Abstract
Minimally invasive surgery, including robotic surgery, has become the standard of care for many abdominal procedures. However, the technical complexity associated with pancreaticoduodenectomy (PD) due to the anatomic location and oncologic characteristics of pancreatic tumors has hindered the widespread application of minimally invasive techniques to this procedure. Recent studies have reported that for experienced surgeons, the application of robotic techniques to PD is associated with equivalent oncologic outcomes and rates of complication when compared to an open operation, and may be associated with accelerated surgical recovery. Despite these encouraging results, robotic PD (RPD) is a procedure attempted by a small group of pancreatic surgeons, leading to the great heterogeneity in the techniques used to perform this operation. Herein we describe our technique for fully RPD and demonstrate its execution with a video supplement.
Keywords: Pancreaticoduodenectomy (PD), robotic surgery, Whipple procedure
Introduction
Pancreaticoduodenectomy (PD), also known as the Whipple, is known to be one of the most challenging and complex abdominal surgical procedures. It remains the only potentially curative procedure for periampullary tumors (1,2). The paramount complexity of this operation can be attributed to the anatomic location of the pancreas within the retroperitoneum, proximity to major visceral vasculature, and the difficult reconstruction required to re-establish gastrointestinal continuity (3-10). In 1994, Gagner and Pomp reported the first successful laparoscopic PD (11-13). Over time, studies have demonstrated the safety and feasibility of laparoscopic pancreatic surgery, as well as reported benefits in terms of postoperative outcomes and equivalent oncologic results when performed by experienced surgeons (14-18). Despite this, only a minority of pancreatic surgeons implemented the laparoscopic approach due to its particular technical challenges, including the difficult nature of the dissection, multiple anastomoses, and steep learning curve.
The development of the robotic surgical platform introduced a three-dimensional view and an extended range of motion, effectively overcoming many of factors limiting a laparoscopic approach to the pancreatic head. In 2003, Giulianotti and colleges reported the first series of robotic PD (RPD) (13). In subsequent years, varied robotic approaches RPD were published, including many hybrid applications of the technology (19). Clearly, despite the technological advantages of the robotic approach, there remains a steep learning curve to the mastery of its technical execution by surgeons. Many institutions remain in the early stages of implementing robotic pancreatic surgery, contributing to the wide variability in how these procedures are performed.
Despite the variation in RPD techniques, data from high volume centers support that RPD equal results in terms of morbidity and mortality, and associated with decreased intraoperative blood loss and length of stay when compared with open approaches (8). Additionally, other studies have demonstrated RPD is associated with equivalent oncologic outcomes in terms of margin positivity and adequate lymphadenectomy when compared with open techniques (20,21). Although the equivalency of RPD to established open techniques has been demonstrated, the robotic approach associated with extended operative times. Here we describe our technique for RPD, share our tips to facilitate the key steps of the procedure, and demonstrate its operative execution in the accompanying video (Figure 1).
Figure 1.

Fully robotic pancreaticoduodenectomy (Whipple procedure) (22). Available online: http://www.asvide.com/articles/1553
Patient selection
Careful patient selection is the critical first step in the successful application of the robotic approach to PD. The potential for conversion to an open approach is relatively high, with an overall conversion rate 17.8% in a recent review (8). While the decision to convert to an open procedure intra-operatively is a reflection of good surgical judgment, there exists a significant expenditure of resources associated with an aborted robotic procedure. This technique should be applied scrupulously with good preoperative as well as intraoperative judgment if it is to benefit outcomes and prove to be cost effective.
The patient’s body habitus, prior abdominal surgery, and etiology of the patient’s disease should be considered strongly. At experienced centers, RPD can be safely and effectively applied to a wide range of benign and malignant lesions of the pancreatic head and neck with varying degrees of vascular involvement (23-25). However, for those surgeons and institutions in the early stages of incorporating RPD into their practice, it is helpful to apply the technique first to small benign and premalignant pancreatic lesions, as these operations tend to be more anatomically straightforward and with minimal risk of vascular invasion (26). Due to these considerations, high quality imaging in the form of a pancreas protocol CT or MRI is required as part of the preoperative workup to determine resectability, as well as identify any aberrant vasculature.
