmic patterns in parasitic infections, until recently we did not know if these behaviors were intrinsic to the parasite or whether parasites were simply responding to rhythmic environmental cues of their host.
What did we learn about Trypanosoma brucei circadian rhythms?
T. brucei is the parasite responsible for causing sleeping sickness in humans. Although no daily oscillation in parasite number has been detected in T. brucei-infected animals [25], we have recently shown that in culture, these extracellular parasites have intrinsic circadian rhythms in gene expression [27]. Such rhythms were identified by initially entraining the parasite population with temperature cycles followed by assessing the transcriptome by RNA-seq of samples every 4 hours for 2 days. Approximately 10% of the transcriptome cycles in free-running conditions in 2 life cycle stages of T. brucei (bloodstream and insect forms). Many of the cycling genes encode proteins involved in metabolic pathways and the overall metabolic activity of the population is indeed synchronous, with intracellular ATP oscillating during the day. This finding suggests that other parasites may also possess endogenous circadian rhythms.
Are all circadian rhythms dependent on a transcription-translation feedback loop?
Most circadian timekeeping mechanisms rely on a transcriptional-translation feedback loop [28]. As mentioned above, in mammals, CLOCK and BMAL1 heterodimers rhythmically bind to promoters across the genome, activating their transcription [15]. This transcriptional-translation feedback loop mechanism of the clock was first described in pioneering studies in fruit flies [29] and fungi [30]. Curiously, in cyanobacteria, the circadian clock can be reconstituted in vitro: the three KaiABC proteins with ATP are sufficient to generate a temperature-compensated circadian KaiC phosphorylation cycle [31], which could potentially suggest that transcription is not important to drive this clock. However, even though transcription and translation of kai genes are not essential for the generation of this KaiC phosphorylation rhythm, feedback regulation of kaiBC transcription appears to be vital for maintaining robust circadian rhythms in vivo [32, 33].
Despite the importance of the transcription-translation feedback loop, there are many other layers of regulation driving circadian rhythms. Posttranscriptional [34] and posttranslational regulation [35], as well as the redox state of NAD (nicotinamide adenine dinucleotide) cofactors [36, 37] have been shown to be important to tune the rhythms. In T. brucei, transcription is believed to be constitutive and gene expression regulated mostly at the posttranscriptional level [38, 39]. The circadian clock in this parasite appears to also be driven mainly by posttranscriptional mechanisms because polycistronic units (PCUs) are not synchronous and individual transcripts within a PCU can be noncyclic or cyclic with different phases of transcript levels [27].
Is entrainment by light necessary to establish circadian rhythmicity?
Light is undoubtedly the strongest and most well studied environmental cue on our planet. It can be perceived directly by photoactive proteins (phytochrome and cryptochrome in plants) [40] and by specialized photoreceptors in the retina (mammals) [41], or indirectly via the redox status of the cell (cyanobacteria) [42]. In fact, highlighting how important light is for life on Earth, photoreceptors have independently evolved multiple times throughout evolution [43]. However, the rhythmicity of the Earth imposes not only light/dark cycles but also temperature and humidity cycles. Plant circadian rhythms can entrain to temperature cycles, in which day and night temperatures differ by 4°C or less [44]. So far ex vivo, peripheral mammalian clocks appear to be irresponsive to light. However, when in culture, fibroblasts, red blood cells, lung, liver, and kidney are very sensitive to temperature changes [5, 45–48]. They can entrain to low-amplitude temperature cycles that mimic the range of circadian variation of body temperature rhythms.
Parasites, by living inside a host, may have evolved similar entrainment mechanisms to a mammalian peripheral clock, since throughout their life cycle parasites also have little exposure to light. We showed that T. brucei parasites (both mammalian-bloodstream and insect-procyclic forms) are able to entrain to temperature cycles differing by only 5°C, whereas the bloodstream forms do not appear to entrain to light/dark cycles [27]. It is possible, however, that—similar to peripheral clocks—other stimuli can also entrain these and other parasites, such as nutrient availability [49], hormones [50, 51], and perhaps even rhythmic immune pressure [52].
What are the selective advantages for parasites to have a circadian clock?
Having circadian clocks is advantageous for organisms. Their longevity and growth rate are improved when these organisms are in a daily environment with a period that resonates with their endogenous circadian clock. Plants, by being able to anticipate the daily changes of their environment, have increased growth, survival, and competitive advantage [53]. In cyanobacteria, the clock mutants whose circadian clock has a shorter period than 24 hours, are less fit to live in a 24-hour day [54]. When mice are exposed to chronic jetlag, and therefore their circadian clock continuously disrupted, they have a shorter lifespan [55].
