Abstract
Introduction
Noncompaction cardiomyopathy is a rare phenotype of cardiomyopathy associated with severe cardiac arrhythmia and thromboembolic complications.
Case Presentation
A 55-year-old woman presented with frank pulmonary edema and received a diagnosis of noncompaction cardiomyopathy.
Discussion
Left ventricular noncompaction cardiomyopathy is increasingly being diagnosed because of advances in imaging modalities. It is important to differentiate this new phenotype of cardiomyopathy from others because its diagnosis, management, and prognosis differ. We reviewed the literature and summarized the diagnostic criteria, associated complications, initial and long-term management, and the recommendation for family screening.
INTRODUCTION
Noncompaction of the left ventricular (LV) myocardium is caused by arrest in embryonic endomyocardial morphogenesis. It is rare and can occur in association with congenital heart diseases and neuromuscular disorders, and as part of genetic syndromes.1
Noncompaction cardiomyopathy has an incidence of about 0.05% in the adult population.2 It is characterized by numerous and prominent trabeculations and intertrabecular recesses that extend between the LV cavity and subendocardium without any communication with the coronary circulation.3
We report a case of LV noncompaction cardiomyopathy (LVNC) in a woman who presented with frank pulmonary edema. Additionally, we conducted a review of the literature on the clinical presentation, diagnosis, and management of this rare entity.
CASE PRESENTATION
Presenting Concerns
A 55-year-old African American woman with a medical history of hypertension presented with progressive shortness of breath, cough with copious pink frothy sputum, and chest pain that started on the day of presentation. Her blood pressure was 168/104 mmHg, heart rate was 132/min, respiratory rate was 38/min, and oxygen saturation level was 88% on room air. On physical examination, she was dyspneic at rest, with bilateral coarse crackles; bilateral pitting pedal edema was noted.
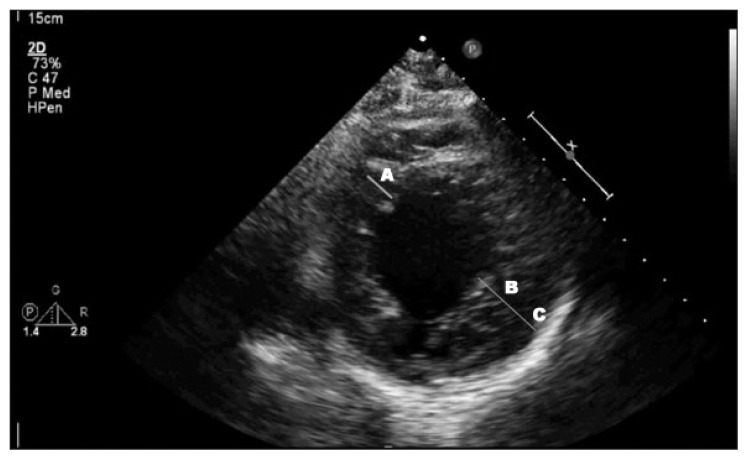
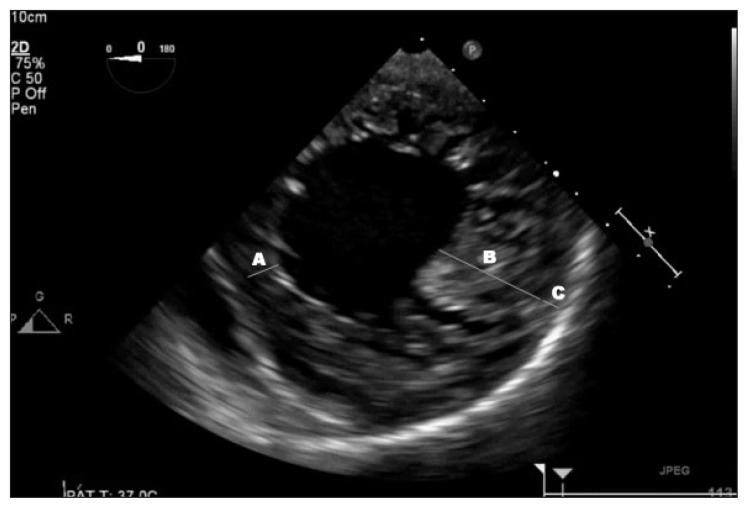
Results of laboratory studies revealed an N-terminal pro-brain-type natriuretic peptide level of 5911 pg/mL, a troponin level of 0.022 ng/mL that peaked at 2.360 ng/mL, and a lactic acid level of 14.7 mmol/L. A chest x-ray and a chest computed tomography (CT) scan that was performed to evaluate for pulmonary embolism showed bilateral pulmonary edema. An electrocardiogram revealed a new left bundle branch block (LBBB), and QTc was 442 milliseconds. A transthoracic echocardiogram showed a severely enlarged LV (Figure 1) with a left ventricular ejection fraction (LVEF) of 30% to 35%, severe posterolateral eccentric mitral regurgitation, and moderate to severe aortic regurgitation. The transesophageal echocardiogram revealed severe aortic regurgitation, moderate mitral regurgitation, and an endocardial wall that was highly suggestive of an LVNC (Figure 2). Findings of left-sided cardiac catheterization revealed normal coronary arteries and a congenital right-sided aortic arch without aneurysmal dilation, which was also confirmed on the chest CT scan.