Clinical summary
The patient in this video was a 68-year-old obese male who presented with several months of indigestion, weight loss and newly diagnosed diabetes mellitus. Subsequent imaging demonstrated a 3.2 cm × 2.3 cm mass in the head of the pancreas with associated dilatation of the main pancreatic duct to 5 mm. The mass was noted to abut the portal vein. Furthermore, the patient was noted to have an accessory right hepatic artery originating from the celiac axis. Endoscopic ultrasound and fine needle biopsy diagnosis of pancreatic ductal adenocarcinoma. Preoperative workup included a high quality abdominal CT scan showing resectable cancer and no evidence of distant metastases. Like other patients presenting with resectable disease at our institution, this patient was enrolled in a clinical trial which he received a combination of cyclophosphamide, nivolumab and a study vaccine in the neoadjuvant setting.
The patient underwent RPD and had an uneventful recovery. On postoperative day (POD) 1, the nasogastric tube was removed and the patient was started on sips of water and ice chips. Deep vein thrombosis prophylaxis was initiated and maintained throughout the hospital course. On POD 2 the Foley catheter was removed and he was able to void spontaneously. By POD 4, he was tolerating a regular diet with pancreatic enzyme supplementation. His JP drains were removed on POD 5 and 6. The average length of stay for a RPD at our institution is 7 days.
Setup
For maximum effectiveness and optimal outcomes after RPD, the importance of a surgical team (i.e., anesthesiologist, bedside assistant, scrub tech and circulator) with robotics experience cannot be overstated. We routinely place a nasogastric tube to decompress the stomach.
After induction of general anesthesia, the patient was placed in a supine and split leg position. A 15 mmHg pneumoperitoneum is established using a Veress needle or a Hasson technique. A 12 mm trocar was placed at the umbilical site and the robotic camera was introduced for abdominal exploration to rule out gross carcinomatosis, liver metastasis and extensive intraabdominal adhesions. If no prohibitive anatomy or pathology was identified, four 8 mm robotic trocars were placed (Figure 2) under direct visual guidance. After placement of all trocars, the da Vinci Xi system was docked from the patient’s left side, with the assistant at the bedside to facilitate instrument exchange.
Figure 2.
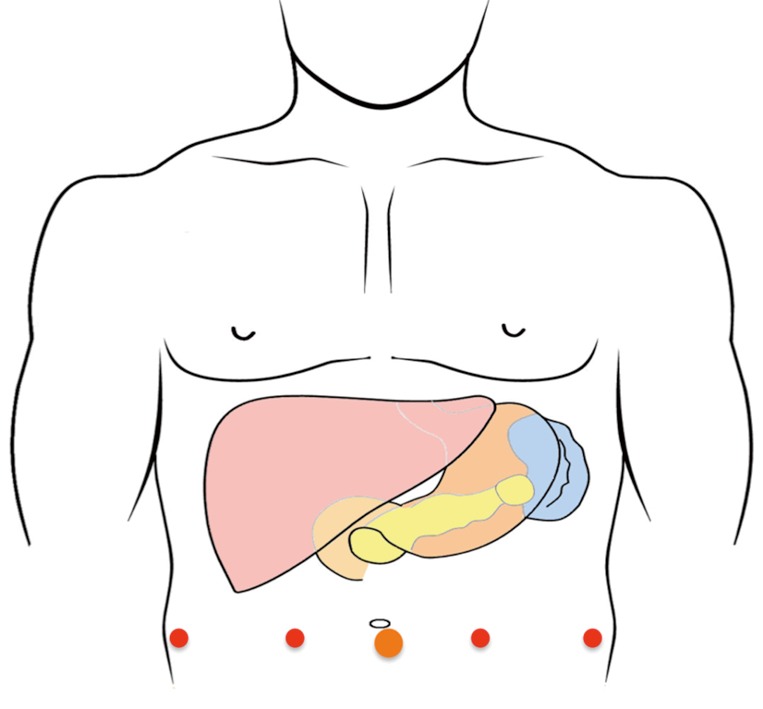
Trocar placement.
Exposure and dissection
In general, we perform the dissection starting with the bile duct, followed by the stomach, pancreas and jejunum. A robotic vessel sealer was used to divide the ligamentum teres and falciform ligament. This viable tissue flap may be used later to cover biliary anastomosis and the gastroduodenal artery (GDA) stump. We entered the lesser sac by dividing the gastro-colic ligament on the greater curvature of the stomach, exposing the pancreas. Once the superior and inferior borders of the pancreas were delineated, dissection of the porta hepatis was performed. We dissected the cystic duct and artery within Calot’s triangle. After confirming the critical view, we double clipped them with Hem-o-lock clips and transected them with the robotic scissor. We isolated the common hepatic duct and transected it above the confluence of the cystic and common bile duct. Of note, the aberrant anatomy of the hepatic artery should be considered here to avoid injury on a replaced right or accessory right hepatic artery, as was the case in our patient.
