Abstract
Background
Chronic rocking dizziness, often described as the feeling of being on a boat, is classically triggered by prolonged exposure to passive motion. Patients with this motion-triggered sensation of rocking, which is also known as mal de debarquement syndrome, often develop new onset headaches along with the dizziness. Chronic rocking dizziness has also been noted in vestibular migraine, occurring without a motion trigger. We sought to clarify the association between both motion-triggered (MT) and non-motion-triggered (non-MT) chronic rocking dizziness and headache history.
Methods
Our methods included questionnaire and interview study of subjects with either MTor non-MT chronic rocking dizziness.
Results
Onset of headaches was earlier in patients with non-MT rocking dizziness (median 26 years: MT; 16 years: non-MT). In MT subjects, there was a bimodal peak of age of onset of headache (20–29 years and 40–49 years). Most headache met criteria for migraine in both groups. By the time that chronic dizziness occurred, both groups had a comparable prevalence of migraine headache (41%: MT; 46%: non-MT). Pre-existing headache usually worsened after the onset of dizziness.
Discussion
Though rocking dizziness does not meet current criteria for vestibular migraine, migraine physiology may predispose to, develop in, or worsen with the onset of chronic rocking dizziness.
Keywords: Rocking dizziness, mal de debarquement syndrome, vestibular migraine, chronic subjective dizziness
Background
The relationship between headache and dizziness is necessarily complex as both are subjective experiences with large quantitative and qualitative variability. However, more unique forms of dizziness that are associated with migraine headaches may reveal distinct pathways for how dizziness and headache can be functionally related.
Mal de debarquement syndrome (MdDS) is the term applied to the classical motion-triggered (MT) form of dizziness that occurs after exposure to prolonged passive motion (1). This phenomenon of post-motion exposure rocking dizziness is generally short lived and is common even in healthy young males (2,3). However, this dizziness can sometimes persist for months or years with the most common demographic for persistent post-MT rocking dizziness being women between the ages of 40 and 50 years (4). Patients who develop this particular kind of dizziness describe the sensation as a feeling of “rocking on a boat.”
Non-motion-triggered (non-MT) rocking dizziness has been described as a symptom in other clinically defined syndromes. 1) Cohen et al. described rocking dizziness as a symptom in 41% of patients with vestibular migraine, but the criteria for diagnosing vestibular migraine was considered to be “inclusive” in this paper (5). It is unclear whether these patients would have met the most recently proposed criteria for vestibular migraine (6). 2) Patients diagnosed with chronic subjective dizziness (CSD) have been reported to describe a rocking and swaying sensation similar to those with classic MdDS but the prevalence of migraine in this group was reported to be no higher than population baseline (about 16.5%). CSD is likely more heterogeneous than MdDS, as it is divided into psychogenic, otogenic, and interactive groups. A hallmark difference between MdDS and CSD is that symptoms of CSD are worsened by exposure to motion, whereas the rocking of MdDS improves with motion (7). 3) Rocking dizziness has been linked to phobic postural vertigo since many patients with rocking dizziness develop intolerance to visual complexity, a key feature of phobic postural vertigo. Phobic postural vertigo, however, is usually an episodic disorder with clear environmental triggers and a high comorbidity with agoraphobia, with or without concurrent panic disorder (8). Detailed psychiatric profiling of patients with chronic rocking dizziness remains to be performed, but the current literature on MT chronic dizziness does not support a primary psychogenic origin.
In addition to sharing a common description of dizziness, we have found that many patients with MdDS develop headache with the onset of this syndrome and that a large proportion of patients with non-MT chronic rocking dizziness already have a personal history of headaches. However, a comparison of these two groups has not been presented in the literature to clarify the relationship that each has to headaches, in particular, migraine.
Therefore, in order to help clarify the relationship between chronic rocking dizziness and headache, we contacted, surveyed, and interviewed subjects whose predominant dizziness symptom was that of a chronic sensation of rocking. Our goals were to determine whether there are any significant differences between patients who develop MT rocking dizziness (MdDS) and those who develop non-MT rocking dizziness in terms of their clinical history of dizziness or headaches.
Methods
The following study design was performed in compliance with the Institutional Review Board of the University of California Los Angeles (UCLA), and specifically reviewed by Medical IRB 3, which oversees neuroscience research. Two-hundred-eighty-three participants with chronic rocking dizziness were recruited for this questionnaire study and were predominantly identified through a University Neurology database spanning the years 1985–2011. Of this total, about two-thirds were recruited from this database. The remaining one-third of the participants had contacted the investigators to participate in the study after hearing about research on MdDS at UCLA from other sources. Potential subjects were screened by reviewing their medical record, if available, or by an initial screening interview to determine their diagnosis. The study period extended from July 2011 to June 2012.
