Abstract
Background
There are gender-specific variations in the epidemiology and clinical course of hepatitis C virus (HCV) infection. However, few long term longitudinal studies have examined trends in the incidence and prevalence of serious liver complications among women compared with men with HCV infection.
Methods
We used the Veterans Administration (VA) Corporate Data Warehouse to identify all veterans with positive HCV viremia from January 2000 to December 2013. We calculated gender-specific annual incidence and prevalence rates of cirrhosis, decompensated cirrhosis and hepatocellular cancer (HCC) adjusting for age, diabetes, HIV and alcohol use. We also calculated the average annual percent change (AAPC) for each outcome by gender using piecewise linear regression in the Joinpoint software.
Results
We identified 264,409 HCV-infected veterans during 2000–2013, of whom 7162 (2.7%) were women. There were statistically significant increases over time in the incidence rates of cirrhosis, decompensated cirrhosis, and HCC for both men and women. The annual adjusted incidence rates of cirrhosis, decompensated cirrhosis and HCC were higher in men than women for all study years. However, these complications increased at a similar rate in both groups. Specifically, the AAPC for cirrhosis was 13.1 and 15.2, while it was 15.6 and 16.9 for decompensated cirrhosis and 21.0 and 25.3 for HCC in men and women, respectively (all test of parallelism not significant). The results were similar in the prevalence analyses although AAPCs were slightly smaller for each outcome.
Conclusion
We found an ongoing upward trend in the incidence and prevalence of HCV complications in this cohort of HCV-infected women. This increase in cirrhosis complications in women with active HCV infection is similar to those in men. With cure from HCV now becoming a reality, most of the projected burden of HCV is potentially preventable. However, benefits of HCV treatment will need to extend to all patients in order to stem the rising tide of HCV complications.
Keywords: HCV, veterans, Cirrhosis, HC
Introduction
Chronic hepatitis C virus (HCV) infection is a progressive condition1 that is the most common cause of cirrhosis, hepatocellular cancer (HCC) and liver disease deaths in the U.S. There are gender related variations in the epidemiology and clinical course of HCV infection. For example, men are at least 1.7 times more likely to be infected with HCV than women.2 Among individuals with chronic HCV, men are also at a higher risk of progression to advanced hepatic fibrosis, cirrhosis and decompensated liver disease than women.3–5 Studies suggest that estrogen hormone may have a protective role against hepatic fibrosis by inhibiting stellate cells, which are responsible for fibrogenesis in the liver.6;7 However, this possible biological advantage for women seems to be limited to younger premenopausal women and is attenuated in older and post-menopausal women. Indeed, post-menopausal women may have a more accelerated progression of fibrosis than men.8–10
The prevalence of liver-related sequelae of chronic HCV such as cirrhosis, decompensated liver disease, and HCC has been increasing in recent years11, in parallel with the aging of the chronically infected HCV cohort in the U.S. However, it is unknown if the burden of HCV-related complications differed between women and men.
Few long-term studies, none of which were conducted in the U.S., have examined temporal trends in the incidence and prevalence of liver complications among women with HCV infection compared with men. Recent data from Japan—where HCV epidemic preceded the U.S. epidemic by 30–40 years—showed an increase in the complications from HCV (e,g., HCC) among women following the decline of these complications in men. For example, the male/female ratio of HCC cases in Japan was 4.5 in 1984–1985 but declined to 2.5 in 2002–2003 due to an increase of HCC in women.12;13 Similar trends have been seen in several European regions such as France.5 Combined, these data suggest that the burden of cirrhosis and HCC in HCV infected women may just be lagging behind that of HCV infected men.
The US Department of Veterans Affairs (VA) is the largest provider of health care to HCV-infected individuals in the U.S. VA is also a semi-closed system making long-term studies of incidence and prevalence of HCV complications in large cohorts feasible. Using a national sample of U.S. Veterans with active HCV, including over 5,800 women, we examined gender-related differences in the incidence and prevalence of cirrhosis, decompensated cirrhosis, and HCC between 2000 and 2013.
METHODS
Data Source
We used the VA Corporate Data Warehouse (CDW) to identify a cohort of veterans with chronic HCV infection who used one of 129 VA healthcare centers nationwide. CDW includes ICD-9 codes for inpatient and outpatient encounters, results from Alcohol Use Disorders Identification Test (AUDIT-C) screens, laboratory data, pharmacy data, and the Vital Status file which captures death and corresponding date.
Study Design and Population
We identified all veterans in the CDW data who had a positive HCV RNA test between January 2000 and December 2013.
Study Outcomes
Outcomes of interest were ascertained between January 2000 and December 2013 and included cirrhosis defined by previously validated ICD-9 codes14 (571.2, 571.5, 571.6, or decompensated cirrhosis), decompensated cirrhosis (070.71, 070.41, 070.44, 070.0, 070.20, 070.21, 070.22, 070.23, 070.42, 070.43, 070.49, 070.6, 572.2, 348.3, 348.31, 348.39, 456.0, 456.1, 456.20, 456.21, 572.3, 572.4, 789.5, 789.59) and HCC (155.0 without 155.1). All-cause mortality and date of death were obtained from the VA Vital Status File.
Potential Confounders
We examined several confounding variables including date of birth, age at first VA visit, race/ethnicity, diabetes, alcohol use disorder, and HIV co-infection. We defined alcohol use disorder by combination of at least two ICD-9 codes and/or positive AUDIT-C screen at least 6 months apart any time during the study follow up. We relied on ICD-9 codes for diabetes and HIV diagnosis.
