Abstract
The response of immune cells to pathogens is often associated with changes in the flux through basic metabolic pathways. Indeed, in many cases changes in metabolism appear to be necessary for a robust immune response. The Liver X receptors (LXRs) are members of the nuclear hormone receptor superfamily that regulate gene networks controlling cholesterol and lipid metabolism. In immune cells, particularly in macrophages, LXRs also inhibit pro-inflammatory gene expression. This Review will highlight recent studies that connect LXR-dependent control of lipid metabolism to regulation of the immune response.
Keywords: Metabolism, Inflammation, Lipids, Cholesterol, Transcription, Nuclear Receptors
Introduction
Over the last several years the importance of metabolic flux to the function of the immune system has become increasingly apparent. The liver X receptors, LXRα (NR1H3) and LXRβ (NR1H2), are members of the nuclear hormone receptor superfamily of ligand-activated transcription factors [1]. Pharmacological and genetic studies indicate that LXRs function as a critical signaling node linking lipid metabolism, inflammation and immune cell function. This review will first describe the LXRs and genetic networks they control. Recent studies suggesting roles for LXRs in coupling cholesterol transport and fatty acid metabolism to inflammatory signaling will then be highlighted.
1. DNA Binding and Transcriptional Control
LXRs bind to DNA as heterodimers with a second sub-group of the nuclear receptor superfamily, the retinoid X receptors (RXRs) [1]. The two LXR subtypes, LXRα and LXRβ, are expressed in an overlapping but non-identical pattern. LXRα is the dominant subtype expressed in the liver and is also highly expressed in tissues that play roles in cholesterol metabolism including intestine, adipose, kidney and adrenals. On the other hand LXRβ is widely expressed [2]. In hematopoietic cells LXRα is restricted to the myeloid lineage while LXRβ can be found in all cell types [3]. Initial studies using electrophoretic shift mobility assays and promoter analyses identified direct repeats of the classic nuclear receptor binding motif AGGTCA separated by 4 nucleotides (DR4) or inverted repeats of the same sequence separated by 1 nucleotide (IR1) as high affinity binding sites for LXR-RXR heterodimers [1]. These sequence preferences have largely been confirmed by genome-wide chromatin immunoprecipitation sequence experiments [4–6].
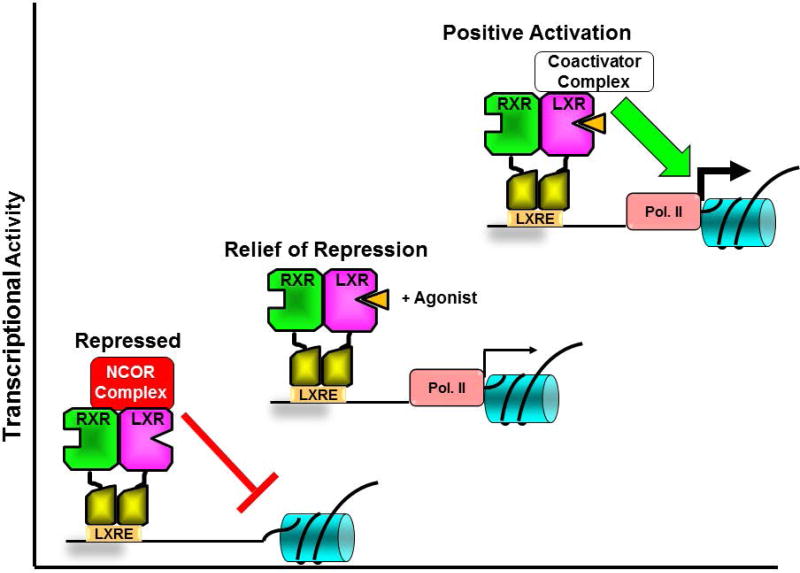
Studies in vitro and in vivo have identified cholesterol derivatives including oxidized forms of cholesterol (oxysterols), cholesterol precursors (e.g. desmosterol) and plant sterols (e.g. stigmosterol) as endogenous LXR agonists that directly bind to the LXR ligand binding domain (LBD) [7–9]. Binding of agonists to the LXR LBD mediates a conformational change largely driven by a mobile alpha helix at the carboxyl terminus (helix 12) that decreases the affinity of LXR for transcriptional corepressor proteins and increases the affinity for transcriptional coactivators [10, 11]. The transcriptional corepressors NCOR1 and NCOR2 (also referred to as the silencing mediator of retinoid and thyroid receptors; SMRT) are the major ligand-dependent corepressors that interact with nuclear receptors. In biochemical assays LXRs demonstrate higher affinity for NCOR1 relative to NCOR2 [12]. A large number of transcriptional coactivator proteins including the steroid receptor coactivators [13], PPARgamma coactivator 1alpha [14] and nuclear receptor coactivator 6 (NCOA6) [15, 16] have been shown to make ligand-dependent interactions with LXRs. Thus binding of agonist couples relief of repression via corepressor release to positive transcriptional activation via coactivator recruitment (Figure 1). Genetic studies in immune cells, however, suggest a more complex picture of LXR-dependent gene regulation. Depending on the particular LXR target gene, deletion of LXRα and LXRβ in bone marrow derived macrophages can result in up-regulation (the expected result if LXR deletion leads to decreased corepressor recruitment), down-regulation or no change (I. Schulman unpublished observations).