Subjects were included in the study if the following criteria were met: 1) Primary symptom of chronic rocking dizziness lasting at least one month; 2) for MT subjects, symptoms must have occurred within two days of disembarking from a boat, airplane, or land vessel with motion exposure lasting at least two continuous hours; 3) for non-MT subjects, no prolonged motion exposure of more than two continuous hours must have occurred within one month of the onset of their symptoms; 4) normal peripheral vestibular function testing with either an electronystagmogram or videonystagmogram; 5) there was no other cause for the rocking dizziness after an evaluation by a neurologist or otolaryngologist with appropriate testing.
All subjects provided written informed consent and were provided a Subject Bill of Rights document. If they indicated a willingness to be contacted after completing the questionnaire, they were interviewed over the telephone to clarify any answers. Only clear and unambiguous answers were tabulated.
Age of onset of rocking dizziness, headache, number of distinct episodes lasting at least one month, triggers, and effect of motion on rocking perception were ascertained. In order to determine a diagnosis of migraine headaches, subjects were asked about each component of the International Classification of Headache Disorders (ICHD) II criteria for migraine, i.e. severity, duration, quality, sidedness, number, and associated symptoms of photophobia, phonophobia, nausea, and visual aura (9), as well as other neurologic symptoms experienced during headaches. For the purposes of the study, the term vertigo was narrowly defined as the illusion of spinning. These scores were tabulated by a research assistant who did not have knowledge of the subjects’ previous diagnoses.
Statistical analysis
Two-sample unpaired t tests were performed for all continuous variables. Wilxcon Rank Sum tests were performed for the age of onset of headache and dizziness as they followed a non-parametric distribution. Fisher’s exact tests were calculated for categorical variables such as sex and presence of certain aura features. A Kruskal-Wallis equality of populations rank test followed by a chi-squared test to account for ties in rank was performed to evaluate differences in the effect of motion on symptoms, number of episodes, and continuation of symptoms at time of study. All p values reported are two tailed. An alpha level of 0.05 was used as a cut-off for significance, with Bonferroni correction for multiple comparisons when significance was achieved. Percentages were rounded off to the nearest whole numbers. Therefore, percentage totals may not equal 100%.
Results
A total of 135 questionnaires were returned. Three subjects returned cards indicating that they did not wish to participate. Twenty-two envelopes were returned for wrong addresses. After removing subjects who had rocking dizziness for less than one month, those in whom a clear distinction between MT and non-MT symptoms could not be determined, and those in whom adequate information to confirm a diagnosis of migraine headache was not available, 117 subjects remained. In total, 76 subjects with MT and 41 subjects with non-MT chronic rocking dizziness were included in the final analysis.
Demographics
Both groups showed a female predominance of more than 80% and an average age of onset of the first lifetime episode of chronic rocking dizziness in the fifth decade (Table 1). The median age of onset of rocking dizziness was several years younger in the non-MT group but this was not statistically significant.
Table 1.
Clinical features of subjects with motion-triggered (MT) and non-motion-triggered (non-MT) chronic rocking dizziness.
| Motion triggered | Non-motion triggered | ||
|---|---|---|---|
| Number of subjects | 76 | 41 | p value |
| Percent female | 62 (82%) | 34 (83%) | 1.00 |
| Age of onset of rocking dizziness | |||
| Median | 45 yrs | 40 yrs | 0.30 |
| Range | 12–69 yrs | 16–71 yrs | |
| Motion trigger | |||
| Sea travel | 50 (66%) | ||
| Air travel | 27 (36%) | ||
| Land travel | 7 (9%) | ||
| Number of distinct episodes ≥ one month | |||
| One | 46 (61%) | 24 (59%) | 0.19 |
| Two | 11 (14%) | 1 (2%) | |
| Three | 7 (9%) | 0 (0%) | |
| Four | 3 (4%) | 1 (2%) | |
| Five | 4 (5%) | 1 (2%) | |
| >Five | 1 (1%) | 6 (15%) | |
| Unknown/unsure | 4 (5%) | 8 (20%) | |
| Duration of first episode | |||
| Mean ± s.d. | 37.0 ± 47.6 mo | 79.9 ± 69.6 mo | <0.001 |
| Median | 18.0 mo | 58.0 mo | <0.001 |
| Range | 1–204 mo | 19–255 mo | |
| Symptoms at time of study | |||
| Continuing | 57 (75%) | 35 (85%) | 0.25 |
| Resolved | 19 (25%) | 4 (10%) | |
| Unknown | 0 (0%) | 2 (5%) | |
| Effect of re-exposure to passive motion | n = 75 | n = 40 | |
| Decrease rocking | 65 (87%) | 28 (70%) | 0.042 |
| No change | 9 (12%) | 8 (20%) | |
| Increase rocking | 1 (1%) | 4 (10%) | |
s.d.: standard deviation; yrs: years; mo: months.