We defined yearly time-varying variables indicating years with diagnosed HCV, and for patients with cirrhosis, decompensated cirrhosis and HCC, years with diagnosed cirrhosis, decompensated cirrhosis, and HCC, respectively (see analysis section for details). We also defined a time varying covariate for cumulative inpatient and outpatient visits per year as a surrogate for healthcare utilization.
Statistical Analyses
We examined gender differences in the yearly cumulative incidence and prevalence of cirrhosis, decompensated cirrhosis, HCC, and all-cause mortality.
For each year, we classified patients with either a new (incident case) or prior diagnosis (prevalent case) of cirrhosis, decompensated cirrhosis or HCC.
Incidence
We conducted a retrospective cohort study to evaluate the cumulative incidence of each outcome (cirrhosis, decompensated cirrhosis, HCC, death) through discrete-time survival15 using the pooled logit modeling.{Hernan, 2010 181 /id} We specified a separate model for each of the 4 outcomes. For each analysis, patients were followed from their index date until the occurrence of the specific study outcome or December 31, 2013. We accounted for the competing risk of all-cause mortality in the analyses for cirrhosis, decompensated cirrhosis, and HCC by estimated cause specific hazards. We evaluated a range of models including only gender (unadjusted model), and then adding patient age, race/ethnicity, alcohol use disorder, and HIV co-infection, and models that included both time-invariant and yearly time-varying effects. We used the AIC statistic to identify the model with the best fit for the data.16 For all 4 outcomes, the best fitting model included all variables, specifically, gender, age at index date, birth cohort, race/ethnicity, diabetes, alcohol use disorder, HIV-coinfection (time invariant factors) and 2 time-varying covariates for years with diagnosed HCV and cumulative visits for each year. For decompensated cirrhosis and HCC analyses, we also included a time varying covariate for years in care with known cirrhosis. Similarly, for all-cause mortality, we included time varying covariates for years with known cirrhosis and HCC. For models which did not converge under standard estimation due to small outcomes in some instances, we imposed a ridge penalty to achieve estimation.17
We computed the mean marginal predictions18 (adjusted incidence) and associated cluster-robust 95% confidence intervals (CI) by gender as well as subgroups based on gender combined with age (45, 45–65, and >65 year ). Predictive margins are a type of standardization, in which the predicted values from the regression models are averaged over the covariate multivariate distribution in the population represented by the cohort.{Roalfe, 2008 182 /id}
We evaluated temporal trends in incidence rates using the Joinpoint program (National Cancer Institute. Version 4.2.1; http://surveillance.cancer.gov/joinpoint/). Briefly, Joinpoint employs a piecewise linear regression approach to determine whether rates over time are best described by a single line or multiple linear segments (i.e., none or ≥1 joinpoints).19 We allowed a maximum of three joinpoints with a minimum of four linear segments per group. The best joinpoint model (i.e., where addition of further joinpoints did not improve model fit) was identified using log-transformed data.20 We obtained the annual percentage change (APC) in incidence rates over a single linear segment along with 95% CI for each segment. We also computed the average APC (AAPC) as a weighted average of the APC’s from the joinpoint model, with the weights equal to the length of the APC intervals. We compared the trends between two subgroups by employing a test of parallelism using the Monte Carlo permutation test.
Prevalence analyses
Using a cross-sectional design, we calculated the annual prevalence of each outcome for each year we had full outcomes data (2000 to 2013). For each year, we defined prevalent cirrhosis based on either a new or prior diagnosis of cirrhosis during that particular year. We used the same method to define prevalent decompensated cirrhosis and HCC. We used logistic regression models with fixed effects for year and with cluster robust standard-error calculation for multiple observations over time among some patients.21 The final adjusted models included all other covariates as described above, with the exception of HIV and time varying covariates for years with known cirrhosis and HCC. We computed mean marginal predictions of prevalence across calendar years for both gender and gender and age subgroups. Last, we evaluated trends in prevalence rates over time using the Joinpoint program as described above.
RESULTS
We identified 264,409 HCV-infected veterans including 7162 (2.7%) women. Women on average were younger than men, (mean 48.3 years [SD 9.1] vs.54.0 years [SD 8.1], respectively) (Table 1). Women were slightly more likely to be white (56.9% vs. 54.4%) but had a lower prevalence of alcohol use disorder, diabetes and HIV compared to men. These differences persisted during the 11 years of follow up. The proportion of patients who received antiviral treatment during study follow-up was not different among men and women (Table 1).
Table 1.