We routinely perform portal lymphadenectomy, which helps skeletonize the proper hepatic artery and portal vein. Once isolated, we traced the proper hepatic to the common hepatic artery. The root of the right gastric artery was identified, clipped and transected with the vessel sealer. Next, the GDA takeoff from the hepatic artery was isolated. The GDA was test clamped to ensure adequate hepatic perfusion via the common hepatic artery. Once adequate perfusion was confirmed, the GDA was ligated with a 2-0 silk tie, double clipped with Hem-o-lock clips, and transected with scissors. The vessel sealer was used to take the omentum down from the greater and lesser curves of the stomach. An Endo-GIA stapler was used to divide the stomach approximately 5 cm proximal to the pylorus. The choice of a green or black staple cartridge depends on the thickness of the stomach.
We continued the dissection to mobilize the hepatic artery away from the portal vein along the superior edge of the pancreas. During this part of the portal vein dissection, care should be taken to avoid uncontrolled division of the superior pancreaticoduodenal vein on the lateral side of the portal vein. Dissection was continued along the inferior edge of pancreas. With the portal vein dissected out above the pancreas, the superior mesenteric vein (SMV) could be easily identified. A retropancreatic tunnel on top of the portal vein was carefully created with blunt dissection. We used an umbilical tape to suspend the pancreatic neck to avoid injury to the portal vein during the transection of the pancreatic neck. We continued the sharp dissection to mobilize the pancreatic neck and head off the portal vein. Dissection was continued along the SMV. The first tributary to the SMV is the gastrocolic trunk of Henle, which drains the right gastroepiploic vein and the right superior colic vein. The Henle trunk was carefully dissected out, clipped and transected with scissors. Once the SMV was isolated, the omentum and transverse colon were free from the head of the pancreas.
The Kocher maneuver was performed to mobilize the pancreatic head and duodenum from the retroperitoneum. The inferior vena cava and the abdominal aorta were exposed. The left renal vein often located across the ventral side of the aorta was carefully identified. The root of the superior mesenteric artery (SMA) was above the left renal vein and needed to be very carefully preserved. The ligament of Treitz can often be divided on the right side of the root of mesentery if the patient has minimal amount of intra-abdominal fat. When the patient is obese, it is easier to identify the ligament of Treitz at its normal position and divide it before the Kocher maneuver. After the first portion of the jejunum was pulled to the right upper quadrant, it was transected using an Endo-GIA stapler. The vessel sealer was used to divide the mesentery along the jejunum until the uncinate of the pancreas.
The transection of the uncinate along the right side of the SMA was the most challenging part. The current robotic vessel sealer is bulky and not suitable for fine dissection. When the tumor is not involving the uncinate, the vessel sealer can be used to divide the tissue along the right side of SMA. If the tumor is close to the uncinate, we often use combination of hook cautery and bipolar Maryland clamp to transect the tissue along the SMA. The inferior pancreaticoduodenal artery was identified, isolated, clipped and transected with the vessel sealer. The dissection along the SMA was performed from caudal to cephalad direction. In this particular case, the accessory right hepatic artery was identified as coming from the celiac axis and preserved.
Finally, the gallbladder was taken down from the liver cystic plate using the hook cautery. A large Endo-Catch bag was used to retrieve the Whipple specimen.
Reconstruction
After the specimen was removed from the peri-umbilical port site, we re-established the pneumoperitoneum. Hemostasis was confirmed before the reconstruction. The order of anastomoses performed during the RPD was the same as that in the open procedure, in which the pancreaticojejunostomy (PJ) was performed first, followed by the hepaticojejunostomy (HJ), and finally the gastrojejunostomy (GJ).
