Abstract
Purpose
To test the ability of MION-47 enhanced MRI to identify tissue macrophage infiltration in a rabbit model of aortic valve sclerosis (AVS).
Materials and Methods
The aortic valves of control and cholesterol-fed New Zealand White rabbits were imaged in vivo pre- and 48 h post-intravenous administration of MION-47 using a 1.5 Tesla (T) MR clinical scanner and a CINE fSPGR sequence. MION-47 aortic valve cusps were imaged ex vivo on a 3.0T whole-body MR system with a custom gradient insert coil and a three-dimensional (3D) FIESTA sequence and compared with aortic valve cusps from control and cholesterol-fed contrast-free rabbits. Histopathological analysis was performed to determine the site of iron oxide uptake.
Results
MION-47 enhanced the visibility of both control and cholesterol-fed rabbit valves in in vivo images. Ex vivo image analysis confirmed the presence of significant signal voids in contrast-administered aortic valves. Signal voids were not observed in contrast-free valve cusps. In MION-47 administered rabbits, histopathological analysis revealed iron staining not only in fibrosal macrophages of cholesterol-fed valves but also in myofibroblasts from control and cholesterol-fed valves.
Conclusion
Although iron oxide labeling of macrophage infiltration in AVS has the potential to detect the disease process early, a macrophage-specific iron compound rather than passive targeting may be required.
Keywords: aortic valve sclerosis, macrophage infiltration, iron oxide, contrast-enhanced MRI
Aortic stenosis is a common, potentially lethal heart valve disease affecting 3–5% of the population over age 75 (1–3). Despite significant clinical consequences, no medical management currently exists for the precursor disease, aortic valve sclerosis (AVS), which is seen in 25% of the population over age 65 (1,2). As a result, surgical valve replacement is the only available approach and is implemented when symptoms develop, despite an operative mortality of approximately 5% (2,4).
In 1994, Otto et al clearly demonstrated that the sclerotic aortic valve process was actually an inflammatory disorder that has specific histopathological features which resemble atherosclerosis. These include lipid and collagen deposition, macrophage and T-lymphocyte infiltration, and eventually calcification (5). Given these common features, pharmaceutical therapies were suggested based on their success in the management of atherosclerosis. However, two large clinical studies assessing the value of statins (HMG-CoA reductase inhibitors) in the prevention of AVS progression have reported negative findings resulting in suggestions that the success or failure of treatments may be based on the timing of treatment initiation (6–11). An ongoing difficulty in these trials has been the inability of imaging techniques to identify the valvular abnormalities at an early stage (10,11). Patients included in the clinical studies were required to have echocardiographic evidence of at least a mild transvalvular gradient. Such findings indicate a substantially reduced valve orifice along with early valvular calcification. Unfortunately, once the calcification process has begun pharmaceutical intervention is less likely to be beneficial (2). Early detection is evidently necessary, and we believe MR imaging could be a valuable tool in the assessment of early valve disease. In this study, we examine the potential of an MR technique of cellular imaging to identify the inflammatory process in the valves as inspired by the current evaluation of vascular atherosclerosis.
Targeting using intravenously administered iron oxide contrast has become a widely accepted means of enhancing the visualization of macrophages in atherosclerotic lesions (12–15). Intravenously administered iron oxide particle uptake has been shown to be an early marker for atherosclerosis-associated inflammatory changes in the vessel wall and to precede the luminal narrowing detectable by conventional MR angiography (12–15). Active intraplaque macrophages readily phagocytose iron oxide particles within the plaque resulting in passive targeting of these inflammatory cells (14). Once internalized, iron oxide-based contrast agents generate local magnetic field inhomogeneities that shorten T2-T2* relaxation rates resulting in signal loss on MR images (12,14,16,17). In addition to the success of superparamagnetic nanoparticles in identifying sclerotic plaques, the appeal of these contrast agents is that they have completed phase III clinical trials and thus currently have the greatest potential for translation into the clinical arena (12–15,18,19).