Figure 1.
Parasternal short-axis view of transthoracic echocardiogram with measurement of wall thickness showing A) normal nonaffected wall, B) noncompacted subendocardial layer, and C) thinner and compacted subepicardial layer. Ratio of B:A is greater than 2.
Figure 2.
Transesophageal echocardiogram with wall thickness measurement showing A) normal nonaffected wall, B) noncompacted subendocardial layer, and C) thinner and compacted subepicardial layer. Ratio of B:A is greater than 2.
Therapeutic Intervention and Treatment
The patient was emergently intubated on presentation because of impending respiratory failure. She was started on an intravenous regimen of furosemide, 40 mg every 12 hours, and other supportive managements, including anticoagulation with heparin infusion and clopidogrel, 75 mg daily, because of an elevated troponin level and a new LBBB, but these treatments were discontinued after the coronary angiogram revealed normal coronary arteries. The patient improved greatly the next day and was extubated. Therapy with metoprolol, 12.5 mg twice daily, and ramipril, 5 mg daily, was started. A wearable defibrillator was provided in anticipation of future evaluation for cardiac resynchronization therapy with defibrillator if the LVEF remained low.
Follow-up and Outcomes
The patient’s symptoms improved substantially, and she was discharged with prescribed guideline-directed medical therapy for heart failure as well as outpatient follow-up for evaluation of her severe aortic regurgitation. On the basis of the transesophageal echocardiogram finding of possible LVNC, cardiac magnetic resonance imaging (MRI) was performed after her discharge, which confirmed LVNC of the LV apex that was best appreciated on the short-axis image (Figure 3). The compacted myocardium was 7.1 mm, whereas the noncompacted myocardium was 21 mm, resulting in a noncompacted-to-compacted ratio of approximately 3.0.
Figure 3.
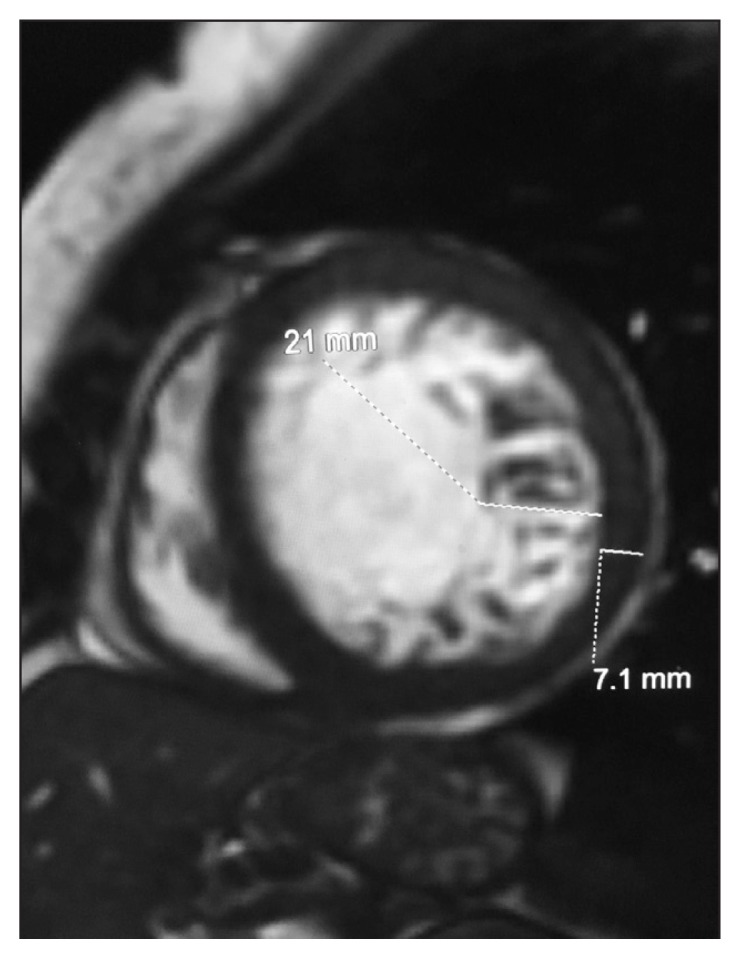
Cardiac magnetic resonance image showing short-axis of left ventricular apical myocardium. Compacted myocardium was 7.1 mm (bottom dashed line), and the noncompacted myocardium was 21 mm (top dashed line), resulting in a noncompacted-to-compacted ratio of approximately 3.0.