In the MT group, sea travel was the most common trigger (66%), followed by air travel (36%) and land travel (9%). Sea travel included cruises, ferries, rafting, and small boats. Air travel was always on a commercial airline. Land travel could include cars, buses, or trains. In some subjects, there was more than one trigger associated with the onset of rocking dizziness so the total exceeded 100%.
Episode features
For most subjects, there was only one clear lifetime episode of rocking dizziness lasting at least one month (61% MT; 59% non-MT, chi-squared = 1.684, p = 0.19), but this first episode was significantly longer in subjects with non-MT onset of dizziness than for those with MT symptoms (median: 18 months MT; 58 months non-MT, p = <0.001). Consistent with the chronic nature of this disorder, most subjects were still experiencing their dizziness at the time of the survey (75% MT; 85% non-MT, chi-squared = 1.309, p = 0.25) (Table 1).
A classic phenomenon in MT chronic rocking dizziness is the temporary reduction of the rocking perception with re-exposure to passive motion, such as driving or riding in a car (10,11). Therefore, subjects were asked what effect driving had on their symptoms. In the MT group, the majority experienced reduction in the rocking perception with driving (87%), with only one subject reporting feeling worse (1%). In the non-MT group, driving reduced symptoms in most subjects (70%) but also worsened the rocking in a small group (10%), leading to an overall difference in motion effects in the two populations (chi-squared with ties = 4.105, p = 0.042).
Association with headache
Subjects were asked whether they were ever significantly bothered by headaches in their lifetime and at what age these started. They were asked whether specific features or associated symptoms of their headache changed after rocking dizziness began.
The median age of onset of headache was significantly lower in subjects with non-MT rocking dizziness (median 26 years MT; 16 years non-MT, p = 0.005). In both groups, more than half of the subjects reported experiencing significant headache in their lifetimes (57% MT; 59% non-MT) with the majority qualifying for a diagnosis of migraine (41% MT; 46% non-MT). As a proportion of the headaches experienced by the subjects, 72% of MT and 79% of non-MT headaches were migraines.
In subjects with MT dizziness, 18% had migraine headaches before the onset of dizziness, whereas 27% of non-MT subjects had prior migraine headaches. In both groups, new headaches that met the criteria for migraine also started with the onset of their chronic rocking dizziness (Table 2).
Table 2.
Percentage of headache and specific migraine features in motion-triggered (MT) and non-motion-triggered (non-MT) chronic rocking dizziness.
| Motion triggered | Non-motion triggered | ||
|---|---|---|---|
| Number of subjects | 76 | 41 | p value |
| Any significant headache | 43 (57%) | 24 (59%) | 1.00 |
| Age of onset of any significant headache (median) | 26 yrs | 16 yrs | 0.005 |
| Migraine headache, total | 31 (41%) | 19 (46%) | 0.70 |
| Migraine headache onset with dizziness | 17 (22%) | 8 (20%) | 0.82 |
| Migraine headache prior to dizziness | 14 (18%) | 11 (27%) | 0.35 |
| Visual aura, total | 22 (29%) | 18 (44%) | 0.15 |
| Visual aura w/o migraine headache | 12 (16%) | 9 (22%) | 0.45 |
| Visual aura w/migraine headache | 10 (13%) | 9 (22%) | 0.29 |
| Visual aura onset with dizziness | 5 (7%) | 4 (10%) | 0.72 |
| Other auras during migraine headache | of 31 total migraine | of 19 total migraine | |
| Confusion | 14 (45%) | 5 (26%) | 0.24 |
| Diplopia | 6 (19%) | 2 (11%) | 0.69 |
| Dysarthria | 7 (23%) | 3 (16%) | 0.72 |
| Unilateral weakness | 1 (3%) | 2 (11%) | 0.55 |
| Bilateral weakness | 2 (7%) | 3 (16%) | 0.36 |
| Unilateral sensory loss | 4 (13%) | 3 (16%) | 1.00 |
| Bilateral sensory loss | 3 (10%) | 0 (0%) | 0.28 |
| Hand incoordination | 10 (32%) | 3 (16%) | 0.32 |
| Tremor | 3 (10%) | 2 (11%) | 1.00 |
| Gait impairment | 11 (36%) | 6 (32%) | 1.00 |
| Vertigo | 4 (13%) | 2 (11%) | 1.00 |
| Tinnitus | 10 (32%) | 7 (37%) | 0.77 |
In both groups, there were a significant proportion of subjects who reported experiencing at least two lifetime episodes of visual auras lasting between five and 60 minutes (29% MT: 44% non-MT). In 7% of MT and 10% of non-MT subjects, the onset of the visual aura coincided with the onset of rocking dizziness. There were no significant differences in the prevalence of other potential aura symptoms between the two groups (Table 2).