Characteristics of patients with chronic HCV infection (N=264,409)
| Variable, N % | Women (N=7,162) | Men (N=257,247) | p-value |
|---|---|---|---|
| Age (mean ± std) | 48.0 (±9.1) | 53.4 (±8.2) | <.0001 |
| Race | |||
| White | 4011 (56.0%) | 136597 (53.1%) | <.0001 |
| Black | 2141 (29.9%) | 79300 (30.8%) | |
| Other | 171 (2.4%) | 4626 (1.8%) | |
| Missing | 839 (11.7%) | 36724 (14.3%) | |
| Alcohol use | <.0001 | ||
| Yes | 2361 (33.0%) | 115532 (44.9%) | |
| No | 4801 (67.0%) | 141715 (55.1%) | |
| HIV | <.0001 | ||
| Yes | 193 (2.7%) | 10591 (4.1%) | |
| No | 6969 (97.3%) | 246656 (95.9%) | |
| Diabetes | <.0001 | ||
| Yes | 2166(30.2%) | 100648(39.1%) | |
| No | 4996(69.8%) | 156599(60.9%) | |
| Antiviral treatment | 0.52 | ||
| No | 5469 (76.4%) | 197262 (76.7%) | |
| Yes | 1693 (23.6%) | 59985 (23.3%) | |
Cumulative incidence of cirrhosis and related complications
Cirrhosis
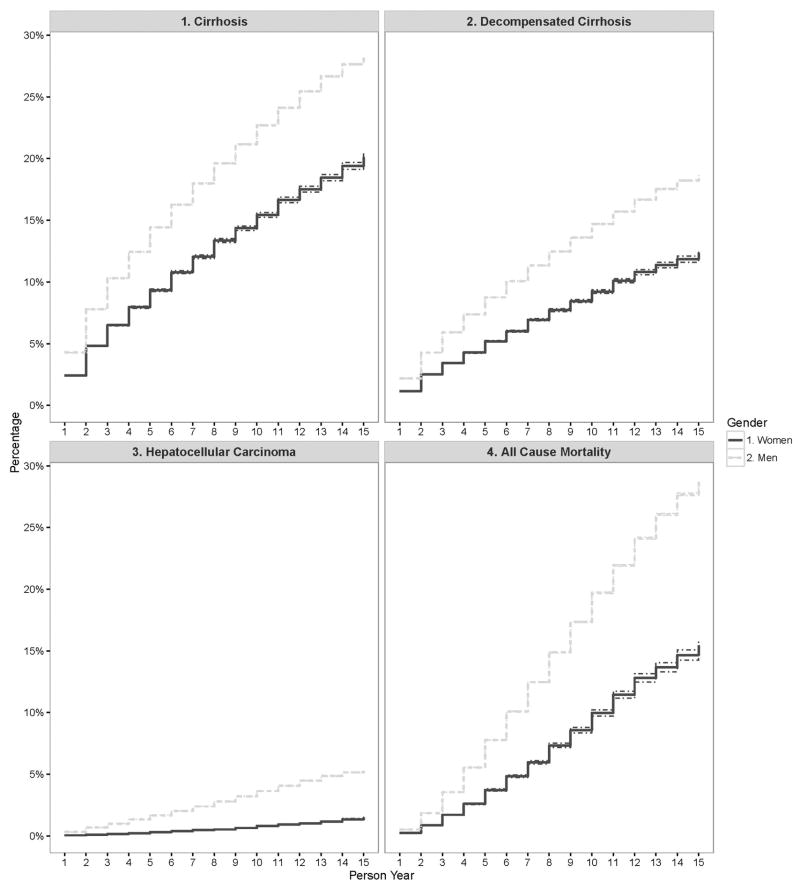
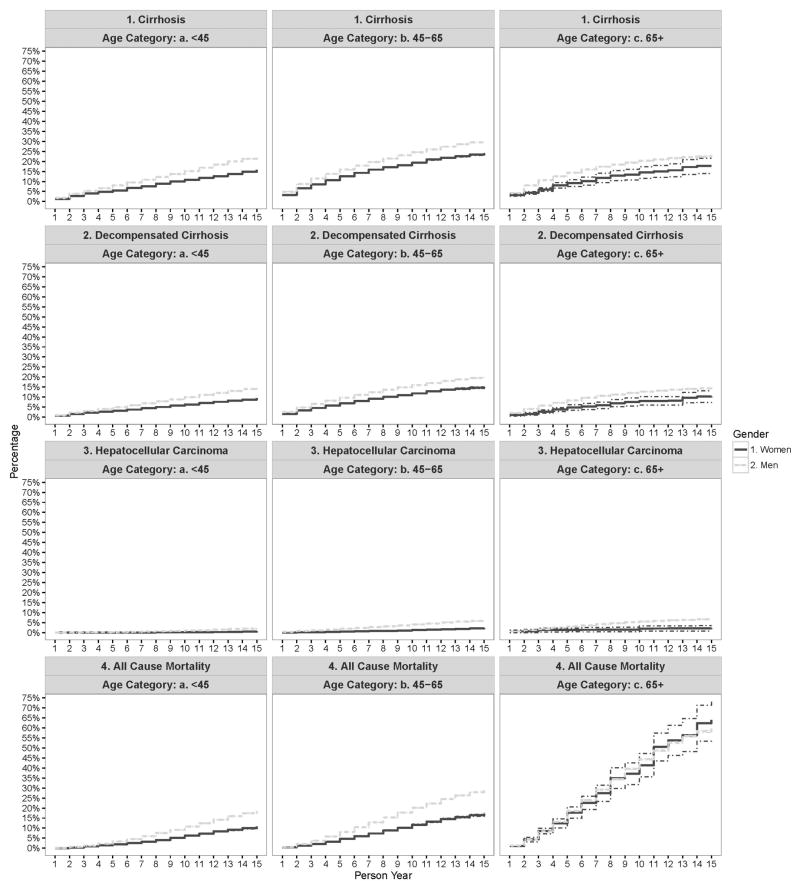
Figure 1 shows the adjusted cumulative incidence of cirrhosis over time. The incidence rates of cirrhosis was higher in men than women for the entire study period. For example, in the first year after index, 2.4% (95%CI: 2.4% – 2.5%) of women vs. 4.3% (95%CI: 4.3% – 4.3%) of men had a new diagnosis of cirrhosis. After 15 years of follow-up, the cumulative incidence for cirrhosis was 20.1% (95%CI: 19.8% – 20.4%) in women vs. 28.2% (95%CI: 28.1% – 28.2%) in men. The results did not change when we stratified the analyses by age at cohort entry; the incidence rate of cirrhosis was higher in men than in women for most of the follow up regardless of patients’ age (Appendix Figure 2).