In aortic valve sclerosis, an inflammatory macrophage infiltrate represents the early phase of the valvular sclerotic process, occurring before calcification (5). If aortic valve disease can be detected earlier (which is not possible at present) then disease progression may be prevented. In this study, a cholesterol-fed rabbit model was used to test the efficacy of identifying macrophage infiltration in early stage AVS using iron oxide-enhanced MRI.
MATERIALS AND METHODS
Animals
Animals were cared for in accordance with the guidelines of the Canadian Council on Animal Care. Ten male age-matched New Zealand White rabbits (1.6–1.8 kg) were fed 100 g/day of rabbit chow supplemented with low-level cholesterol (0.125–0.25% w/w) for 30 months to promote aortic valve sclerosis development. Five additional rabbits served as controls and were fed normal chow.
Contrast Agent
Monocrystalline iron-oxide nanoparticles (MION-47, Center for Molecular Imaging Research, Harvard Medical School, Charlestown, MA) is a blood pool agent with a mean particle size of 27.5 ± 6.8 nm (20,21). The plasma half-life of MION-47 is 11.4 ± 0.6 h in mice and the r1- and r2-relaxivities are 21.9 ± 3.6 mM−1s−1 and 44.6 ± 7.1 mM−1s−1, respectively, in an aqueous solution at 37°C and 0.47T (21). MION-47 was administered intravenously to five cholesterol-fed and two control-fed anaesthetized rabbits at a concentration of 11.2 mg Fe/kg in 10 mL of saline over a 15-min period.
In Vivo MRI Protocol
Five cholesterol-fed and two control-fed rabbits were imaged before and 48 h post-MION-47 administration. Imaging was performed under general anesthesia with an initial intramuscular injection of ketamine (23.4 mg/kg), xylazine (1.3 mg/kg), and glycopryolate (0.01 mg/kg), followed by intravenous administration of 1:10 dilution of the initial injection in saline by means of the marginal ear vein at a rate of 7–12 mL/h. Rabbits were imaged in the supine position using a 1.5 Tesla (T) MR clinical scanner (CV/i cardiac MRI scanner, GE Healthcare) interfaced with a two-channel radiofrequency phased array surface coil. All in vivo imaging was executed using CINE fast spoiled gradient echo (fSPGR) sequences gated retrospectively to the cardiac cycle (peripheral trigger, arrhythmia rejection window = 30, minimum trigger delay, 30 cardiac phases, 2 segments per view). The gating signal was obtained using a finger plethysmograph attached to the rabbit’s ear. The rabbit aortic valve was examined using three oblique sagittal slices each transecting one of the three aortic valve cusps (field of view = 8 cm, matrix 256 × 128, in-plane resolution 0.31× 0.62 mm, slice thickness = 2 mm, TR = 15.5 ± 1.4 ms, TE = 9.0 ± 0.6 ms, flip angle = 20°, bandwidth = ± 31.25 kHz, number of excitations = 6, 3 slices).
Preparation of Ex Vivo Specimens
Seventy-two hours after the administration of MION-47, the 15 rabbits (with or without contrast) were killed with an intravenous ketamine injection (100 mg). Rabbits were perfused with Hank’s Balanced Salt Solutions (HBSS) containing heparin (1 U/mL) after which aortic valve cusps were excised and submerged in 10% formalin for 1–2 h. Specimens were stored in 1 × phosphate buffered saline (PBS) at 4°C until preparation for ex vivo MR imaging. The noncoronary cusps of each rabbit were embedded in 1% agarose in 1 × PBS in a 5-mL plastic vial.