Relative age of onset of headache and dizziness
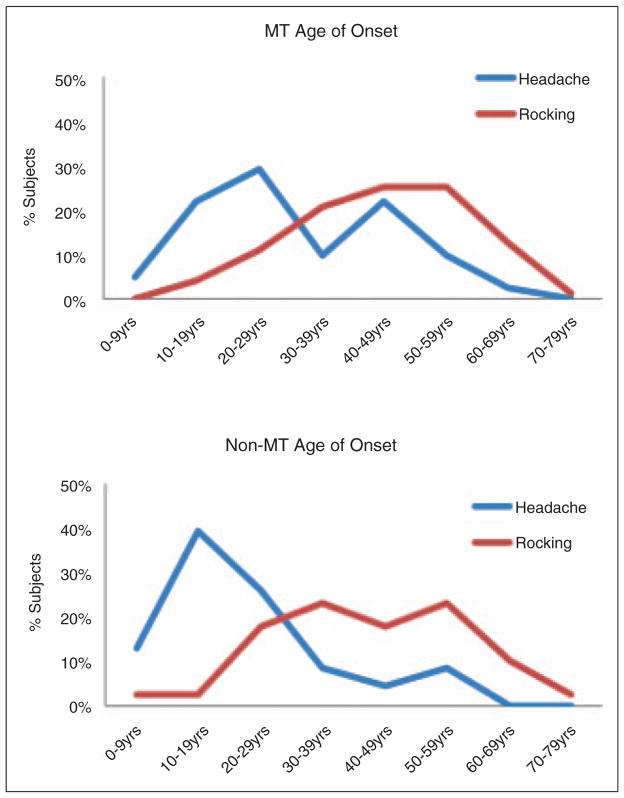
When the age of onset of rocking dizziness was compared to the age of onset of headache in the two groups, the MT group showed two peaks of onset of headache with the second peak coinciding with the onset of rocking dizziness in about half of the subjects. In the non-MT group, the headaches more frequently preceded the onset of dizziness (Figure 1)
Figure 1.
Age of onset of headache and chronic rocking dizziness in subjects with MT and non-MT symptoms, presented in decades.
MT: motion triggered; non-MT: non-motion-triggered.
Change in headache frequency with dizziness onset
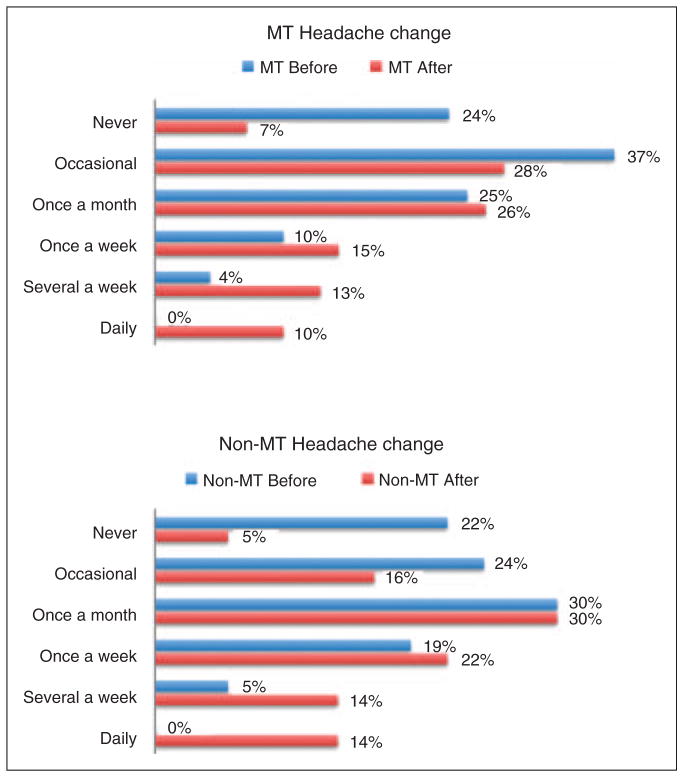
Because existing headaches can also change in frequency with the onset of dizziness, we asked subjects to describe the frequency of headache they experienced. They were given six choices and were asked to choose the response that best described the frequency of their headaches: 1: Never; 2: Occasionally (about one to two times per year); 3: Once a month, on average; 4: Once a week, on average; 5: Several times a week; 6: Daily. They were asked to give a score for before and after the onset of rocking dizziness.