Approximately 3 weeks after discharge, the patient presented with complaints of being shocked by her wearable defibrillator. An electrocardiogram showed sinus rhythm at 89/min, an isolated premature ventricular contraction, LBBB, QRS duration of 185 milliseconds, and QTc of 510 milliseconds. Polymorphic ventricular tachycardia (VT; torsades de pointes), which was appropriately treated with the application of 150 J from the wearable defibrillator, was noted on analysis of the device results. Laboratory studies revealed a serum potassium level of 2.6 mmol/L and a magnesium level of 1.8 mmol/L. Her electrolyte levels were repleted. She was subsequently started on anticoagulation therapy because of an elevated risk of systemic thromboembolism associated with LVNC.
The patient subsequently underwent aortic valve replacement with a 19-mm aortic valve bioprosthesis (Trifecta, St Jude Medical Inc, St Paul, MN) and a mitral valve repair and annuloplasty using a 25-mm flexible adjustable annuloplasty ring (Attune, St Jude Medical Inc, St Paul, MN). After surgery, polymorphic VT developed with subsequent ventricular fibrillation arrest, requiring defibrillation and amiodarone therapy. She achieved return of spontaneous circulation after several rounds of cardiopulmonary resuscitation. She subsequently had a long and complicated hospital course, including another sternotomy with resection of the anterior leaflet of the mitral valve and replacement of the valve with a 25-mm porcine aortic valve bioprosthesis (Epic, St Jude Medical Inc, St Paul, MN) for severe treatment of mitral regurgitation.
The patient’s clinical status gradually improved, and she was enrolled in cardiac rehabilitation with plans for outpatient implantation of a biventricular implantable cardioverter-defibrillator (ICD). However, one day during her hospital stay, she had a cardiac arrest secondary to ventricular fibrillation, from which she could not be resuscitated (Table 1).
Table 1.
Timeline of the case
| Date | Relevant medical history and interventions | ||
|---|---|---|---|
| Summaries from initial and follow-up visits | Diagnostic testing | Interventions | |
| November 25, 2016 | A 55-year-old African American woman with a history of hypertension and smoking 1–2 cigarettes per d for 10 years presented with progressive shortness of breath, cough with copious pink frothy sputum, and chest pain that started on the day of presentation. Initial diagnostic assessment was acute respiratory failure because of acute pulmonary edema and new-onset congestive heart failure. History also included 1–2 glasses of wine twice a week and paternal diabetes mellitus. |
Laboratory and imaging studies (Nov 25): NT pro-BNP, 5911 pg/mL; troponin, 0.022 ng/mL that peaked at 2.360 ng/mL ECG: New LBBB; QTc, 442 milliseconds CT of chest: Bilateral pulmonary edema, right-sided aortic arch Transthoracic echocardiography (Nov 28): LVEF, 30%–35%; moderate to severe aortic regurgitation, severe mitral regurgitation Transesophageal echocardiography (Nov 29): LVEF, 25%–30%; severe aortic regurgitation, moderate mitral regurgitation, endocardial wall highly suggestive of left ventricular noncompaction Cardiac catheterization (Nov 30): No substantial coronary artery disease |
Patient was emergently intubated and started on intravenous diuresis. Guideline-directed medical therapy for heart failure (β-blocker and ACE inhibitor) was gradually introduced once acute pulmonary edema resolved. She was subsequently extubated. A low-salt, low-fat diet was started. Smoking cessation counseling was initiated. A wearable defibrillator was provided before her discharge on December 2, 2016. |
| December 23, 2016 | Outpatient tests | Cardiac MRI: Showed left ventricular noncompaction | |
| December 25, 2016 | Patient presented with syncope and subsequent shock from her wearable defibrillator. | Wearable defibrillator interrogation (Dec 25): Polymorphic ventricular tachycardia | Serum electrolyte levels were corrected. Wearable defibrillator was continued in anticipation of CRT-D implantation if LVEF remained low after 3 months of optimal medical therapy for heart failure. Anticoagulation therapy was started because of increased risk of thromboembolism with noncompaction diagnosed on cardiac MRI. Patient was discharged home on December 27, 2016. |
| January 30, 2017 | Patient presented for aortic valve replacement and mitral valve repair. She had a prolonged and complicated hospital course because of acute respiratory failure requiring mechanical ventilator support. | Aortic valve replacement and mitral valve repair (February 20). Mitral valve replacement (March 10). Cardiac rehabilitation was initiated, and her clinical status improved. | |
| March 26, 2017 | Sudden cardiac arrest developed because of ventricular fibrillation, and she could not be resuscitated. | ||
ACE = angiotensin-converting enzyme; CRT-D = cardiac resynchronization therapy with defibrillator; CT = computed tomography; ECG = electrocardiography; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; MRI = magnetic resonance imaging; NT pro-BNP = N-terminal pro-brain-type natriuretic peptide.