Ex Vivo MRI protocol
Ex vivo MR imaging was conducted on a 3.0T GE EXCITE whole-body MR system with a custom-built gradient insert coil (inner diameter 17.5 cm, maximum gradient strength 500 mTesla/m and peak slew rate 1500 Tesla/m/s) using a customized solenoid radiofrequency coil. Specimens were visualized in cross-section using a three-dimensional (3D) FIESTA pulse sequence (TR = 9.4 ms, TE = 4.6 ms, flip angle = 20°, bandwidth = ±16 kHz, 4 phase cycles, number of excitations = 2) at 100 × 100 × 200 μm3 resolution.
In Vivo MR Image Analysis
In vivo MR image analysis was performed off-line using the Ontario Consortium of Cardiac Imaging (OCCI) Viewer. Using the ruler tool of the viewer, three blinded observers examined oblique axial images of each cusp pre- and 48 h post-contrast administration. Thickness measurements were performed in the middle third of each cusp. Measurements from all three cusps were averaged to generate final thickness measurements for control and cholesterol-fed rabbit cusps pre- and post-MION-47. Contrast-to-noise ratio between the blood and aortic valve cusp (contrast-to-noise ratio [CNR] = SNRblood − SNRvalve) was also calculated for control and cholesterol-fed rabbit valves pre- and post-MION-47 administration.
Ex Vivo MR Image analysis
To analyze the percent area of voids seen in ex vivo MR images, five random slices of each cusp in cross-section were analyzed using Adobe Photoshop CS2 (version 8.0, Adobe Systems Inc). The outline of the valve was manually traced to determine total pixel area and void area was determined by thresholding. The number of positive pixels was reported as a percentage of valve pixel area. Percent areas from each group of rabbits were averaged to generate means for control and cholesterol-fed rabbit cusps with and without MION-47 contrast.
Histopathological Analysis
After ex vivo MR imaging, valve cusps were embedded in OCT by freezing in liquid-nitrogen cooled isopentane. Tissue blocks were serially sectioned, and sections were subjected to single labeling immunohistochemistry using Dako envision HRP+ system (horseradish peroxidase; Dako Canada, Mississauga, ON) according to the manufacturer’s instructions using the following monoclonal antibodies: anti-rabbit activated macrophage RAM11 (1:50, Dako Canada, Mississauga, ON), anti-smooth muscle α-actin clone 1A4 (1:100, Sigma-Aldrich Canada, Oakville, ON), and anti-CD31 clone JC/70A (1:50, Abcam Inc., Cambridge, MA). The secondary antibody used was goat anti-mouse IgG (H+L)-HRP conjugate (1:200, Bio-Rad Laboratories, Mississauga, ON). After antibody staining, slides were counterstained with Perl’s Prussian Blue to colocalize iron oxide uptake. Histological images were captured with an Axiovision 2IE microscope with an Axiocam 12MP camera and Axiovision 4.3 image acquisition software (Carl Zeiss, Canada).
Statistical Analysis
All data was analyzed with one-way analysis of variance followed by Tukey’s post hoc test using Graph-Pad Instat (GraphPad Software, Inc.). Values represent mean ± standard error. P < 0.05 indicated a significant difference between groups.
RESULTS
In Vivo MR Imaging
In precontrast images, control and cholesterol-fed aortic valves were visually distinct (Fig. 1a) with cholesterol-fed rabbit valve cusps displaying an average thickness significantly greater (P < 0.05) than control (0.46 ± 0.03 mm and 0.36 ± 0.02 mm, respectively, Fig. 1b). The average pre-MION-47 CNRs differentiating blood and the aortic valve cusp were calculated as 12.4 and 14.91 for control and cholesterol-fed rabbits, respectively.
Figure 1.