Figure 2 shows the change in headache frequency for MT and non-MT subjects. In both groups, there was a shift toward an increased frequency of headaches after the onset of rocking dizziness.
Figure 2.
Change in headache frequency in MT and non-MT subjects before (blue) and after (red) the onset of chronic rocking dizziness.
MT: motion triggered; non-MT: non-motion-triggered.
In order to determine which groups of subjects were contributing to the increased frequency of headaches, all subjects were grouped according to their initial headache frequency. Change in headache frequency, whether increased, decreased, or no change, were counted for each group. The average change in score was noted.
Overall, most subjects noted either an increase or no change in headache frequency with the onset of the rocking dizziness. However, most of the increase in headache frequency was experienced by subjects who had never or only occasionally experienced headache prior to the onset of dizziness (Table 3). Only 11 of 117 subjects reported a decrease in headache frequency after the onset of rocking dizziness.
Table 3.
Change in headache frequency as a function of prior headache frequency.
| Pre-rocking dizziness headache frequency | Change in headache frequency
|
||||
|---|---|---|---|---|---|
| # of Subjects | Increased | No change | Decreased | Average change in score | |
| 1 = Never | 24 | 18 (75%) | 6 (25%) | 0 (0%) | 1.94 |
| 2 = Occasional | 34 | 17 (50%) | 16 (47%) | 1 (3%) | 0.99 |
| 3 = Once a month | 28 | 13 (46%) | 11 (39%) | 4 (14%) | 0.73 |
| 4 = Once a week | 14 | 3 (21%) | 7 (50%) | 4 (29%) | 0.02 |
| 5 = Several a week | 5 | 2 (40%) | 1 (20%) | 2 (40%) | −0.40 |
| 6 = Daily | 0 | 0 | 0 | 0 | NA |
Discussion
In trying to understand the biological links between headache and dizziness, it is probably not wise to group all forms of migraine-related dizziness together and demand a common behavior among them. The spectrum of dizziness associated with migraine is wide, and detailed study of each type may open a window into understanding a particular component of migraine physiology.
In this study, we found that the baseline prevalence of migraine headache in MT rocking dizziness (MdDS) was not much higher than averages reported for United States (US) women. A migraine prevalence of about 17%–18% in women has remained relatively consistent over the last two decades (12,13). This is similar to our finding in a previous smaller study of subjects with MdDS (10). Once the rocking dizziness syndrome settled in, however, the percentage of subjects meeting criteria for migraine was significantly higher than population baseline. As shown in Figure 1, there were two peaks of onset of headache in this group: one at a typical younger age, and one two decades later. The second peak coincided exactly with the onset of the rocking dizziness in about half of the subjects, suggesting that headaches are part of this syndrome in a significant proportion of these patients. This bimodal peak has also been described in migraine headache alone, suggesting that perhaps there is a common vulnerability to both migraine headaches and MdDS that occurs at this stage of life (14). Given the peak incidence of MdDS in the fifth decade and predominance of women experiencing this syndrome, there are also likely to be age-, hormonal-, and neuroplasticity-related brain changes that are relevant risk factors.
In contrast, a prior history of headaches does appear to be one possible risk factor in the development of non-MT rocking dizziness. This is supported by a prior finding that even in subjects with classical MdDS, a personal history of migraine is much more prevalent in those who go on to develop spontaneous recurrences of the same rocking dizziness after an MT episode (10). Although we had expected that the prevalence of migraine would always be higher in the non-MT group at every decade, we found that the overall prevalence of headache in both MT and non-MT rocking dizziness were quite similar by the time that rocking dizziness had developed. The main difference between these groups was the significantly earlier age of onset of headache in the non-MT group.