DISCUSSION
LVNC is a rare clinical entity that was first described in 1969 by Feldt et al after the discovery of a biventricular spongy myocardium with complete situs inversus in a 3-month-old female infant.4,5 However, there has been an increase in awareness and growing interest since the first isolated LVNC case and 2 case series published in 19846 and 1990,3 respectively. Its true prevalence is unknown, but it varies from 0.014% to 0.24% depending on the patient population being studied.7,8 The condition can occur in association with congenital heart diseases,1 as noted in this patient, who was also found to have a congenital right-sided aortic arch (Table 1).
LVNC has no predilection for a specific age group. Cases have been diagnosed in utero9 and as late as age 94 years.10 It appears to be more common in males than females.3,7,11 There is considerable variability in the genetic patterns and the phenotypical and clinical presentations. Both familial and sporadic forms have been described.11 The familial type is more common in the pediatric age group compared with adults and follows an X-linked, autosomal-dominant, or mitochondrial inheritance pattern.11,12 During the fifth and eighth week of embryonic development of the normal heart, the myocardium is compacted and the coronary arteries are formed.13 An arrest of the endomyocardial morphogenesis at this stage is thought to be the basis for myocardial noncompaction. This theory, however, was challenged by Bleyl et al,14 who reported 3 cases without myocardial noncompaction on intrauterine echocardiograms but later were diagnosed with LVNC.
The clinical presentation of LVNC varies widely. Patients may remain asymptomatic for years, although symptoms can develop as early as childhood. Symptomatic presentation includes LV systolic and diastolic dysfunction, heart failure, arrhythmias, thromboembolic events, and sudden cardiac death.15,16 In the systematic review conducted by Bhatia et al,15 the most common symptom prompting referral was shortness of breath (63%), and approximately 30% of patients had New York Heart Association Class III or IV congestive heart failure with a mean LVEF of 36%. In the study, heart failure was the most common cause of morbidity, and 15 patients were subsequently referred for heart transplant. In addition, most arrhythmias in patients with LVNC were VT and atrial fibrillation,15 with the prevalence of VT approaching 40%, and sudden cardiac death resulting in more than 55% of LVNC-related deaths. These presentations were also encountered in our patient. She presented with florid heart failure, and her LVEF was approximately 30%. Before her readmission, she had an episode of torsades de pointes, which was appropriately treated with the application of 150 J from the wearable defibrillator. Given the polymorphic nature of the VT and prolonged QTc, the event was thought to be triggered by electrolyte abnormality rather than a primary VT. However, it is known that LVNC may provide a pro-arrhythmogenic substrate for development of VT because the continuity between the endocardium and the deep intertrabecular recesses creates a pathway for reentrant circuits.7 A substantial number of patients will also experience systemic thromboembolism.15
The diagnosis of LVNC is based on the LV morphology. An echocardiogram is the test of choice for initial evaluation. Several diagnostic criteria are widely accepted for diagnosing noncompacted myocardium. The most extensive echocardiographic criteria were proposed by Jenni et al.17 The criteria include 1) an excessively thickened LV wall presenting with 2 layers of differing structure; 2) a noncompacted-to-compacted wall thickness ratio greater than 2:1 at end-systole; 3) communication of the deep intertrabecular recesses with the ventricular cavity, identified by color Doppler echocardiography; 4) absence of coexisting cardiac abnormalities; and 5) presence of multiple prominent trabeculations. In their proposal, Chin et al3 focused on the LV free wall at end-diastole in parasternal short-axis and apical views. Noncompaction is likely when the ratio of the distance from the epicardial surface to the trough of the trabecular recess and the distance from the epicardial surface to the peak of trabeculation is 