Figure 1.
Adjusted cumulative incidence of cirrhosis, decompensated cirrhosis, hepatocellular carcinoma and all cause mortality in HCV-infected individuals seen in the US Department of Veteran Affairs from 2000–2013.
The joinpoint analysis showed that cumulative incidence increased sharply during the initial 3 years of patient follow up for both genders (APC for women: 51.5%, 95%CI: 27.8 – 79.7%, p<0.01; men: 43.4%, 95%CI: 24.3 – 65.3%, p<0.01) and rose more slowly in the later years (APC for women in year 7–15=6.9%, 95%CI: 95% CI 6.3 – 7.6%, p<0.01; men in years 10–15=4.8%, 95%CI: 3.8 – 5.8%, p<0.01) (Table 2). The AAPC for cumulative incidence was 15.2% (95% CI, 12.8–17.6%) for women and 13.1% (95% CI, 11.4–14.9%) for men (Table 2). Despite the between group differences in the incidence rates at any given time point, the trends in the adjusted incidence of cirrhosis were not different in both genders (p-value for test for parallelism=0.99).
Table 2.
Temporal trends in the incidence of HCV-related outcomes among veterans with hepatitis C virus infection by gender
| Outcome | Men | Women | ||||
|---|---|---|---|---|---|---|
| APC % (95% CI)+ | AAPC % (95% CI)+ | Segment | APC % (95% CI)+ | AAPC% (95% CI)+ | P-value* | |
| Cirrhosis | ||||||
| Year 1–3 | 43.4 (24.3–65.3) | 13.1 (11.4–14.9) | Year 1–3 | 51.5 (27.8–79.7) | 15.2 (12.8–17.6) | 0.99 |
| Year 3–6 | 15.8 (12.3–19.3) | Year 3–7 | 16.4 (13.9–19.0) | |||
| Year 6–10 | 8.6 (7.3–9.9) | Year 7–15 | 6.9 (6.3–7.6) | |||
| Year 10–15 | 4.8 (3.8–5.8) | ---- | ---- | |||
| Decompensated cirrhosis | ||||||
| Year 1–3 | 52.2 (27.1–82.3) | 15.6 (13.0–18.2) | Year 1–3 | 53.7 (24.6–89.6) | 16.9 (13.9–19.9) | 0.97 |
| Year 3–7 | 17.1 (15.0–19.3) | Year 3–7 | 19.0 (16.2–21.9) | |||
| Year 7–15 | 7.2 (6.2–8.2) | Year 7–15 | 8.1 (7.1–9.2) | |||
| Hepatocellular cancer | ||||||
| Year 1–3 | 63.4 (37.0–95.0) | 21.0 (18.6–23.4) | Year 1–5 | 47.3 (36.3–59.3) | 25.3 (22.5–28.1) | 0.72 |
| Year 3–6 | 25.2 (20.1–30.4) | Year 5–15 | 17.4 (15.4–19.4) | |||
| Year 6–10 | 15.9 (13.8–18.0) | ---- | ---- | |||
| Year 10–15 | 8.7 (6.8–10.6) | ---- | ---- | |||
APC – Annual percent change
AAPC – Average annual percent change
CI – confidence interval
All p-values are <0.01 indicating there is a significant change over time for each segment
P-value for test for parallelism comparing males vs. females
Decompensated cirrhosis
The trends for adjusted incidence rates of decompensated cirrhosis were similar to the trends in cirrhosis. Although the incidence rates of decompensated cirrhosis remained higher in men than in women for all follow up years, they increased at a similar rate in both groups (AAPC for women=16.9%, 95%CI: 13.9–19.9%; men=15.6%, 95% CI: 13.0–18.2%) (p-value for test for parallelism=0.97) (Table 2). The cumulative incidence of decompensated cirrhosis was 12.4% (95%CI: 12.1% – 12.6%) in women and 18.6% (95%CI: 18.6% – 18.7%) in men after 15 years of follow-up.
HCC
The incidence rate of HCC after the first year of follow-up was 0.06% (95%CI: 0.06% – 0.06%) for women vs. 0.3% (95%CI: 0.3% – 0.3%) in men (Figure 1). The cumulative incidence rate over the entire study follow up increased to 1.49% (95%CI: 1.4% – 1.5%) in women vs. 5.3% (95%CI: 5.3% – 5.4%) in men after 15 years of follow-up. The incidence rate of HCC remained low in all patients younger than 45 year at cohort entry. For the remaining patients 45 years and older, HCC incidence was higher in men than in women for most of the follow up period (Figure 1). Joinpoint showed that the greatest increase in the incidence of HCC for both genders was seen in the initial 1 to 5 years of follow up (APC for women in year 1–5: 47.0%, 95%CI: 36.3–59.3%, p<0.01; men in year 1–3: 63.4%, 95%CI: 37.0–95.0%, p<0.01). HCC incidence continued to rise albeit more slowly in the later years (APC for women in year 5–15=17.4%, 95%CI: 15.4 – 19.4%, p<0.01; men in years 10–15=8.7%, 95%CI: 6.8 – 10.6%, p<0.01) (Table 2). The AAPC for cumulative HCC incidence was 25.3% (95%CI: 22.5–28.1%) for women and 21.0% (95%CI: 18.6–23.4%) for men during the study duration (p-value for test for parallelism=0.72).