The terminology used to describe the association between migraine and dizziness has changed through the years, but the International Headache Society, in collaboration with the Committee for Classification of Vestibular Disorders of the Barany Society, has recently settled on the term vestibular migraine. A diagnosis of definite vestibular migraine will be included in the appendix of the International Classification of Headache Disorders third edition (15). This definition includes recurrent episodes of either vertigo or head motion-induced dizziness with nausea, with acute symptoms limited to five minutes to 72 hours.
The definition of vertigo for these criteria was derived directly from the consensus document for the Committee for the Classification of Vestibular Disorders of the Barany Society, which created the International Classification of Vestibular Disorders (ICVD) criteria (16). These criteria separate vertigo from other dizziness, with vertigo defined as a sense of self-motion when no actual self-motion is occurring, and dizziness defined as spatial disorientation without a false sense of motion. Under the ICVD criteria, symptoms triggered by prolonged passive motion “as occurs following sea voyages” are categorized under both “Other triggered vertigo” (ICVD 1.2.7) as well as “Other triggered dizziness,” (ICVD 2.2.7).
The Consensus document on vestibular migraine outlined restrictions on the kinds of vertigo that can contribute to a diagnosis of vestibular migraine to include those that are spontaneous, or triggered by positional, vision, or head motion changes (15). It specifically excluded sound-induced, Valsalva-induced, orthostatic, and other triggered vertigo (16). Head motion-induced nausea was included in the criteria for vestibular migraine but other forms of dizziness were specifically excluded in order to enhance specificity (6). Therefore, the phenomenon of MdDS was technically excluded in the diagnostic umbrella of vestibular migraine.
Despite the high temporal correlation between migraine onset and rocking dizziness in some subjects, the behavior of rocking dizziness whether motion induced or not, also does not fall into the current conceptualization of vestibular migraine as an episodic disorder (6). Similar to the diagnosis of migraine headaches, a diagnosis of definite vestibular migraine requires at least five episodes lasting less than 72 hours. In contrast, rocking dizziness usually starts as a chronic disorder with a single episode potentially lasting months or years. However, if the diagnosis of vestibular migraine is revised to include a chronic subtype, then many patients with non-MT rocking dizziness may be able to fit into these criteria.
There are several other distinctions between rocking dizziness and other forms of vertigo or dizziness, however. 1) The dizziness of MdDS or other rocking dizziness is almost never described as true rotational vertigo, is generally not associated with nausea, and is not associated with the peripheral vestibular deficits occasionally seen in vestibular migraine (4,16–18). In this study, only six total subjects specifically reported experiencing rotational vertigo during their migraine headaches. 2) Rocking dizziness usually improves with exposure to motion, as shown in this study, which is opposite the general pattern reported for vestibular migraine (10,11,19). 3) In a survey study, selective serotonin reuptake inhibitors and benzodiazepines were reported to be the most effective symptomatic treatments for rocking dizziness, whereas tricyclic amines frequently worsened symptoms (10). Although rigorous controlled trials on treatment for rocking dizziness are lacking, this pattern is not consistent with evidence through retrospective studies showing that medications effective in preventing migraine headaches may also be effective in preventing recurrent migraine-related vertigo spells (10,18,20–22).
Patients who present with rocking dizziness from MTs versus non-MTs are almost indistinguishable from a symptom standpoint, which has led some clinicians to refer to patients with non-MT chronic rocking dizziness as exhibiting an “MdDS-like” or “spontaneous MdDS” syndrome (23). A small proportion of patients with non-MT dizziness may experience worsened symptoms with motion while this is quite unusual in the MT group. Nevertheless, the majority of subjects in both groups reported temporary improvement in the perception of rocking when passively moved. It is possible given these similar features that these two groups have converged on the same abnormal brain physiology despite entering via different mechanisms. That migraine physiology can be induced by rather innocuous stimuli like prolonged periodic motion may be a clue as to the nature of the intrinsic vulnerability of this brain state.