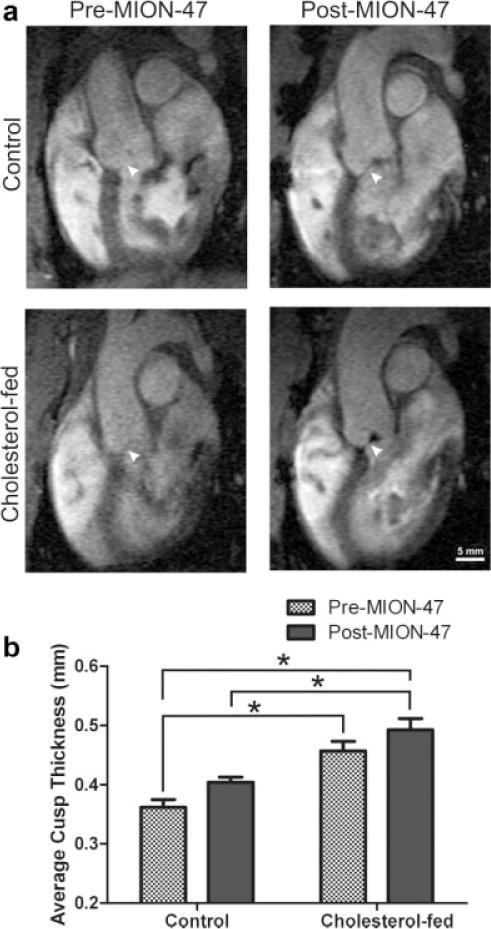
a: Retrospectively gated cine fSPGR oblique sagittal images of control and cholesterol-fed rabbit aortic valve right coronary cusps pre- and 48 h post- intravenous administration of 11.2 mg Fe/kg MION-47. b: Average cusp thickness of control and cholesterol-fed rabbit aortic valve cusps pre- and 48 h post-MION-47 administration. Data expressed as mean ±SEM. *P < 0.05.
The visibility of cholesterol-fed rabbit aortic valve cusps was enhanced in 48 h post-MION-47 administered (postcontrast) MR images compared with precontrast images from the same animal (Fig. 1a). Unexpectedly, the control animals also showed enhancement (Fig. 1a). Areas of hypointensity (negative contrast) could be detected in postcontrast images that were not present precontrast; however, the precise depth of these signal voids within the valve tissue could not be determined. Postcontrast administration average cusp thickness for control and cholesterol-fed rabbit valves were 0.40 ± 0.02 mm and 0.49 ± 0.002 mm, respectively. Thus, the measurable thickness difference between control and cholesterol-fed valve cusps persisted in postcontrast images (P < 0.05), however, the delineation of control versus diseased valve cusp thickness was not improved by the addition of MION-47. The addition of MION-47 improved the CNR for control and cholesterol-fed rabbit images equally with average values of 23.4 and 22.9, respectively.
Ex Vivo MR Imaging
The presence of signal voids in both MION-47 administered control and cholesterol-fed rabbit valves was confirmed by 3D FIESTA ex vivo imaging (Fig. 2a), and regions of hypointensity were shown to be predominantly localized to the fibrosal (aortic) side of the cusp. Quantification of signal void area showed significant increases in average void area with MION-47 administration when compared with contrast-free tissue from both control and cholesterol-fed rabbits (P < 0.05; Fig. 2b). No significant void area was observed for control or cholesterol-fed rabbit valves without MION-47. In MION-47 administered rabbits, the difference between the average signal void area detected in cholesterol-fed and control valve tissue was not significant.
Figure 2.
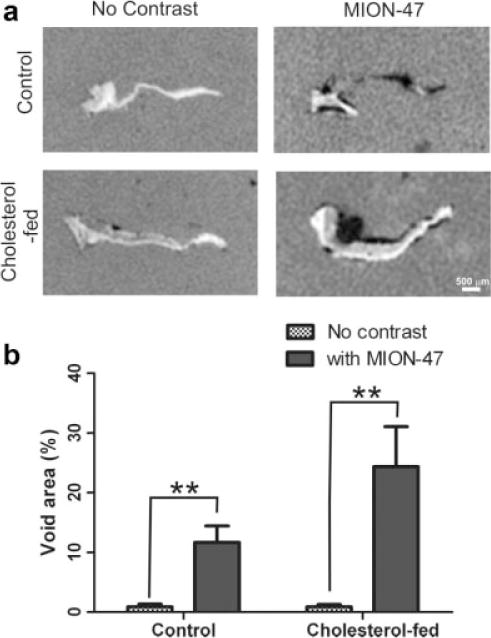
a: The 3D FIESTA ex vivo MR images of control and cholesterol-fed rabbit aortic valve cusps with and without MION-47. b: Average percent void area of control and cholesterol-fed rabbit aortic valve cusps with and without MION-47. Data expressed as mean ±SEM. **P < 0.01.