Figure 1. Transcriptional regulation by LXR-RXR heterodimers.
In the absence of ligands (bottom left) LXR-RXR heterodimers interact with nuclear receptor corepressors (NCOR) and actively repress transcription. Binding of agonists (orange triangle) to LXRs results in a conformational change that releases corepressors (relief of repression, middle) and promotes interactions with positively acting coactivators that increase transcription. The thickness of the arrows in front of RNA polymerase II (RNA Pol II) in the figure signifies the level of transcription.
2. LXRs Function as Intracellular Cholesterol Sensors
As described above, oxysterols including 22-hydroxycholesterol, 24- hydroxycholesterol, 25- hydroxycholesterol, 27- hydroxycholesterol and 24,25-epoxycholesterol as well as precursors in the cholesterol biosynthetic pathway such as desmosterol have been shown to bind directly to the LXR LBD in biochemical assays and to act as agonists in cell-based functional assays [7–9]. Experiments using mice with genetic deletion of oxysterol generating enzymes supports the function of these molecules as LXR agonists in vivo [17]. Disabling cholesterol biosynthesis in the liver by genetic deletion of steroid response element binding protein 2 (SREBP2) also leads to decreased expression of LXR target genes in hepatocytes [18] supporting a requirement cholesterol-derived LXR ligands. Importantly the binding affinities of cholesterol derivatives to LXR are close to the concentrations that these molecules achieve in cells [7–9]. Thus the LXRs are poised to regulate gene expression in response to changes in intracellular cholesterol levels. Binding studies and cell-based assays suggest that fatty acids can also directly bind to LXRs and function as agonists [19] or antagonists [20] depending on the particular study. The ability of LXRs to regulate fatty acid synthesis (section 3b) raises the possibility of feed-back or feed-forward control via direct fatty acid binding, however, no experiments have yet demonstrated a role for fatty acids as LXR ligands in vivo.
3. Genetic Networks Controlled by LXRs
3a. Cholesterol transport
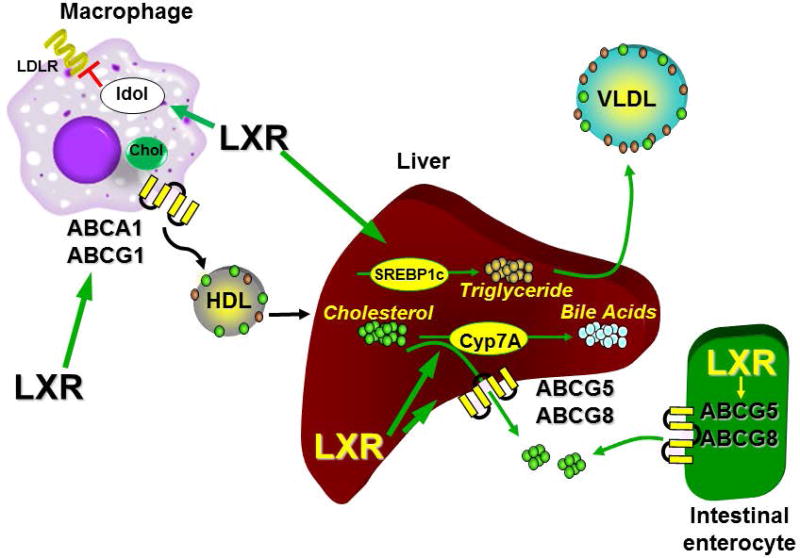
Studies using genetic knockouts and synthetic LXR agonists identified the genes encoding the ATP binding cassette transporters ABCA1 and ABCG1 as well as the gene encoding apolipoprotein E (APOE) as direct LXR target genes with one or more LXR binding sites in the promoters and transcriptional enhancers associated with all 3 genes [21–23]. In peripheral cells such as macrophages ABCA1 and ABCG1 function as cholesterol transporters that mediate the transfer of intracellular cholesterol to high density lipoprotein particles (HDL; Figure 2) [24–26]. APOE also facilitates this process [27, 28]. In contrast, direct LXR-dependent regulation of the gene encoding the inducible degrader of the LDL receptor (IDOL; gene