0.5 or less. The presence of at least 3 different LV apical hypertrabeculations in a single imaging plane is also a diagnostic criterion proposed by Stöllberger and Finsterer.8 The authors further modified this criterion to include a 2-layered myocardium with a ratio of noncompacted to compacted myocardium greater than 2.0 at end-diastole. There are, however, several pitfalls with these echocardiographic parameters that are worth mentioning. It has been shown that about 68% of healthy hearts have prominent LV trabeculations,18 and these trabeculations can also be observed in hypertrophied hearts resulting from dilated, valvular, or hypertensive cardiomyopathy.7 In fact, LV trabeculations can be a normal finding in athletes and African Americans.19 In a study by Kohli et al,20 8% of healthy blacks in the control group and 24% of patients with heart failure in an outpatient general cardiology clinic fulfilled at least 1 or more of the criteria listed earlier. Careful assessment and use of these echocardiographic parameters is therefore warranted to prevent overdiagnosis.
New echocardiographic parameters are presently being considered to aid in the diagnosis of LVNC. Bellavia et al21 showed that there was a reduction in systolic strain, strain rate, and displacement in patients with LVNC independent of the LVEF. These techniques provide additional qualitative and objective assessment of LVNC.
Cardiac MRI, as reported in our case, is a useful diagnostic tool for LVNC. It is particularly important when echocardiographic findings are equivocal or there is inability to obtain a good-quality echocardiogram. It is also useful for assessing the severity of LVNC with the identification and quantification of the degree of fibrosis with delayed gadolinium enhancement.22 There are proposed criteria for diagnosis of noncompaction using cardiac MRI. Petersen et al23 studied 7 patients with a clinical diagnosis of LVNC and found that a ratio between the noncompacted and compacted myocardium greater than 2.3 measured at end-diastole accurately diagnosed LVNC. Although this approach has an 86% sensitivity and 99% specificity in individuals with suspected cardiac disease,24 a sizable number of patients (43%) in the MESA (Multi-Ethnic Study of Atherosclerosis)25 study without cardiac disease or hypertension met this cutoff point in at least 1 segment of the LV, suggesting that the specificity of this proposal is low in patients at low risk of LVNC. Another proposal by Jacquier et al26 is the calculation of LV trabecular mass using steady-state precision short-axis views. An LV trabecular mass greater than 20% of the total LV mass is suggestive of LVNC; however, this approach has been shown to have poor interobserver variability.24 CT angiography can be used when cardiac MRI is contraindicated or when echocardiographic findings are inconclusive. It also provides the benefit of assessing coronary arteries24; however, there is presently no consensus on specific criteria for diagnosing LVNC with CT angiography.
Symptomatic patients with LV systolic dysfunction and heart failure are treated conventionally according to American College of Cardiology/American Heart Association guideline-directed medical therapy. This includes the use of diuretics, β-blockers, and angiotensin-converting enzyme inhibitors. Addition of an aldosterone antagonist and digitalis may also be considered. Cardiac resynchronization therapy with or without ICD should be considered for symptomatic patients with New York Health Association Classes II, III, and IV, with LVEF of 35% or less despite optimal medical therapy, and QRS duration longer than 120 milliseconds in those patients with LBBB QRS morphology and 150 milliseconds or longer in non-LBBB QRS morphology. There has been reported improvement in functional capacity, LVEF and dimensions, and brain-type natriuretic peptide with the use of cardiac resynchronization therapy in LVNC cardiomyopathy.27,28