Histopathological Analysis
Areas of hypointensity on ex vivo MR images corresponded to regions of Perl’s Prussian blue staining in histological valve sections (Fig. 3a,b). As expected, histological analysis revealed punctate iron positive staining predominantly within subendothelial macrophages in cholesterol-fed rabbit tissue (Fig. 3b iv). Control tissue was devoid of macrophages irrespective of the presence or absence of MION-47 (Fig. 3b i & ii). All MION-47 administered rabbit aortic valve tissue displayed iron staining colocalized to smooth muscle α-actin positive myofibroblasts on the fibrosal side of the cusp (Fig. 3d ii & iv). The contrast agent-free rabbits did not exhibit Perl’s Prussian blue positive staining (Fig. 3b ic & ivc).
Figure 3.
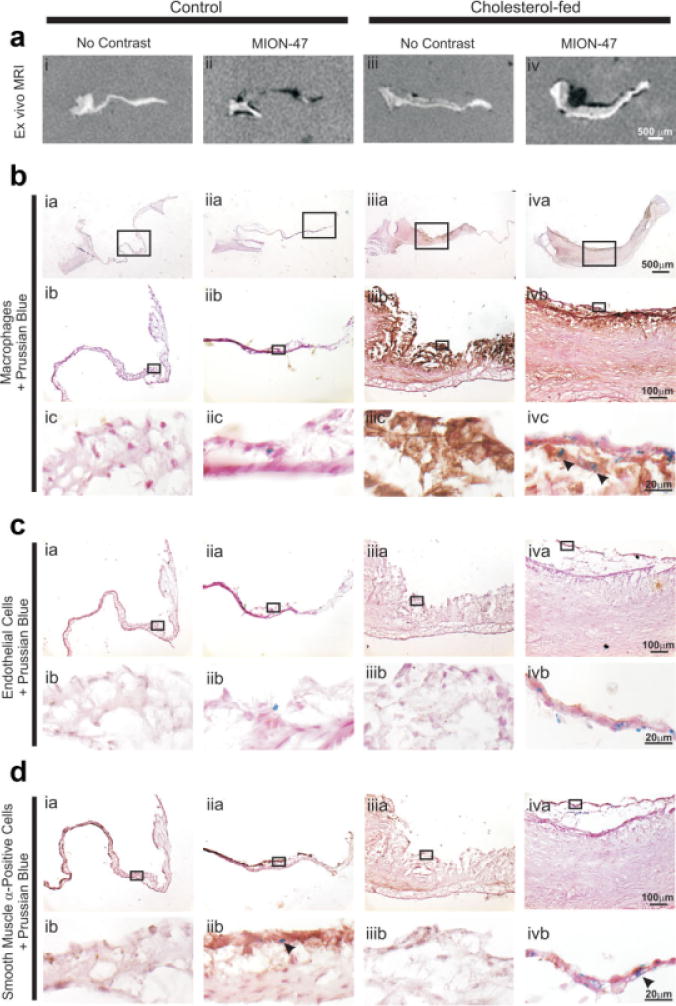
a–d: Immunohistochemical analysis of control and cholesterol-fed rabbit aortic valve cusps with and without MION-47. Immunohistochemistry was performed on aortic valve sections using the following antibodies: anti-RAM11 for macrophage infiltration, anti-CD31 for endothelial cells, anti-smooth muscle α-actin for myofibroblasts. Counterstaining was performed with Perl’s Prussian blue to indicate sites of iron uptake. Boxes indicate the site of magnification of lower panels. Arrowheads indicate colocalized staining. Example scale bars for each magnification can be seen in right hand panels.