symbol MYLIP) a protein that decreases the level of functional LDL receptors on the cell surface acts to limit cholesterol uptake [29] (Figure 2). Therefore in response to increases in intracellular cholesterol and a concomitant rise in cholesterol-derived LXR ligands, LXRs promote a net movement of cholesterol out of cells to HDL particles. HDL traffics cholesterol to the liver where LXRα directly regulates expression of another pair of ATP binding cassette transporters, ABCG5 and ABCG8, which function as heterodimers to mediate excretion of cholesterol to the intestine [22, 30, 31] (Figure 2). In rodents, LXRα also regulates expression of cytochrome P450 7A1 (Cyp7a1) the gene encoding cholesterol 7-hydroxylase which is the rate limiting enzyme in the conversion of cholesterol to bile acids (Figure 2) [32]. Interestingly, the LXR binding identified in the mouse Cyp7a1 promoter is not conserved in humans [33]. Finally, in the intestine, up-regulation of ABCG5 and ABCG8 by LXRs limits the absorption of cholesterol [34, 35] (Figure 2). Taken together LXRs stimulate the movement of cholesterol out of peripheral cells and ultimately out of the body; a process that is referred to as reverse cholesterol transport (RCT). Studies tracing the movement of labeled cholesterol from macrophages to the feces in animals treated with synthetic LXR agonists and in LXR knockout mice are consistent with the pathway described above [36–38]. The RCT pathway, particularly the ABCA1-dependent efflux of cholesterol out of macrophages to HDL may play an important role in the pathology of human cardiovascular disease. Analysis of samples from patients in the Dallas Heart Study indicates that the ability of an individual’s HDL to accept cholesterol from macrophages in an ABCA1-dependent manner (measured in vitro) is inversely correlated with the subsequent incidence of cardiovascular disease [39].
Figure 2. LXRs regulate reverse cholesterol transport (RCT).
Increased levels of cholesterol in peripheral cells such as macrophages results in LXR-dependent up-regulation of genes encoding proteins such as ABCA1 and ABCG1 that facilitate the transport of intracellular cholesterol out of cells to HDL particles. Simultaneous regulation of IDOL leads to down-regulation of the LDL receptors and decreased cholesterol uptake. In the liver LXR-dependent regulation of the genes encoding ABCG5 and ABCG8 promotes the excretion of hepatic cholesterol to the bile while induction of CYP7A, the gene encoding cholesterol 7α hydroxylase, increases the catabolism of cholesterol to bile. Hepatic regulation of the gene encoding SREBP1c as well as genes encoding other enzymes involved in fatty acid synthesis leads to increases in triglyceride levels and promotes secretion of VLDL. LXR regulation of ABCG5.ABCG8 in the intestine limits the absorption of cholesterol favoring a net movement to the feces.