Although there is lack of solid evidence on the impact of appropriate therapy on the progression of noncompaction and improvement of LVEF, regression of LVNC has been reported in selected patients receiving appropriate therapy.29,30 A retrospective study evaluating the effect of β-blocker therapy in LVNC showed significant reduction in LV mass at one year compared with an increase in LV mass noted in patients not receiving a β-blocker; however, the LVEF was unchanged at one year.31 A case of significant improvement in LVEF with aggressive medical therapy (β-blocker, angiotensin-converting enzyme inhibitor, and furosemide) at one year was reported by Lin et al.32
According to the American College of Cardiology/American Heart Association guidelines on device-based therapy for cardiac rhythm abnormalities,33 there are sufficient observational data to indicate that placement of an ICD as a strategy to reduce the risk of sudden death is a reasonable clinical strategy in patients with LVNC. Implantation of an ICD should follow the general guidelines for primary and secondary prevention. In addition, ICD implantation is required if VTs are recorded, and in patients with normal systolic function or delayed gadolinium enhancement on cardiac MRI if an additional risk factor such as family history of sudden cardiac death, nonsustained VT, or previous syncope is present.4
Medically, the treatment of ventricular arrhythmia in LVNC is the same as with other patients.11 Routine anticoagulation therapy with warfarin has been recommended because of the increased risk of systemic thromboembolism in these patients. There is need for close long-term follow-up, and it is recommended that all first-degree relatives of affected patients undergo clinical screening for LVNC.34
CONCLUSION
LVNC is an increasingly recognized condition because of the ubiquity of echocardiography. Patients may remain asymptomatic or present with symptoms ranging from congestive heart failure and abnormal valvular function to ventricular arrhythmias, systemic thromboembolism, and sudden cardiac death, with no predilection for a certain age group. Diagnosis is essential because of the need for aggressive treatment, close follow-up, and clinical screening of all first-degree relatives of affected patients. Echocardiography is the imaging modality of choice. However, cardiac MRI and CT imaging are increasingly becoming acceptable diagnostic tools. A noncompacted-to-compacted myocardium ratio of greater than 2 at end-systole on echocardiogram is the most commonly used diagnostic criterion, and the ratio of noncompacted to compacted myocardium greater than 2.3 at end-diastole on cardiac MRI is suggestive of LVNC.
The treatment strategy includes a combination of guideline-directed medical therapy for the treatment of heart failure and cardiac arrhythmias, and prevention of systemic thromboembolism. Once the condition is diagnosed, aggressive medical management is required, including long-term anticoagulation therapy, because of increased risk of thromboembolism. In patients with normal systolic function, the occurrence of sudden cardiac death, nonsustained VT, or syncope should prompt immediate consideration for an ICD. Clinical screening of all first-degree relatives of affected patients is also recommended. Prospective studies are needed to improve the management and clinical outcome of this patient population.
Tough Organ
The heart is a tough organ: A marvelous mechanism that, mostly without repairs, will give valiant pumping service up to a hundred years.
— Willis John Potts, MD, 1895–1968 American Surgeon
Acknowledgments
The authors are indebted to the anonymous reviewers for providing insightful comments and suggestions for improvements on an earlier draft of the manuscript. All authors contributed equally to the preparation of this manuscript.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Oechslin E, Jenni R. Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur Heart J. 2011 Jun;32(12):1446–56. doi: 10.1093/eurheartj/ehq508. DOI: https://doi.org/10.1093/eurheartj/ehq508. [DOI] [PubMed] [Google Scholar]
- 2.Patil VC, Patil HV. Isolated non-compaction cardiomyopathy presented with ventricular tachycardia. Heart Views. 2011 Apr;12(2):74–8. doi: 10.4103/1995-705X.86019. DOI: https://doi.org/10.4103/1995-705X.86019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation. 1990 Aug;82(2):507–13. doi: 10.1161/01.cir.82.2.507. DOI: https://doi.org/10.1161/01.cir.82.2.507. [DOI] [PubMed] [Google Scholar]