All-cause mortality
A total of 15.5% (95%CI: 15.0% – 15.9%) of women and 28.7% (95%CI: 28.6% – 28.9%) of men died during 15 years of follow-up. Most of the between gender difference in the risk of death was seen in younger (<65 year old) patients; mortality did not differ between men vs. women in the oldest age group (Figure 2). In a discrete-time survival model predicting all cause mortality that included gender as a covariate, women were less likely to die than men [unadjusted discrete time OR = 0.49 (95% CI: 0.46 – 0.52) and adjusted OR = 0.59 (95% CI: 0.55 – 0.62)].
Figure 2.
Adjusted cumulative incidence of cirrhosis, decompensated cirrhosis, hepatocellular carcinoma and all cause mortality stratified by age group in HCV-infected individuals seen in the US Department of Veteran Affairs from 2000–2013.
Prevalence of cirrhosis and related complications
Cirrhosis
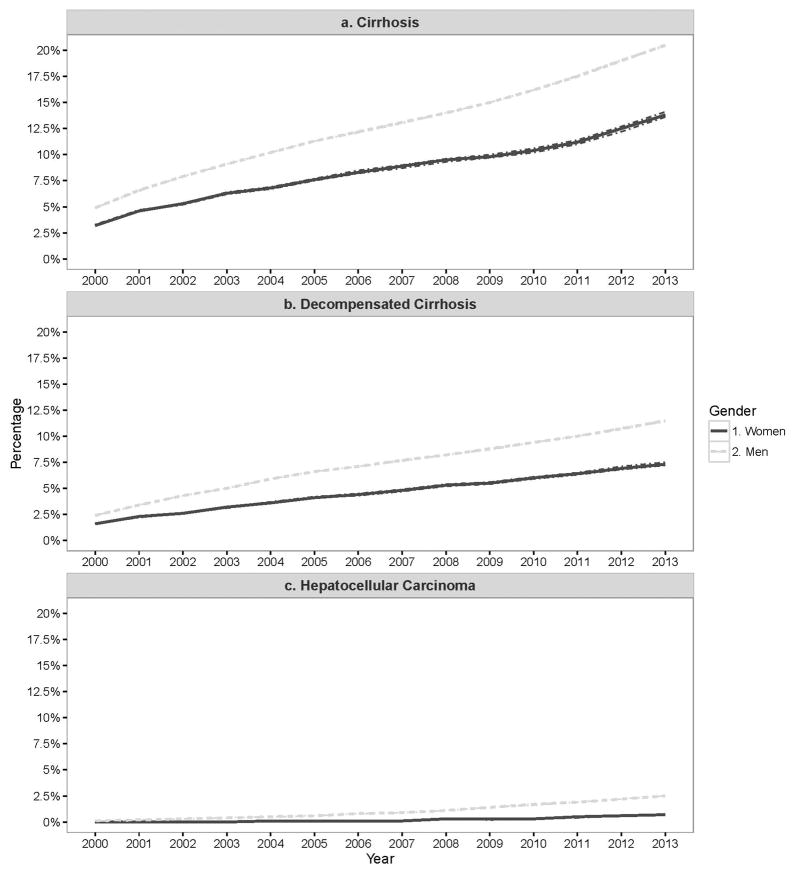
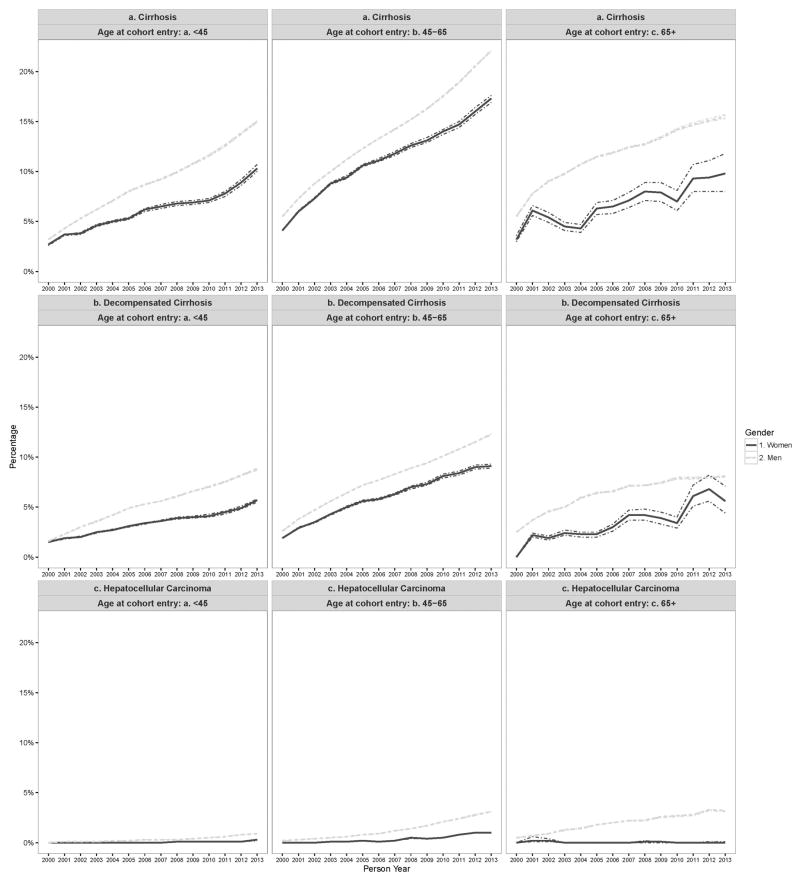
The annual prevalence of cirrhosis was significantly higher in men vs. women for all study years. In 2000, 3.2% (95%CI: 3.2% – 3.3%) of women vs. 4.9% (95%CI: 4.9% – 4.9%) of men had been diagnosed with cirrhosis (Figure 3). By 2013, the prevalence for cirrhosis had risen to 13.8% (95%CI: 13.6% – 14.1%) and 20.5% (95%CI: 20.4% – 20.5%) in women vs. men, respectively. Men had consistently higher prevalence of cirrhosis in all three age groups across all study years. However, the prevalence of cirrhosis increased at a similar rate in both groups (AAPC in women= 11.6%, 95%CI: 10.3 – 12.8% and men=12.5%, 95%CI: 11.9–13.1%) Table 3 (p-value for test for parallelism=0.99).