DISCUSSION
Aortic stenosis is an inflammatory disease process that has considerable clinical consequences and no preventative therapy (1,2,4). The inability of clinical trials to pinpoint a beneficial pharmaceutical therapy has been hampered by the failure of current diagnostic techniques to reliably identify and monitor the disease process in its early “sclerotic” phase (10,11). Early features of AVS are remarkably similar to atherosclerosis, including endothelial dysfunction, subendothelial thickening, and macrophage infiltration (5). Magnetic resonance imaging holds particular promise for the early identification of this disease process because valvular thickening and macrophage infiltration, the two valvular changes that characterize the disorder in its early stages, have the potential to be examined using this modality.
It has previously been demonstrated that MRI has the capability of identifying increased valve thickness as valve disease develops (22). In our study, precontrast valve thickness of control and diseased valve tissue was measurably different, thereby validating this previous finding. Much like atherosclerosis, macrophage infiltration begins early in aortic valve disease and macrophages can accumulate before substantial valve thickening (5). With this in mind, we conducted a proof-of-principle study examining the use of an iron oxide-based contrast agent to image macrophage infiltration in AVS with MRI.
Our investigation of iron oxide-enhanced MR imaging yielded some unexpected results. MION-47 enhanced the in vivo visibility of cholesterol-fed as well as control rabbit aortic valves. This was reflected by improved cusp visibility and higher CNR values in contrast-enhanced images when compared with precontrast images of the same rabbits. Ex vivo imaging successfully confirmed the existence of significant signal voids in MION-47 administered rabbit valves. Because no signal voids were observed in aortic valves without MION-47 administration and contrast agent-free rabbits did not exhibit any positive iron staining, all voids were likely due to MION-47 uptake.
Our results showed that passive targeting of macrophage infiltration in the aortic valve does not provide a clear differentiation between early AVS and normal valves. We did, however, demonstrate the potential of macrophage labeling to provide that information. We showed that the macrophages infiltrating the valve during the sclerotic process will indeed phagocytose iron particles and their presence can be readily detected by MR. Histologically, iron deposition was found within cells on the fibrosal side of the aortic valve cusp immediately beneath the endothelial layer. This observation is consistent with findings for atherosclerotic plaques where iron positive staining has been found primarily in the subendothelial layer of arterial lesions with minimal infiltration deeper into the intima (12–15,18). As expected, macrophages in the fibrosal layer of our cholesterol-fed rabbit aortic valve lesions stained positively for iron. This useful observation was confounded by our simultaneous finding that iron staining myofibroblasts were also found in both cholesterol-fed and control aortic valve cusps, suggesting that, unexpectedly, valvular myofibroblasts also have phagocytic properties. This may have implications in the progress of the valve disease because these phagocytic cells were only found on the fibrosal side of the valve, the preferential site of lesion development.
To the best of our knowledge, phagocytic activity has not been previously reported for valvular myofibroblasts in vivo. We suggest that myofibroblasts under stress may also take on a macrophage-like phenotype as has been observed in vascular smooth muscle cells (18,23). Under normal conditions, local environmental factors such as differences in hemodynamic stress have already been shown to cause asynchronous differentiation of endothelial cell phenotypes on opposite sides of the valve (24). These factors may also result in differential phenotypes in valvular cells in the subendothelial layers of the valve; but further investigation is required to determine causality. Korosoglou and colleagues examined MION-47 uptake in atherosclerotic lesions of hyperlipidemic rabbits and observed particle uptake solely in macrophage-rich atherosclerotic plaques (15). Smooth muscle α-actin–positive cells were not investigated in control or sclerotic tissue. Several other atherosclerosis studies have noted an absence of iron oxide particle uptake in control vascular tissue (12–15) and when investigating iron oxide uptake in atherosclerotic lesions, few of these studies have investigated cell types beyond macrophages. One study, however, conducted by Durand et al found positive Perl’s staining predominantly in macrophages but also in smooth muscle α-actin–positive cells (13).