3b. Fatty acid synthesis
LXRs also directly regulate expression of many genes involved in fatty acid synthesis including the gene encoding the sterol regulatory element binding protein 1c (SREBP1c), the master transcriptional regulator of fatty acid synthesis. Fatty acid synthase (FASN), as well as several desaturases and fatty acid elongases required for the synthesis of long chain polyunsaturated fatty acids are also direct XR targets [32, 40–43] (Figure 2). The net effect of LXR activation is an increase in the levels of long chain polyunsaturated fatty acids (PUFAs), increased triglyceride synthesis and increased hepatic triglyceride secretion in the form of very low density lipoprotein (VLDL) particles (Figure 2) [44, 45]. Indeed treatment of animals including humans with potent synthetic LXR agonists can lead to hypertriglyceridemia and hepatic steatosis [46, 47]. LXRα is the major LXR subtype expressed in the liver [2] and the analysis of single knockout mice indicates that hyperlipidemic effect of LXR agonists is mediated by this subtype [36, 38, 48, 49]. A number of ideas have been put forward to explain coupling of cholesterol mobilization to fatty acid synthesis via LXR activation. First, while free cholesterol is toxic to cells esterification with fatty acids allow for long term storage and may provide a buffer to protect against high intracellular cholesterol levels. Second, a major mechanism to remove cholesterol from the liver is to secrete cholesterol into the blood in VLDL particles which require triglycerides. Additionally, via direct LXR-dependent regulation of the gene encoding lysophosphatidylcholine acyl transferase (LPCAT3) LXR activation leads to increased incorporation of long chain fatty acids into phospholipids which could alter the cholesterol trafficking activity of lipoprotein particles [50–52]. For instance, Breevoort et al. [36] have shown that LXR agonist treatment improves the ability of HDL particles accept cholesterol from macrophages via a pathway that may involve the modulation of HDL phospholipid composition. The role for LXRs in regulating fatty acid synthesis is supported by both genetic knockouts and experiments with synthetic agonists. Nevertheless, increasing cholesterol levels only poorly if at all induce fatty acid synthesis at least in part because increasing cholesterol inhibits the proteolytic processing of SREBP1c to an active transcription factor [6, 53]. Therefore although elevated cholesterol may activate LXRs, the SREBP1c arm controlling fatty acid synthesis is inactive when cholesterol levels are high. Based on these observations it may be more appropriate to consider LXR-dependent regulation of fatty acid synthesis as a pharmacological response to potent synthetic ligands as opposed to a physiological response to elevated cholesterol levels.
4. LXRs and Metabolic Disease
The identification of LXRs as important regulators of lipid metabolism prompted investigations into the potential therapeutic potential of the receptors in diseases associated with dyslipidemia, particularly type II diabetes and cardiovascular disease.
4a. Type II diabetes
Synthetic LXR agonists exhibit significant anti-diabetic activity in well-established rodent models of type II diabetes including db/db mice and high fat diet fed animals. LXR agonists both decrease hyperglycemia and improve insulin sensitivity. Remarkably LXR anti-diabetic activity is manifest even in the face of severe agonist-dependent hyperglycemia [54–57]. A number of mechanisms for LXR-dependent anti-diabetic activity have been put forward including increases in energy utilization by brown adipose [58], regulation of insulin secretion in the pancreas [59] and direct up-regulation of GLUT4, the major insulin stimulated glucose transporter [56]. Perhaps the most definitive study performed by Commerford et al. [55] included euglycemic-hyperinsulinemic clamp measurements of high fat fed rats treated with vehicle or LXR agonist. This study concluded that LXR agonist-dependent inhibition of hepatic glucose production accounts for the majority of LXR anti-diabetic activity. Indeed treatment of animals with synthetic LXR agonists decreases the mRNA levels for phosphoenolpyruvate carboxykinase (PEPCK) and other gluconeogenic enzymes in the liver [54, 56]. The mechanistic basis for the LXR agonist-dependent repression of PEPCK expression remains to be determined. Although LXRs are widely thought of as cholesterol sensors, the diabetes models raise the possibility of a larger role for LXRs in controlling the metabolic transition between fed and fasted states. In the liver LXR activation mimics the response to insulin by simultaneously stimulating fatty acid synthesis and inhibiting glucose production suggesting a potential contribution of LXRs in recognition of the fed state. LXRs have also been suggested to play a direct role in the insulin-dependent induction of fatty acid synthesis [60]. Interestingly, Mitro et al. [57] identified glucose itself as a possible LXR ligand and proposed that LXRs may also function as glucose sensors. This observation, however, has not been extended. Finally, to our knowledge the contributions of the individual LXR subtypes to anti-diabetic activity have not been well explored. LXRα is the major subtype expressed in the liver and thus one might predict that regulating hepatic glucose production is an LXRα-specific activity. As discussed in section 3b, LXRα also selectively mediates the hyperlipidemic effects of synthetic agonists. If glucose lowering is in fact liver- and LXRα-selective, it is difficult to imagine a route to LXR anti-diabetic agents that is not compromised by hyperlipidemia. Chronic inflammation plays an important role the pathogenesis of type II diabetes [61] and as discussed in section 5 LXRs can inhibit inflammation [62–64]. The contribution of anti-inflammatory activity to ability of LXR agonists to improve insulin sensitivity and to decrease hyperglycemia, however, has not been well explored.