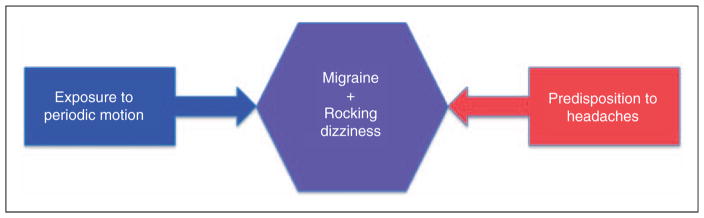
Supporting the possibility of a shared pathway in these two clinical phenomena is that a prior episode of MT rocking dizziness (MdDS) can be a risk factor for what appears to be spontaneous re-emergence of similar rocking dizziness (10). We hypothesize that classic MdDS is inducing activity within this pathway, whereas prior migraine physiology lowers the threshold to unmasking latent activity in the same pathway. Both lead to the same core physiology and symptom complex (Figure 3). Functional imaging studies in MdDS have shown increased resting state connectivity between visual processing areas and the left entorhinal cortex, a major driver of cerebral oscillatory activity, in these subjects (24). This is in the context of reduced connectivity with prefrontal circuits that regulate limbic activity. Continuous vestibular stimulation by exposure to a periodically moving vessel may have entrained these circuits to alter functional connectivity between posterior visual-vestibular processing areas and the entorhinal cortex over time, thus changing the way that new somatosensory information is processed and stored. One collateral effect of this is the induction of migraine headaches in some people. If similar functional changes also exist in patients with non-MT rocking dizziness, where migraine is the dominant risk factor, brain activity mediated through connectivity with the entorhinal cortex may be key in understanding the relationship between dizziness and headache.
Figure 3.
Convergence of risk factors to create a common state of rocking dizziness and migraine.
A limitation to our study is that subjects who referred to their abnormal head sensations as “pressure,” “fullness,” or “heaviness,” but did not consider themselves to be experiencing head pain were not tallied as having headache. However, from the follow-up interviews, it seemed that these were important symptoms that were also typically chronic. These descriptions may be relevant to the overall clinical picture of rocking dizziness and may be important to investigate in future studies.
A second limitation is inherent in any study acquiring historical data, which is that when assessing the change in frequency of headache, there is a risk of recall bias of previous health. The burden of headache in the context of another disruptive symptom may appear to be greater. Completely new headaches would be less likely to suffer from this recall bias, but milder headaches that may not have been noted previously may have become more salient to the patient as the overall burden of illness grew with time.
A final limitation is that as this study was administered through a tertiary-care center, we may have selected for subjects with more severe symptoms, especially those with longer duration of symptoms. Indeed, because both classical MdDS and non-MT dizziness are often not recognized as distinct clinical entities, many patients spend years seeking a diagnosis before their clinical picture is recognized (25).
This study investigated one aspect of a balance syndrome predominantly characterized by abnormal perceptions of rocking. There are certainly many other relevant comorbid factors involved, particularly because only half of the subjects in the study reported headache. Although not discussed in this work, other factors such as anxiety, hormonal changes, and other physiologic stress may also be important associated factors. These other factors are being investigated in separate analyses of this same population. The contribution of headache and specifically migraine to the syndrome of rocking dizziness appears to be quite significant, however, whether or not motion triggers are recognized.
Clinical implications.
Mal de debarquement syndrome (MdDS) is the term applied to the persistent chronic rocking dizziness that occurs after exposure to passive motion. This can be associated with new onset headaches.
A prior history of migraines is frequently seen in patients who develop the spontaneous onset of chronic rocking dizziness without a motion trigger.
In these two groups, headaches often worsen after the onset of rocking dizziness.
Rocking dizziness does not fit the current criteria for vestibular migraine because of its chronic rather than episodic nature. MdDS specifically is considered a form of “other triggered” vertigo or dizziness, which was not included in current conceptualization of vestibular migraine.
Re-exposure to motion reduces symptoms in most patients, but occasionally can worsen symptoms in non-motion triggered situations.
Acknowledgments
The authors thank the subjects who spent many hours completing the questionnaires and undergoing interviews.
Funding
This work was supported by National Institutes of Health/National Institute on Deafness and Other Communications Disorders (NIH/NIDCD) grant R03 DC010451 and the MdDS Balance Foundation Early Career Award.
Footnotes
Conflict of interest
None declared.