T. brucei also has a circadian clock—it’s able to generate circadian rhythmic gene expression in the absence of host cells and in the absence of any stimuli [27]. What is this rhythm for? Why does T. brucei invest its energy in making sure some mRNAs are more abundant at certain times of the day [56]? We observed that many of the genes that undergo circadian expression encode for metabolic enzymes. Inside the mammalian host, nutrient availability also cycles throughout the day. Thus, it is possible that the circadian clock of the parasite coordinates its metabolism to the predictable circadian nutrient changes.
Additionally, anticipating the rhythmic immune response could be beneficial for the parasites. The number of leukocytes in the mouse circulation varies across the day, being mostly recruited into the tissues at the beginning of the mouse active phase [57]. Also at this time there is higher cytokine release when mice are challenged with lipopolysaccharide (LPS) [9]. These, and other, examples show that immune responses are different throughout the day (reviewed in [58]).
Among many possible advantages, anticipating the optimal timing for transmission would be quite an important advantage for a parasite. The vector biting habits for a blood meal follow a circadian rhythm [59–61], and it would be beneficial to have the transmissible forms ready in circulation to match this vector behavior and therefore more efficiently complete the life cycle. In fact, a mismatch in timing between parasite and host rhythms is costly to the malaria parasite replication and transmission [62]. As described above, in some parasitic infections the appearance of transmissible forms follows a rhythmic pattern [21–23], what is still unknown is whether this is driven by an intrinsic circadian clock of the parasite.
Where do we go from here?
It would be interesting to assess if other parasites also have endogenous circadian clocks. In T. brucei, the next step is to identify the molecular timekeeping machinery responsible for driving the circadian oscillations. By disrupting the expression of such molecules and thus perturbing this mechanism, one could test the physiological consequences and possible evolutionary advantages for the parasite in keeping a circadian clock.
Acknowledgments
We thank Victoria Acosta-Rodríguez for insightful discussions.
Funding Statement
This work was supported by an HHMI International Early Career Scientist award (55007419, to LMF) and Fundação para a Ciência e Tecnologia award (IF/01050/2014 to LMF). JST is an Investigator in the Howard Hughes Medical Institute. FRF is a Research Associate in the Howard Hughes Medical Institute. HHMI: http://www.hhmi.org; FCT: http://www.fct.pt. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Yang R, Su Z. Analyzing circadian expression data by harmonic regression based on autoregressive spectral estimation. Bioinformatics. 2010;26(12):i168–74. doi: 10.1093/bioinformatics/btq189 ; PubMed Central PMCID: PMC2881374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms. 2010;25(5):372–80. doi: 10.1177/0748730410379711 ; PubMed Central PMCID: PMC3119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14(24):2289–95. Epub 2004/12/29. S0960982204009157 [pii] doi: 10.1016/j.cub.2004.11.057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119(5):693–705. Epub 2004/11/20. S0092867404010542 [pii] doi: 10.1016/j.cell.2004.11.015 . [DOI] [PubMed] [Google Scholar]
- 5.Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330(6002):379–85. Epub 2010/10/16. 330/6002/379 [pii] doi: 10.1126/science.1195262 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izumo M, Johnson CH, Yamazaki S. Circadian gene expression in mammalian fibroblasts revealed by real-time luminescence reporting: temperature compensation and damping. Proc Natl Acad Sci U S A. 2003;100(26):16089–94. Epub 2003/12/06. doi: 10.1073/pnas.2536313100 ; PubMed Central PMCID: PMC307697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieler J, Cannavo R, Gustafson K, Gobet C, Gatfield D, Naef F. Robust synchronization of coupled circadian and cell cycle oscillators in single mammalian cells. Molecular systems biology. 2014;10:739 doi: 10.15252/msb.20145218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017. doi: 10.1038/nrg.2016.150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(2):582–7. doi: 10.1073/pnas.1106750109 ; PubMed Central PMCID: PMCPMC3258648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian Gene Bmal1 Regulates Diurnal Oscillations of Ly6Chi Inflammatory Monocytes. Science. 2013. doi: 10.1126/science.1240636 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, et al. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192(1):407–17. Epub 2013/12/07. doi: 10.4049/jimmunol.1301982 . [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Rollins D, Ruhn KA, Stubblefield JJ, Green CB, Kashiwada M, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342(6159):727–30. doi: 10.1126/science.1243884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101(15):5339–46. doi: 10.1073/pnas.0308709101 ; PubMed Central PMCID: PMC397382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288(5466):682–5. ; PubMed Central PMCID: PMC1635489. [DOI] [PubMed] [Google Scholar]