- 4.Finsterer J, Stöllberger C, Towbin JA. Left ventricular noncompaction cardiomyopathy: Cardiac, neuromuscular, and genetic factors. Nat Rev Cardiol. 2017 Apr;14(4):224–37. doi: 10.1038/nrcardio.2016.207. DOI: https://doi.org/10.1038/nrcardio.2016.207. [DOI] [PubMed] [Google Scholar]
- 5.Feldt RH, Rahimtoola SH, Davis GD, Swan HJ, Titus JL. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am J Cardiol. 1969 May;23(5):732–4. doi: 10.1016/0002-9149(69)90037-x. DOI: https://doi.org/10.1016/0002-9149(69)90037-x. [DOI] [PubMed] [Google Scholar]
- 6.Engberding R, Bender F. [Echocardiographic detection of persistent myocardial sinusoids]. Z Kardiol. 1984 Dec;73(12):786–8. [Aritcle in German] [PubMed] [Google Scholar]
- 7.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000 Aug;36(2):493–500. doi: 10.1016/s0735-1097(00)00755-5. DOI: https://doi.org/10.1016/s1062-1458(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 8.Stöllberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr. 2004 Jan;17(1):91–100. doi: 10.1016/S0894-7317(03)00514-5. DOI: https://doi.org/10.1016/s0894-7317(03)00514-5. [DOI] [PubMed] [Google Scholar]
- 9.Winer N, Lefèvre M, Nomballais M, et al. Persisting spongy myocardium. A case indicating the difficulty of antenatal diagnosis. Fetal Diagn Ther. 1998 Jul-Aug;13(4):227–32. doi: 10.1159/000020843. DOI: https://doi.org/10.1159/000020843. [DOI] [PubMed] [Google Scholar]
- 10.Sato Y, Matsumoto N, Matsuo S, et al. Isolated noncompaction of the ventricular myocardium in a 94-year-old patient: Depiction at echocardiography and magnetic resonance imaging. Int J Cardiol. 2007 Jun 25;119(1):e32–4. doi: 10.1016/j.ijcard.2007.01.101. DOI: https://doi.org/10.1016/j.ijcard.2007.01.101. [DOI] [PubMed] [Google Scholar]
- 11.Sarma RJ, Chana A, Elkayam U. Left ventricular noncompaction. Prog Cardiovasc Dis. 2010 Jan-Feb;52(4):264–73. doi: 10.1016/j.pcad.2009.11.001. DOI: https://doi.org/10.1016/j.pcad.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Udeoji DU, Philip KJ, Morrissey RP, Phan A, Schwarz ER. Left ventricular noncompaction cardiomyopathy: Updated review. Ther Adv Cardiovasc Dis. 2013 Oct;7(5):260–73. doi: 10.1177/1753944713504639. DOI: https://doi.org/10.1177/1753944713504639. [DOI] [PubMed] [Google Scholar]
- 13.Freedom RM, Yoo SJ, Perrin D, Taylor G, Petersen S, Anderson RH. The morphological spectrum of ventricular noncompaction. Cardiol Young. 2005 Aug;15(4):345–64. doi: 10.1017/S1047951105000752. DOI: https://doi.org/10.1017/s1047951105000752. [DOI] [PubMed] [Google Scholar]
- 14.Bleyl SB, Mumford BR, Brown-Harrison MC, et al. Xq28-linked noncompaction of the left ventricular myocardium: Prenatal diagnosis and pathologic analysis of affected individuals. Am J Med Genet. 1997 Oct 31;72(3):257–65. DOI: https://doi.org/10.1002/(sici)1096-8628(19971031)72:3<257::aid-ajmg2>3.3.co;2-f. [PubMed] [Google Scholar]
- 15.Bhatia NL, Tajik AJ, Wilansky S, Steidley DE, Mookadam F. Isolated noncompaction of the left ventricular myocardium in adults: A systematic overview. J Card Fail. 2011 Sep;17(9):771–8. doi: 10.1016/j.cardfail.2011.05.002. DOI: https://doi.org/10.1016/j.cardfail.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Pignatelli RH, McMahon CJ, Dreyer WJ, et al. Clinical characterization of left ventricular noncompaction in children: A relatively common form of cardiomyopathy. Circulation. 2003 Nov 25;108(21):2672–8. doi: 10.1161/01.CIR.0000100664.10777.B8. DOI: https://doi.org/10.1161/01.CIR.0000100664.10777.B8. [DOI] [PubMed] [Google Scholar]
- 17.Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart. 2001 Dec;86(6):666–71. doi: 10.1136/heart.86.6.666. DOI: https://doi.org/10.1136/heart.86.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyd MT, Seward JB, Tajik AJ, Edwards WD. Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: Implications for evaluation of mural thrombi by two-dimensional echocardiography. J Am Coll Cardiol. 1987 Feb;9(2):323–6. doi: 10.1016/s0735-1097(87)80383-2. DOI: https://doi.org/10.1016/s0735-1097(87)80383-2. [DOI] [PubMed] [Google Scholar]
- 19.Rooms I, Dujardin K, De Sutter J. Non-compaction cardiomyopathy: A genetically and clinically heterogeneous disorder. Acta Cardiol. 2015 Dec;70(6):625–31. doi: 10.2143/AC.70.6.3120173. DOI: https://doi.org/10.2143/AC.70.6.3120173. [DOI] [PubMed] [Google Scholar]