References
- 1.Brown JJ, Baloh RW. Persistent mal de debarquement syndrome: A motion-induced subjective disorder of balance. Am J Otolaryngol. 1987;8:219–222. doi: 10.1016/s0196-0709(87)80007-8. [DOI] [PubMed] [Google Scholar]
- 2.Gordon CR, Spitzer O, Shupak A, et al. Survey of mal de debarquement. BMJ. 1992;304:544. doi: 10.1136/bmj.304.6826.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon CR, Spitzer O, Doweck I, et al. Clinical features of mal de debarquement: Adaptation and habituation to sea conditions. J Vestib Res. 1995;5:363–369. [PubMed] [Google Scholar]
- 4.Cha Y-H. Mal de debarquement. Semin Neurol. 2009;29:520–527. doi: 10.1055/s-0029-1241038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JM, Bigal ME, Newman LC. Migraine and vestibular symptoms—identifying clinical features that predict “vestibular migraine”. Headache. 2011;51:1393–1397. doi: 10.1111/j.1526-4610.2011.01934.x. [DOI] [PubMed] [Google Scholar]
- 6.Lempert T, Newman-Toker D, Seemungal B, et al. Classification of vestibular migraine: Consensus with the International Headache Society. XXVIIth Barany Society meeting; Uppsala, Sweden. 10–13 June 2012. [Google Scholar]
- 7.Staab JP, Ruckenstein MJ. Expanding the differential diagnosis of chronic dizziness. Arch Otolaryngol Head Neck Surg. 2007;133:170–176. doi: 10.1001/archotol.133.2.170. [DOI] [PubMed] [Google Scholar]
- 8.Brandt T. Phobic postural vertigo. Neurology. 1996;46:1515–1519. doi: 10.1212/wnl.46.6.1515. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2. 2004. [Google Scholar]
- 10.Cha Y-H, Brodsky J, Ishiyama G, et al. Clinical features and associated syndromes of mal de debarquement. J Neurol. 2008;255:1038–1044. doi: 10.1007/s00415-008-0837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hain TC, Hanna PA, Rheinberger MA. Mal de debarquement. Arch Otolaryngol Head Neck Surg. 1999;125:615–620. doi: 10.1001/archotol.125.6.615. [DOI] [PubMed] [Google Scholar]
- 12.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 13.Stewart WF, Lipton RB, Celentano DD, et al. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–69. [PubMed] [Google Scholar]
- 14.Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia. 2010;30:1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]
- 15.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: Diagnostic criteria. J Vestib Res. 2012;22:167–172. doi: 10.3233/VES-2012-0453. [DOI] [PubMed] [Google Scholar]
- 16.Cass SP, Furman JM, Ankerstjerne K, et al. Migraine-related vestibulopathy. Ann Otol Rhinol Laryngol. 1997;106:182–189. doi: 10.1177/000348949710600302. [DOI] [PubMed] [Google Scholar]
- 17.Cutrer FM, Baloh RW. Migraine-associated dizziness. Headache. 2005;32:300–304. doi: 10.1111/j.1526-4610.1992.hed3206300.x. [DOI] [PubMed] [Google Scholar]
- 18.Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): Vestibular migraine? J Neurol. 1999;246:883–892. doi: 10.1007/s004150050478. [DOI] [PubMed] [Google Scholar]
- 19.Jeong S-H, Oh S-Y, Kim H-J, et al. Vestibular dysfunction in migraine: Effects of associated vertigo and motion sickness. J Neurol. 2010;257:905–912. doi: 10.1007/s00415-009-5435-5. [DOI] [PubMed] [Google Scholar]
- 20.Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: Report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337–1345. doi: 10.1212/WNL.0b013e3182535d20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baier B, Winkenwerder E, Dieterich M. “Vestibular migraine”: Effects of prophylactic therapy with various drugs. J Neurol. 2009;256:436–442. doi: 10.1007/s00415-009-0111-3. [DOI] [PubMed] [Google Scholar]
- 22.Bikhazi P, Jackson C, Ruckenstein MJ. Efficacy of antimigrainous therapy in the treatment of migraine-associated dizziness. Am J Otol. 1997;18:350–354. [PubMed] [Google Scholar]
- 23.Cha Y-H. Less common neuro-otologic disorders. Continuum (Minneap Minn) 2012;18:1142–1157. doi: 10.1212/01.CON.0000421623.56525.11. [DOI] [PubMed] [Google Scholar]
- 24.Cha Y-H, Chakrapani S, Craig A, et al. Metabolic and functional connectivity changes in mal de debarquement syndrome. PLoS One. 2012;7:e49560. doi: 10.1371/journal.pone.0049560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macke A, Leporte A, Clark BC. Social, societal, and economic burden of mal de debarquement syndrome. J Neurol. 2012;259:1326–1330. doi: 10.1007/s00415-011-6349-6. [DOI] [PubMed] [Google Scholar]