Figure 3.
Adjusted yearly prevalence of cirrhosis, decompensated cirrhosis, hepatocellular carcinoma and all cause mortality in HCV-infected individuals seen in the US Department of Veteran Affairs from 2000–2013.
Table 3.
Temporal trends in the prevalence of HCV-related outcomes among veterans with hepatitis C virus infection by gender
| Outcome | Men | Women | ||||
|---|---|---|---|---|---|---|
| APC (95% CI)+ | AAPC (95% CI)+ | Segment | APC (95% CI)+ | AAPC (95% CI)+ | P-value* | |
| Cirrhosis | ||||||
| 2000–2002 | 32.5 (28.1–37.1) | 12.5 (11.9–13.1) | 2000–2004 | 20.7 (16.0–25.6) | 11.6 (10.3–12.8) | 0.99 |
| 2002–2005 | 15.2 (13.1–17.4) | 2004–2013 | 7.7 (7.0–8.4) | |||
| 2005–2013 | 7.0 (6.8–7.2) | ---- | ---- | |||
| Decompensated cirrhosis | ||||||
| 2000–2002 | 26.3 (21.3–31.6) | 11.3 (10.5–12.1) | 2000–2003 | 22.5 (11.9–34.0) | 10.8 (8.8–12.9) | 0.99 |
| 2002–2005 | 11.8 (9.1–14.7) | 2003–2013 | 7.6 (6.7–8.4) | |||
| 2005–2013 | 7.6 (7.4–7.9) | |||||
| Hepatocellular cancer** | ||||||
| 2000–2010 | 23.0 (20.8–25.3) | 20.8 (19.2–22.4) | --- | --- | --- | --- |
| 2010–2013 | 13.7 (10.7–16.8) | --- | --- | --- | ||
APC – Annual percent change
AAPC – Average annual percent change
CI – confidence interval
All p-values are <0.01 indicating there is a significant change over time for each segment
P-value for test for parallelism comparing males vs. females
no values were reported for women due to no HCC diagnosed in 2000–2003
Decompensated cirrhosis
Temporal trends in the prevalence of decompensated cirrhosis mirrored those observed for cirrhosis. In 2000, the prevalence of decompensated cirrhosis was 1.6% (95%CI: 1.6% – 1.6%) in women and 2.4% (95%CI: 2.4% – 2.4%) in men but increased by 2013 to 7.3% (95%CI: 7.2% – 7.5%) in women and 11.5% (95%CI: 11.4% – 11.5%) in men. The prevalence of decompensated cirrhosis increased at a similar rate in both groups (AAPC in women= 11.3%, 95%CI: 10.5 – 12.1% and men=10.8%, 95%CI: 8.8–12.9%) Table 3 (p-value for test for parallelism=0.99).
HCC
HCC prevalence was higher in men than in women across all years. Over the study years, HCC prevalence remained relatively constant for women (0% in 2000 to 0.7% in 2013) but increased from 0.1% (95% CI: 0.1%–0.1%) to 2.5% (95%CI: 2.5% – 2.5%) in 2013 (AAPC=20.8%, 95% CI: 19.2–22.4 %) in men.
DISCUSSION
We found that the incidence and prevalence of HCV complications was higher in men than in women for all study years. However, the rate of increase in the incidence rates of cirrhosis and decompensated cirrhosis among HCV-infected women is similar to the rate of increase in men. Indeed, the AAPC for the incidence of cirrhosis, decompensated cirrhosis, and HCC was numerically higher in women than in men, although this difference did not reach statistical significance. We also found that the overall mortality was significantly lower in HCV-infected women than in men (Figure 1); the mortality rate and corresponding 95% CI did not overlap during the entire follow up. The longer survival, coupled with the increasing incidence of HCV complications in women, suggests that HCV infected women will likely have a progressively larger contribution to the overall burden of cirrhosis and its related complications.
A mathematical model based on the prevalence and natural history of HCV in the U.S. general population of HCV-infected individuals showed that most of the current and near-term burden in HCV-related cirrhosis was disproportionately attributed to men. However, the trends are expected to change after 2020;22 with the number of men with cirrhosis declining (due to early maturation of the cohort and competing risks of mortality); whereas the number of women with cirrhosis continuing to increase for at least another decade. Our study is the first to provide direct and contemporary estimates of gender specific time trends in the burden of cirrhosis and its complications from the largest assembled group of patients with active HCV anywhere in the world– including over 5800 women.