Overall, our study indicates that to target macrophages in the aortic valve with MRI will require a specifically targeted contrast agent. In the molecular imaging field, current work is being performed to develop such agents by modifying particle coating materials (14,25). For example, Smith and colleagues have developed a biochemically derivatized annexin V SPIO to target apoptotic cells, activated platelets, and nonapoptotic lipid-loaded cells such as foam cells in atherosclerotic lesions (26). Targeted SPIO produced negative contrast in atherosclerotic plaque at a concentration 200-fold lower than nonspecific SPIO and were shown to be preferentially taken up by macrophages. Other agents have been more specifically targeted to macrophages: Lipinski and colleagues developed a gadolinium containing immunomicelle linked to antibodies against macrophage scavenger receptor-A types I and II (27). The targeted immunomicelles significantly enhanced ApoE knockout murine aortas compared with untargeted micelles, while immunomicelle uptake was not detected in wild-type murine aortas without macrophage containing atherosclerotic lesions.
In conclusion, early identification of macrophage infiltration in the sclerotic aortic valve is an exciting frontier for molecular imaging. Iron oxide uptake in valvular myofibroblasts was an unexpected observation that reflects the complexity of aortic valve pathophysiology and warrants further investigation. Macrophage infiltration remains a key feature of early aortic valve disease and a valuable target for diagnostic imaging. A noninvasive technique that could identify earlier characteristics of valve disease such as macrophage infiltration would assist in patient identification at a time where pharmaceutical intervention may be beneficial. MRI has multiplanar imaging capabilities, excellent soft tissue contrast, and is safe for repetitive assessments, making it an ideal candidate for long-term evaluations of therapeutic interventions.
Acknowledgments
The authors thank Dr. Maria Drangova for in vivo MR protocol development, the Rutt lab for technical support, and the Weissleder lab for contrast agent development.
Contract grant sponsor: Heart and Stroke Foundation of Ontario; Contract grant number: T6332.
References
- 1.Stewart MD, Siscovick MD, Lind MS, et al. Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol. 1997;29:630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien KD. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more) Arterioscler Thromb Vasc Biol. 2006;26:1721–1728. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 3.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 4.Yeo K, Low R. Aortic stenosis: assessment of the patient at risk. J Magn Reson Imaging. 2007;20:509–516. doi: 10.1111/j.1540-8183.2007.00297.x. [DOI] [PubMed] [Google Scholar]
- 5.Otto C, Kuusisto J, Reichenbach D, Gown A, O’Brien KD. Characterization of the early lesion of ’degenerative’ valvular aortic stenosis. Histological and immunohistochemical studies. Circulation. 1994;90:844–853. doi: 10.1161/01.cir.90.2.844. [DOI] [PubMed] [Google Scholar]
- 6.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol. 2001;88:693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 7.Chua D, Kalb K. Statins and progression of calcified aortic stenosis. Ann Pharmacother. 2006;40:2195–2199. doi: 10.1345/aph.1H206. [DOI] [PubMed] [Google Scholar]