- 15.Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, et al. Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science. 2012. Epub 2012/09/01. science.1226339 [pii] doi: 10.1126/science.1226339 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci U S A. 2012. Epub 2012/03/21. 1118726109 [pii] doi: 10.1073/pnas.1118726109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robles MS, Cox J, Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10(1):e1004047 doi: 10.1371/journal.pgen.1004047 ; PubMed Central PMCID: PMCPMC3879213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robles MS, Humphrey SJ, Mann M. Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab. 2017;25(1):118–27. doi: 10.1016/j.cmet.2016.10.004 . [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Mauvoisin D, Martin E, Atger F, Galindo AN, Dayon L, et al. Nuclear Proteomics Uncovers Diurnal Regulatory Landscapes in Mouse Liver. Cell Metab. 2017;25(1):102–17. doi: 10.1016/j.cmet.2016.10.003 ; PubMed Central PMCID: PMCPMC5241201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawking F, Worms MJ, Gammage K. Host temperature and control of 24-hour and 48-hour cycles in malaria parasites. Lancet. 1968;1(7541):506–9. . [DOI] [PubMed] [Google Scholar]
- 21.Mouahid G, Idris MA, Verneau O, Theron A, Shaban MM, Mone H. A new chronotype of Schistosoma mansoni: adaptive significance. Trop Med Int Health. 2012;17(6):727–32. Epub 2012/04/20. doi: 10.1111/j.1365-3156.2012.02988.x . [DOI] [PubMed] [Google Scholar]
- 22.Theron A. Hybrids between Schistosoma mansoni and S. rodhaini: characterization by cercarial emergence rhythms. Parasitology. 1989;99 Pt 2:225–8. Epub 1989/10/01. . [DOI] [PubMed] [Google Scholar]
- 23.Thurston JP. The periodicity of microfilariae. I. The distribution of microfilariae in the body. Trans R Soc Trop Med Hyg. 1951;45(3):307–28. . [DOI] [PubMed] [Google Scholar]
- 24.Southworth GC, Mason G, Seed JR. Studies on frog trypanosomiasis. I. A 24-hour cycle in the parasitemia level of Trypanosoma rotatorium in Rana clamitans from Louisiana. J Parasitol. 1968;54(2):255–8. Epub 1968/04/01. . [PubMed] [Google Scholar]
- 25.Hawking F. Circadian rhythms of Trypanosoma congolense in laboratory rodents. Trans R Soc Trop Med Hyg. 1978;72(6):592–5. Epub 1978/01/01. . [DOI] [PubMed] [Google Scholar]
- 26.Cornford EM, Freeman BJ, MacInnis AJ. Physiological relationships and circadian periodicities in rodent trypanosomes. Trans R Soc Trop Med Hyg. 1976;70(3):238–43. . [DOI] [PubMed] [Google Scholar]
- 27.Rijo-Ferreira F, Pinto-Neves D, Barbosa-Morais NL, Takahashi JS, Figueiredo LM. Trypanosoma brucei metabolism is under circadian control. Nat Microbiol. 2017;2:17032 doi: 10.1038/nmicrobiol.2017.32 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6(7):544–56. doi: 10.1038/nrg1633 ; PubMed Central PMCID: PMC2735866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hardin PE, Hall JC, Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990;343(6258):536–40. Epub 1990/02/08. doi: 10.1038/343536a0 . [DOI] [PubMed] [Google Scholar]
- 30.Aronson BD, Johnson KA, Loros JJ, Dunlap JC. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Science. 1994;263(5153):1578–84. Epub 1994/03/18. . [DOI] [PubMed] [Google Scholar]
- 31.Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, et al. Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science. 2005;308(5720):414–5. doi: 10.1126/science.1108451 . [DOI] [PubMed] [Google Scholar]
- 32.Ito H, Mutsuda M, Murayama Y, Tomita J, Hosokawa N, Terauchi K, et al. Cyanobacterial daily life with Kai-based circadian and diurnal genome-wide transcriptional control in Synechococcus elongatus. Proc Natl Acad Sci U S A. 2009;106(33):14168–73. doi: 10.1073/pnas.0902587106 ; PubMed Central PMCID: PMCPMC2729038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishiura M, Kutsuna S, Aoki S, Iwasaki H, Andersson CR, Tanabe A, et al. Expression of a gene cluster kaiABC as a circadian feedback process in cyanobacteria. Science. 1998;281(5382):1519–23. . [DOI] [PubMed] [Google Scholar]
- 34.Kojima S, Shingle DL, Green CB. Post-transcriptional control of circadian rhythms. Journal of cell science. 2011;124(Pt 3):311–20. doi: 10.1242/jcs.065771 ; PubMed Central PMCID: PMCPMC3021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107(7):855–67. . [DOI] [PubMed] [Google Scholar]