We found an ongoing upward trend in the incidence and prevalence of cirrhosis and decompensated cirrhosis. HCC also increased slightly but to a smaller degree, specifically in women. With cure from HCV now becoming a reality, most of the projected burden of HCV is potentially preventable. However, benefits of HCV treatment are limited only to patients who have been tested, know that they infected with HCV, and have access to affordable treatment.23 In the U.S, HCV infection remains undiagnosed in over 50% of all persons with HCV disease24, Access to highly effective yet expensive direct acting antiviral (DAA) treatment remains a challenge. As many as 40% of HCV-infected Americans are uninsured or underinsured.25 With the recent expansion of Medicaid,26 many of these individuals may now be able to access healthcare but their access to DAAs remains difficult because of restrictions on treatment, particularly for those with non-advanced hepatic fibrosis.27–29 These barriers, combined with the recent increases in the rate of new HCV infections,23;30 suggest that the new HCV treatments may not dramatically reverse the underlying trends in the burden of HCV related complications at least in the short term (next 5 to 10 years). Furthermore, in other countries where access to HCV testing and treatment is limited, we expect the burden of HCV to continue to rise and substantially contribute to the worldwide prevalence of liver disease.
The increasing burden of HCV complications in women is concerning. Studies show that women are less likely to receive antiviral treatment than men although the reasons underlining this difference, and the extent to which those reasons are modifiable (e.g., lack of patient education) are poorly understood. In a prospective study of 4084 Veterans with HCV (120 women), there was a non-significant trend towards lower treatment acceptance rate among women (OR=0.89, 95% CI=0.5–1.7).31 In a survey of community residents in New South Wales Australia, Grebely et al. found that women with HCV were less likely to receive antiviral treatment than men.32 Agostini reported similar disparity in a national sample of French patients.33 In a U.S. study, Charlebois et al. noted a trend towards lower treatment in women who participated in a community based program for drug use.34 Women also undergo liver transplantation at lower rates than men35. These and our recent data suggest that the degree of under-treatment may be greater in women than in men and may fuel the rising trends of HCV complications and suboptimal outcomes in women with HCV.36
Our study has several strengths, including the large sample size, long period of follow-up, use of previously validated definitions for cirrhosis and HCC, and examination of demographic and clinical variables that may impact the burden of cirrhosis in HCV. Moreover, most of the patients with HCV in the VA are diagnosed as a result of a system-wide screening program, rather than after development of complications from liver disease. The presence of this unique HCV screening mechanism makes our sample a relatively unbiased cohort. Finally, the availability of laboratory data allowed us to identify a cohort of patients with confirmed chronic HCV infection.
Our study is limited by the observational retrospective nature of its design. Several unmeasured patient characteristics could have affected our results. Although we had information on antiviral treatment in our database, we opted not to include this variable in our analysis given similar treatment rate in the two study groups and the low efficacy of previous interferon based treatment used during the study time frame.37 It is plausible that the rate of increase in HCV complications may decline with the wide dissemination of DAA in the VA and future studies will monitor these trends. Our results were derived from diagnosed HCV-infected patients (men and women) who sought care in the VA health care system, and although the generalizability of the biologic process of cirrhosis progression probably extends from these veterans to other HCV-infected individuals in the VA as well as nonveterans, further research would be needed to confirm that. Indeed, there is a high rate of alcohol misuse in women with HCV in the VA (32.9% reported in this study). We were also limited by the sensitivities and specificities of the ICD-9 coding system for our outcomes, which may vary between VA and non-VA practitioners, thus limiting the generalizability of overall rates of cirrhosis and its complications to patients with HCV outside of the VA.
Chronic HCV infection carries high morbidity in women and men alike. HCV infected women are living longer than men and may further increase their contribution to the burden of HCV-related comorbidities
Figure 4.
Adjusted yearly prevalence of cirrhosis, decompensated cirrhosis, hepatocellular carcinoma and all cause mortality stratified by age group in HCV-infected individuals seen in the US Department of Veteran Affairs from 2000–2013.
Acknowledgments
Funding: The research reported here was supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Service (VA IIR 13-059). Dr. Kanwal and El-Serag are Physician Research Scientists and Drs. Kramer and White are Research Scientists at the Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413), Michael E. DeBakey VA Medical Center, Houston, TX. This work is also partly funded by NIH grant T32 DK083266-01A1, NIH/National Institute of Diabetes and Digestive and Kidney Disease to Hashem El-Serag and Center Grant P30 DK56338.
Abbreviations
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- DLD
decompensated liver disease
- VA
Veterans Affairs
- CDW
Corporate Data Warehouse
- ICD-9
International Classification of Disease, 9th Revision
- AUD
alcohol use disorder
Footnotes
Disclosures: No conflicts of interest exist.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the United States government.