- 8.Bellamy MF, Pellikka PA, Klarich KW, Tajik AJ, Enriquez-Sarano M. Association of cholesterol levels, hydroxymethylglutaryl coenzyme-a reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol. 2002;40:1723–1730. doi: 10.1016/s0735-1097(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 9.Moura LM, Ramos SF, Zamorano JL, et al. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol. 2007;49:554–561. doi: 10.1016/j.jacc.2006.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 11.Rossebo AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 12.Ruehm SG, Corot C, Vogt P, Kolb S, Debatin JF. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation. 2001;103:415–422. doi: 10.1161/01.cir.103.3.415. [DOI] [PubMed] [Google Scholar]
- 13.Durand E, Raynaud JS, Bruneval P, et al. Magnetic resonance imaging of ruptured plaques in the rabbit with ultrasmall superparamagnetic particles of iron oxide. J Vasc Res. 2007;44:119–128. doi: 10.1159/000098484. [DOI] [PubMed] [Google Scholar]
- 14.Briley-Saebo K, Mulder W, Mani V, et al. Magnetic resonance imaging of vulnerable atherosclerotic plaques: current imaging strategies and molecular imaging probes. J Magn Reson Imaging. 2007;26:460–479. doi: 10.1002/jmri.20989. [DOI] [PubMed] [Google Scholar]
- 15.Korosoglou G, Weiss RG, Kedziorek DA, et al. Noninvasive detection of macrophage-rich atherosclerotic plaque in hyperlipidemic rabbits using “positive contrast” magnetic resonance imaging. J Am Coll Cardiol. 2008;52:483–491. doi: 10.1016/j.jacc.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yancy A, Olzinski A, Hu T, et al. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: critical determinants of atherosclerotic plaque labeling. J Magn Reson Imaging. 2005;21:432–442. doi: 10.1002/jmri.20283. [DOI] [PubMed] [Google Scholar]
- 17.Hyafil F, Laissy JP, Mazighi M, et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: relationship between signal loss and macrophage infiltration. Arterioscler Thromb Vasc Biol. 2006;26:176–181. doi: 10.1161/01.ATV.0000194098.82677.57. [DOI] [PubMed] [Google Scholar]
- 18.Briley-Saebo K, Mani V, Hyafil F, Cornily J, Fayad Z. Fractionated feridex and positive contrast: in vivo MR imaging of atherosclerosis. Magn Reson Med. 2008;59:721–730. doi: 10.1002/mrm.21541. [DOI] [PubMed] [Google Scholar]
- 19.Anzai Y, Piccoli CW, Outwater EK, et al. Evaluation of neck and body metastases to nodes with ferumoxtran 10-enhanced MR imaging: phase III safety and efficacy study. Radiology. 2003;228:777–788. doi: 10.1148/radiol.2283020872. [DOI] [PubMed] [Google Scholar]
- 20.Shen T, Weissleder R, Papisov M, Bogdanov AJ, Brady T. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med. 1993;29:599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- 21.Wunderbaldinger P, Josephson L, Weissleder R. Tat peptide directs enhanced clearance and hepatic permeability of magnetic nanoparticles. Bioconjug Chem. 2002;13:264–268. doi: 10.1021/bc015563u. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton AM, Rogers KA, Drangova M, et al. The in vivo diagnosis of early stage aortic valve sclerosis using magnetic resonance imaging in a rabbit model. J Magn Reson Imaging. 2009;29:825–831. doi: 10.1002/jmri.21729. [DOI] [PubMed] [Google Scholar]
- 23.Nahrendorf M, Zhang H, Hembrador S, et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly KA, Shaw SY, Nahrendorf M, et al. Unbiased discovery of in vivo imaging probes through in vitro profiling of nanoparticle libraries. Integr Biol. 2009;1:311–317. doi: 10.1039/b821775k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith B, Heverhagen J, Knopp M, et al. Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI) Biomed Microdevices. 2007;9:719–727. doi: 10.1007/s10544-007-9081-3. [DOI] [PubMed] [Google Scholar]
- 27.Lipinski M, Amirbekian V, Frias J, et al. MRI to detect atherosclerosis with gadolinium-containing immunomcelles targeting the macrophage scavenger receptor. Magn Reson Med. 2006;56:601–610. doi: 10.1002/mrm.20995. [DOI] [PubMed] [Google Scholar]