- 20.Kohli SK, Pantazis AA, Shah JS, et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: Time for a reappraisal of diagnostic criteria? Eur Heart J. 2008 Jan;29(1):89–95. doi: 10.1093/eurheartj/ehm481. DOI: https://doi.org/10.1093/eurheartj/ehm481. [DOI] [PubMed] [Google Scholar]
- 21.Bellavia D, Michelena HI, Martinez M, et al. Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart. 2010 Mar;96(6):440–7. doi: 10.1136/hrt.2009.182170. DOI: https://doi.org/10.1136/hrt.2009.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett CE, Freudenberger R. The current approach to diagnosis and management of left ventricular noncompaction cardiomyopathy: Review of the literature. Cardiol Res Pract. 2016;2016:5172308. doi: 10.1155/2016/5172308. DOI: https://doi.org/10.1155/2016/5172308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen SE, Selvanayagam JB, Wiesmann F, et al. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005 Jul 5;46(1):101–5. doi: 10.1016/j.jacc.2005.03.045. DOI: https://doi.org/10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Gati S, Rajani R, Carr-White GS, Chambers JB. Adult left ventricular noncompaction: Reappraisal of current diagnostic imaging modalities. JACC Cardiovasc Imaging. 2014 Dec;7(12):1266–75. doi: 10.1016/j.jcmg.2014.09.005. DOI: https://doi.org/10.1016/j.jcmg.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Kawel N, Nacif M, Arai AE, et al. Trabeculated (noncompacted) and compact myocardium in adults: The Multi-Ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012 May 1;5(3):357–66. doi: 10.1161/CIRCIMAGING.111.971713. DOI: https://doi.org/10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquier A, Thuny F, Jop B, et al. Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J. 2010 May;31(9):1098–104. doi: 10.1093/eurheartj/ehp595. DOI: https://doi.org/10.1093/eurheartj/ehp595. [DOI] [PubMed] [Google Scholar]
- 27.Oginosawa Y, Nogami A, Soejima K, et al. Effect of cardiac resynchronization therapy in isolated ventricular noncompaction in adults: Follow-Up of four cases. J Cardiovasc Electrophysiol. 2008 Sep;19(9):935–8. doi: 10.1111/j.1540-8167.2008.01161.x. DOI: https://doi.org/10.1111/j.1540-8167.2008.01161.x. [DOI] [PubMed] [Google Scholar]
- 28.Stöllberger C, Blazek G, Bucher E, Finsterer J. Cardiac resynchronization therapy in left ventricular hypertrabeculation/non-compaction and myopathy. Europace. 2008 Jan;10(1):59–62. doi: 10.1093/europace/eum245. DOI: https://doi.org/10.1093/europace/eum245. [DOI] [PubMed] [Google Scholar]
- 29.Wong PH, Fung JW. Regression of non-compaction in left ventricular non-compaction cardiomyopathy by cardiac contractility modulation. Int J Cardiol. 2012 Feb 9;154(3):e50–1. doi: 10.1016/j.ijcard.2011.06.040. DOI: https://doi.org/10.1016/j.ijcard.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 30.Stöllberger C, Keller H, Finsterer J. Disappearance of left ventricular hypertrabeculation/noncompaction after biventricular pacing in a patient with polyneuropathy. J Card Fail. 2007 Apr;13(3):211–4. doi: 10.1016/j.cardfail.2006.11.007. DOI: https://doi.org/10.1016/j.cardfail.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Franke J, Pribe-Wolferts R, et al. Effects of β-blocker therapy on electrocardiographic and echocardiographic characteristics of left ventricular noncompaction. Clinical Research in Cardiology. 2015 Mar;104(3):241–9. doi: 10.1007/s00392-014-0778-z. DOI: https://doi.org/10.1007/s00392-014-0778-z. [DOI] [PubMed] [Google Scholar]
- 32.Lin T, Milks MW, Upadhya B, Hundley WG, Stacey RB. Improvement in systolic function in left ventricular non-compaction cardiomyopathy: A case report. J Cardiol Cases. 2014 Dec;10(6):231–4. doi: 10.1016/j.jccase.2014.08.004. DOI: https://doi.org/10.1016/j.jccase.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein AE, DiMarco JP, Ellenbogen KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008 May 27;51(21):e1–62. doi: 10.1016/j.jacc.2008.02.032. DOI: https://doi.org/10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA Heart Failure Society of America. Genetic evaluation of cardiomyopathy—a Heart Failure Society of America practice guideline. J Card Fail. 2009 Mar;15(2):83–97. doi: 10.1016/j.cardfail.2009.01.006. DOI: https://doi.org/10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]