We performed a retrocolic standard end-to-side PJ in 2 layers. The posterior edge of the pancreatic neck was secured to the bowel with a running 3-0 V-Loc suture. A pin-hole enterotomy was made next to the pancreatic duct. The duct-to-mucosa anastomosis was performed using 5-0 PDS sutures applied in an interrupted fashion. The caliber of the main pancreatic duct dictates the number of sutures on the PJ and the size of a pancreatic stent. We routinely use pediatric feeding tube as pancreatic stent to across the duct-to-mucosa anastomosis. In general, 4–6 interrupted sutures are sufficient to secure the duct-to-mucosa anastomosis. The PJ was finished with a second anterior line of running 3-0 V-Loc suture between the pancreas and jejunum.
Approximately 5 cm distal to the PJ, an end-to-side HJ was created. The 5-0 PDS sutures were placed in an interrupted fashion to secure the hepatic duct to jejunum. In this case, a total of 12 interrupted sutures were placed to form the HJ. The number of sutures should be dictated by the caliber of the dilated CBD. If the diameter of the hepatic duct were larger than 5 mm in diameter, we would choose the running PDS suture for this anastomosis.
Lastly, the jejunum on the left side of the mesenteric root was identified for GJ anastomosis. We performed an antecolic side-to-side isoperistaltic GJ utilizing a 60 mm blue load Endo-GIA stapler. In order to achieve a tension-free GJ anastomosis, it may be necessary to divide the omentum for obese patients. The enterotomy site was closed with 3-0 V-Loc in 2 layers in a running fashion. In this case, the GJ anastomosis was covered with an omental flap and the ligamentum teres flap was utilized to cover the GDA stump.
We routinely use two 19-French Blake drains through the existing lateral robotic port sites. We find it helpful to have a systematic convention in which the right-sided drain was positioned posterior to the PJ anastomosis and the left-sided drain anterior to the PJ anastomosis.
Tips, tricks and pitfalls
Selection of anatomically and pathologically favorable candidate patients is key for successful execution of RPD, especially for those new to the technique.
Once isolated, the GDA should be test clamped prior to division to assess hepatic perfusion via the common hepatic artery alone; if adequacy of flow is in question, a Doppler may be introduced for further confirmation.
Following retro-pancreatic tunneling, an umbilical tape is passed through the tunnel and used to retract the gland anteriorly to facilitate a controlled pancreatic division.
To prevent excess blood loss during final mobilization, care should be taken to actively identify the inferior pancreaticoduodenal vein on the anterior surface of the PV as well as small branches of SMA perfusing the specimen.
We perform a 2 layer end to side PJ: the pancreatic neck is secured to the bowel with a running 3-0 V-Loc suture and the pancreatic duct is secured to the jejunal mucosa with 4–6 interrupted 5-0 PDS sutures.
The HJ is completed in an end to side fashion with 10–12 interrupted 5-0 PDS sutures; the number of sutures is dictated by the caliber of the dilated CBD.
The tissue flap created on division of the ligamentum teres at the beginning of the case may be utilized for protection of the GDA stump.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Dai R, Turley RS, Blazer DG. Contemporary review of minimally invasive pancreaticoduodenectomy. World J Gastrointest Surg 2016;8:784-91. 10.4240/wjgs.v8.i12.784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He J, Ahuja N, Makary MA, et al. 2564 resected periampullary adenocarcinomas at a single institution: trends over three decades. HPB (Oxford) 2014;16:83-90. 10.1111/hpb.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doula C, Kostakis ID, Damaskos C, et al. Comparison Between Minimally Invasive and Open Pancreaticoduodenectomy: A Systematic Review. Surg Laparosc Endosc Percutan Tech 2016;26:6-16. 10.1097/SLE.0000000000000228 [DOI] [PubMed] [Google Scholar]