- 36.Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342(6158):1243417 doi: 10.1126/science.1243417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293(5529):510–4. Epub 2001/07/07. doi: 10.1126/science.1060698 . [DOI] [PubMed] [Google Scholar]
- 38.Figueiredo LM, Cross GA, Janzen CJ. Epigenetic regulation in African trypanosomes: a new kid on the block. Nat Rev Microbiol. 2009;7(7):504–13. doi: 10.1038/nrmicro2149 . [DOI] [PubMed] [Google Scholar]
- 39.Clayton CE. Gene expression in Kinetoplastids. Curr Opin Microbiol. 2016;32:46–51. doi: 10.1016/j.mib.2016.04.018 . [DOI] [PubMed] [Google Scholar]
- 40.Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8(3):217–30. doi: 10.1038/nrg2049 . [DOI] [PubMed] [Google Scholar]
- 41.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76–81. doi: 10.1038/nature01761 ; PubMed Central PMCID: PMCPMC2885907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ivleva NB, Gao T, LiWang AC, Golden SS. Quinone sensing by the circadian input kinase of the cyanobacterial circadian clock. Proc Natl Acad Sci U S A. 2006;103(46):17468–73. doi: 10.1073/pnas.0606639103 ; PubMed Central PMCID: PMCPMC1859952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory S. Gavelis PJKaBSL. How exaptations facilitated photosensory evolution: Seeing the lightby accident. Bioessays. 2017;39, 7, 1600266. doi: 10.1002/bies.201600266 [DOI] [PubMed] [Google Scholar]
- 44.Michael TP, Salome PA, McClung CR. Two Arabidopsis circadian oscillators can be distinguished by differential temperature sensitivity. Proc Natl Acad Sci U S A. 2003;100(11):6878–83. doi: 10.1073/pnas.1131995100 ; PubMed Central PMCID: PMCPMC164540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. Coupling governs entrainment range of circadian clocks. Molecular systems biology. 2010;6:438 doi: 10.1038/msb.2010.92 ; PubMed Central PMCID: PMCPMC3010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol. 2002;12(18):1574–83. . [DOI] [PubMed] [Google Scholar]
- 47.Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5(2):e34 doi: 10.1371/journal.pbio.0050034 ; PubMed Central PMCID: PMCPMC1783671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469(7331):498–503. Epub 2011/01/29. nature09702 [pii] doi: 10.1038/nature09702 ; PubMed Central PMCID: PMC3040566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93(6):929–37. . [DOI] [PubMed] [Google Scholar]
- 50.Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289(5488):2344–7. . [DOI] [PubMed] [Google Scholar]
- 51.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A. 2007;104(9):3450–5. doi: 10.1073/pnas.0611680104 ; PubMed Central PMCID: PMCPMC1802007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian Clock Proteins and Immunity. Immunity. 2014;40(2):178–86. doi: 10.1016/j.immuni.2014.02.002 . [DOI] [PubMed] [Google Scholar]
- 53.Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, et al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309(5734):630–3. doi: 10.1126/science.1115581 . [DOI] [PubMed] [Google Scholar]
- 54.Ouyang Y, Andersson CR, Kondo T, Golden SS, Johnson CH. Resonating circadian clocks enhance fitness in cyanobacteria. Proc Natl Acad Sci U S A. 1998;95(15):8660–4. ; PubMed Central PMCID: PMCPMC21132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16(21):R914–6. doi: 10.1016/j.cub.2006.09.058 ; PubMed Central PMCID: PMC1635966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang GZ, Hickey SL, Shi L, Huang HC, Nakashe P, Koike N, et al. Cycling Transcriptional Networks Optimize Energy Utilization on a Genome Scale. Cell reports. 2015;13(9):1868–80. doi: 10.1016/j.celrep.2015.10.043 ; PubMed Central PMCID: PMCPMC4680985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37(2):290–301. doi: 10.1016/j.immuni.2012.05.021 ; PubMed Central PMCID: PMC3428436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nature reviews Immunology. 2013;13(3):190–8. doi: 10.1038/nri3386 ; PubMed Central PMCID: PMCPMC4090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones MD, Ford MG, Gillett JD. Light-on and light-off effects on the circadian flight activity in the mosquito Anopheles gambiae. Nature. 1966;211(5051):871–2. . [DOI] [PubMed] [Google Scholar]
- 60.Taylor B, Jones MD. The circadian rhythm of flight activity in the mosquito Aedes aegypti (L.). The phase-setting effects of light-on and light-off. The Journal of experimental biology. 1969;51(1):59–70. . [DOI] [PubMed] [Google Scholar]
- 61.Rund SS, O'Donnell AJ, Gentile JE, Reece SE. Daily Rhythms in Mosquitoes and Their Consequences for Malaria Transmission. Insects. 2016;7(2). doi: 10.3390/insects7020014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Donnell AJ, Schneider P, McWatters HG, Reece SE. Fitness costs of disrupting circadian rhythms in malaria parasites. Proc Biol Sci. 2011; 278(1717):2429–36. doi: 10.1098/rspb.2010.2457 . [DOI] [PMC free article] [PubMed] [Google Scholar]