Reference List
- 1.Moyer VA. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:349–357. doi: 10.7326/0003-4819-159-5-201309030-00672. [DOI] [PubMed] [Google Scholar]
- 2.Uhanova J, Tate RB, Tataryn DJ, Minuk GY. A population-based study of the epidemiology of hepatitis C in a North American population. J Hepatol. 2012;57:736–742. doi: 10.1016/j.jhep.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Deuffic S, Buffat L, Poynard T, Valleron AJ. Modeling the hepatitis C virus epidemic in France. Hepatology. 1999;29:1596–1601. doi: 10.1002/hep.510290528. [DOI] [PubMed] [Google Scholar]
- 4.Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003;80:137–146. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 6.Guy J, Peters MG. Liver disease in women: the influence of gender on epidemiology, natural history, and patient outcomes. Gastroenterol Hepatol (N Y ) 2013;9:633–639. [PMC free article] [PubMed] [Google Scholar]
- 7.Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719–727. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- 8.Di MV, Lebray P, Myers RP, et al. Progression of liver fibrosis in women infected with hepatitis C: long-term benefit of estrogen exposure. Hepatology. 2004;40:1426–1433. doi: 10.1002/hep.20463. [DOI] [PubMed] [Google Scholar]
- 9.Codes L, Asselah T, Cazals-Hatem D, et al. Liver fibrosis in women with chronic hepatitis C: evidence for the negative role of the menopause and steatosis and the potential benefit of hormone replacement therapy. Gut. 2007;56:390–395. doi: 10.1136/gut.2006.101931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Villa E, Vukotic R, Camma C, et al. Reproductive status is associated with the severity of fibrosis in women with hepatitis C. PLoS One. 2012;7:e44624. doi: 10.1371/journal.pone.0044624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La VC. Trends in mortality from hepatocellular carcinoma in Europe, 1980–2004. Hepatology. 2008;48:137–145. doi: 10.1002/hep.22312. [DOI] [PubMed] [Google Scholar]
- 13.Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44(Suppl 19):102–107. doi: 10.1007/s00535-008-2251-0. [DOI] [PubMed] [Google Scholar]
- 14.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 15.Kleinbaum D, Klein M. Survival Analysis. Springer; New York: 2012. [Google Scholar]
- 16.Lumley T, Scott A. AIC and BIC for modeling with complex survey data. Journal of Survey Statistics and Methodology. 2015;3:1–18. [Google Scholar]
- 17.Hastie T, Ibshirani R, Friedman J. The Elements of Statistical Learning. New York, NY: Springer New York; 2009. [Google Scholar]
- 18.Santos CA, Fiaccone RL, Oliveira NF, et al. Estimating adjusted prevalence ratio in clustered cross-sectional epidemiological data. BMC Med Res Methodol. 2008;8:80. doi: 10.1186/1471-2288-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu B, Huang L, Tiwari RC, Feuer EJ, Johnson KA. Modelling population-based cancer survival trends using join point models for grouped survival data. J R Stat Soc Ser A Stat Soc. 2009;172:405–425. doi: 10.1111/j.1467-985X.2009.00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 21.Skinner C, de Toledo Vieira M. Variance estimation in the analysis of clustered longitudinal survey data. Survey Methodology. 2007;33:3–11. [Google Scholar]
- 22.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–21. 521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 23.Ward JW, Mermin JH. Simple, Effective, but Out of Reach? Public Health Implications of HCV Drugs. N Engl J Med. 2015;373:2678–2680. doi: 10.1056/NEJMe1513245. [DOI] [PubMed] [Google Scholar]
- 24.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 25.Stepanova M, Kanwal F, El-Serag HB, Younossi ZM. Insurance status and treatment candidacy of hepatitis C patients: analysis of population-based data from the United States. Hepatology. 2011;53:737–745. doi: 10.1002/hep.24131. [DOI] [PubMed] [Google Scholar]
- 26.Haley SJ, Kreek MJ. A window of opportunity: maximizing the effectiveness of new HCV regimens in the United States with the expansion of the Affordable Care Act. Am J Public Health. 2015;105:457–463. doi: 10.2105/AJPH.2014.302327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oregon Medicaid Restricts HCV Therapy Access....A Symptom of a Failing Healthcare System That Denies Human Dignity & Life. 2015 Available at: http://natap.org/2014/HCV/080514_01.htm.
- 28.Hepatitis C prior authorization and treatment policy. 2015 Available at: http://www.hca.wa.gov/medicaid/pharmacy/Documents/HepC_PriorAuthTreatmentPolicy.pdf.
- 29.The Risky Business of Limiting Medicaid Access to Sovaldi. 2015 Available at: http://www.governing.com/topics/health-human-services/gov-hepatitis-coverage-solvaldi-lawsuits.html.
- 30.http://www.cdc.gov/hepatitis/hcv/statisticshcv.htm#section3. 5-19-2016. 10-19-2016.
- 31.Bini EJ, Brau N, Currie S, et al. Prospective multicenter study of eligibility for antiviral therapy among 4,084 U.S. veterans with chronic hepatitis C virus infection. Am J Gastroenterol. 2005;100:1772–1779. doi: 10.1111/j.1572-0241.2005.41860.x. [DOI] [PubMed] [Google Scholar]
- 32.Grebely J, Bryant J, Hull P, et al. Factors associated with specialist assessment and treatment for hepatitis C virus infection in New South Wales, Australia. J Viral Hepat. 2011;18:e104–e116. doi: 10.1111/j.1365-2893.2010.01370.x. [DOI] [PubMed] [Google Scholar]
- 33.Agostini H, Castera L, Melin P, Cattan L, Roudot-Thoraval F. HEPACOM: multicenter, observational prospective study of outcome and monitoring of HCV positive antiviral-naive patients managed in the French health care system. Gastroenterol Clin Biol. 2007;31:1074–1080. doi: 10.1016/s0399-8320(07)78338-0. [DOI] [PubMed] [Google Scholar]
- 34.Charlebois A, Lee L, Cooper E, Mason K, Powis J. Factors associated with HCV antiviral treatment uptake among participants of a community-based HCV programme for marginalized patients. J Viral Hepat. 2012;19:836–842. doi: 10.1111/j.1365-2893.2012.01648.x. [DOI] [PubMed] [Google Scholar]
- 35.Mindikoglu AL, Emre SH, Magder LS. Impact of estimated liver volume and liver weight on gender disparity in liver transplantation. Liver Transpl. 2013;19:89–95. doi: 10.1002/lt.23553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanwal F, Kramer JR, El-Serag HB, et al. Race and Gender Differences in the Use of Direct Acting Antiviral Agents for Hepatitis C Virus. Clin Infect Dis. 2016;63:291–299. doi: 10.1093/cid/ciw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–325. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]