- 4.Joyce D, Morris-Stiff G, Falk GA, et al. Robotic surgery of the pancreas. World J Gastroenterol 2014;20:14726-32. 10.3748/wjg.v20.i40.14726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Memeo R, Sangiuolo F, de Blasi V, et al. Robotic pancreaticoduodenectomy and distal pancreatectomy: State of the art. J Visc Surg 2016;153:353-9. 10.1016/j.jviscsurg.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 6.Memeo R, Tzedakis S, de Blasi V, et al. Robotic Pancreaticoduodenectomy: Operative Steps (With Video). Surg Laparosc Endosc Percutan Tech 2016;26:e91-4. 10.1097/SLE.0000000000000304 [DOI] [PubMed] [Google Scholar]
- 7.Parisi A, Desiderio J, Trastulli S, et al. Robotic pylorus-preserving pancreaticoduodenectomy: Technical considerations. Int J Surg 2015;21 Suppl 1:S59-63. 10.1016/j.ijsu.2015.06.061 [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Cai H, Meng L, et al. Minimally invasive pancreaticoduodenectomy: A comprehensive review. Int J Surg 2016;35:139-46. 10.1016/j.ijsu.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 9.Zeh HJ, 3rd, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg 2011;45:323-40. 10.1016/j.yasu.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 10.Zhang YH, Zhang CW, Hu ZM, et al. Pancreatic cancer: Open or minimally invasive surgery? World J Gastroenterol 2016;22:7301-10. 10.3748/wjg.v22.i32.7301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker EH, Ross SW, Seshadri R, et al. Robotic pancreaticoduodenectomy for pancreatic adenocarcinoma: role in 2014 and beyond. J Gastrointest Oncol 2015;6:396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao CH, Wu YT, Liu YY, et al. Systemic Review of the Feasibility and Advantage of Minimally Invasive Pancreaticoduodenectomy. World J Surg 2016;40:1218-25. 10.1007/s00268-016-3433-1 [DOI] [PubMed] [Google Scholar]
- 13.Wright GP, Zureikat AH. Development of Minimally Invasive Pancreatic Surgery: an Evidence-Based Systematic Review of Laparoscopic Versus Robotic Approaches. J Gastrointest Surg 2016;20:1658-65. 10.1007/s11605-016-3204-1 [DOI] [PubMed] [Google Scholar]
- 14.Coratti A, Di Marino M, Coratti F, et al. Initial Experience With Robotic Pancreatic Surgery: Technical Feasibility and Oncological Implications. Surg Laparosc Endosc Percutan Tech 2016;26:31-7. 10.1097/SLE.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 15.Croner RS. Robotic Pancreatic Resections: Feasibility and Advantages. Indian J Surg 2015;77:433-5. 10.1007/s12262-015-1391-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng L, Lin S, Li Y, et al. Systematic review and meta-analysis of robotic versus open pancreaticoduodenectomy. Surg Endosc 2016. [Epub ahead of print]. 10.1007/s00464-016-5371-2 [DOI] [PubMed] [Google Scholar]
- 17.Place TL, Nau P, Mezhir JJ. Minimally invasive pancreatectomy for cancer: a critical review of the current literature. J Gastrointest Surg 2015;19:375-86. 10.1007/s11605-014-2695-x [DOI] [PubMed] [Google Scholar]
- 18.Zureikat AH, Postlewait LM, Liu Y, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg 2016;264:640-9. 10.1097/SLA.0000000000001869 [DOI] [PubMed] [Google Scholar]
- 19.Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas 2010;39:160-4. 10.1097/MPA.0b013e3181bd604e [DOI] [PubMed] [Google Scholar]
- 20.Corcione F, Pirozzi F, Cuccurullo D, et al. Laparoscopic pancreaticoduodenectomy: experience of 22 cases. Surg Endosc 2013;27:2131-6. 10.1007/s00464-012-2728-z [DOI] [PubMed] [Google Scholar]
- 21.Walsh RM, Chalikonda S, How I. Do It: Hybrid Laparoscopic and Robotic Pancreaticoduodenectomy. J Gastrointest Surg 2016;20:1650-7. 10.1007/s11605-016-3170-7 [DOI] [PubMed] [Google Scholar]
- 22.Galvez D, Sorber R, Javed AA, et al. Fully robotic pancreaticoduodenectomy (Whipple procedure). Asvide 2017;4:244. Available online: http://www.asvide.com/articles/1553
- 23.Adam MA, Choudhury K, Dinan MA, et al. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer: Practice Patterns and Short-term Outcomes Among 7061 Patients. Ann Surg 2015;262:372-7. 10.1097/SLA.0000000000001055 [DOI] [PubMed] [Google Scholar]
- 24.Baker EH, Ross SW, Seshadri R, et al. Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot 2016;12:554-60. 10.1002/rcs.1688 [DOI] [PubMed] [Google Scholar]
- 25.Piedimonte S, Wang Y, Bergman S, et al. Early experience with robotic pancreatic surgery in a Canadian institution. Can J Surg 2015;58:394-401. 10.1503/cjs.003815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boone BA, Zenati M, Hogg ME, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg 2015;150:416-22. 10.1001/jamasurg.2015.17 [DOI] [PubMed] [Google Scholar]