4b. LXRs and atherosclerosis
A critical event in the development of atherosclerotic cardiovascular disease is the recruitment of macrophages to the underlying epithelial layer of blood vessel walls and the uncontrolled uptake of oxidized/modified forms of cholesterol. Continued accumulation of oxidized cholesterol by macrophages and an associated inflammatory response leads to foam cell formation and the initiation of atherosclerosis [65]. Reversing the process of macrophage cholesterol accumulation has been held out as a potential novel treatment for cardiovascular disease, however other than injectable forms of apolipoprotein A1 (APOA1) [66], no drugs that enhance the movement of cholesterol out of macrophages have been validated in the clinic. The ability of LXRs to promote macrophage cholesterol efflux and drive the RCT pathway (Figure 2) stimulated great interest in the therapeutic potential of LXR ligands for the treatment of cardiovascular disease. Studies using animal models of atherosclerosis, particularly Apoe−/− and Ldlr−/− mice, demonstrated that LXR agonists can reduce the development of atherosclerosis, block the progression of existing disease and even promote disease regression [44, 67–69]. Single knockout of LXRα in Apoe−/− or Ldlr−/− mice increases atherosclerosis while knockout of LXRβ has no effect, assigning all of the physiological anti-atherogenic activity defined genetically to the alpha subtype [44]. Nevertheless, depending on the study LXRβ alone is able to completely [70] or partially [44] compensate for the loss of LXRα in mediating the anti-atherogenic response to potent synthetic LXR agonists. The hyperlipidemic activity of LXR agonists has presented a major hurdle to successful progression of LXR agonists through clinical trials [47]. The finding that pharmacological activation of LXRβ alone is sufficient to reduce atherosclerosis while LXRα mediates the hyperlipidemic effects of LXR agonists has prompted the search for LXRβ-selective agonists. The identification of subtype-selective LXR ligands has proven difficult due to the high amino acid identity in ligand binding pocket of the two receptors (only 1 amino acid differs between the two). Nevertheless several small molecules with selectively for LXRβ in biochemical and cell-based assays have been described [71, 72] although the specificity necessary to limit hyperlipidemia upon multiple dosing in humans has not been achieved [47].
Tissue-specific knockout of LXRs in the hematopoietic system (transplant of LXR null bone marrow into lethally irradiated Ldlr−/− mice) increases atherosclerosis and the anti-atherogenic activity of LXR agonists is lost in these animals [68]. Notably, lipid-laden macrophages can be observed in the spleens of Ldlr−/− mice with LXR null hematopoietic cells after exposure to a high fat, high cholesterol Western diet [73]. On the other hand, transgenic over expression of LXRα selectively in myeloid cells [74] or treatment with nanoparticles targeting synthetic LXR ligands to macrophages [75] have been shown to reduce atherosclerosis in animal models. This combination of genetic and pharmacological studies suggests that activation of LXRs in macrophages is necessary and sufficient to reduce atherosclerosis, at least in pre-clinical models. Furthermore the requirement for macrophage LXR activity has led to the hypothesis that the ability of LXRs to promote the efflux of cholesterol from macrophages, the first step in the RCT pathway (Figure 2), accounts for the anti-atherogenic activity of LXR agonists.
Although promoting macrophage cholesterol efflux is an obvious mechanism for the anti-atherogenic activity of LXR ligands, Kappus et al. [76] demonstrated that LXR agonists still reduce atherosclerosis when the major cholesterol transporters ABCA1 and ABCG1 are deleted from myeloid cells. Similarly, Zhang et al. found that LXR agonists still decrease atherosclerosis in liver-specific LXRα knockout mice even though the ability of LXR agonists to stimulate RCT is lost under these conditions [38]. Thus while enhancing LXR activity in hematopoietic cells can reduce atherosclerosis, the genetic pathways responsible for this activity remain to be determined. In this regard Tall and colleagues have uncovered a role for cholesterol metabolism in controlling the proliferation of hematopoietic stem cells. Decreased expression of APOE, ABCA1 or ABCG1 or prolonged hypercholesterolemia, all conditions that increase intracellular cholesterol levels, increases myelopoiesis resulting in an elevated number of macrophages that can contribute to atherosclerotic lesion formation [77–79]. A direct role for LXRs in myelopoiesis has not yet been explored. Nevertheless, we have not detected differences in circulating monocytes when LXR positive and LXR null mice are compared (I. Schulman unpublished observations). Bensinger et al. have also demonstrated a role for LXRβ-dependent regulation of ABCG1 expression in the control of T cell proliferation [80] raising the possibility that LXR activity in hematopoietic cells other than members of the myeloid lineage contributes to atherosclerosis. Such a proposal, however, is difficult to reconcile with genetic studies specifically linking LXRα and not LXRβ to atherosclerosis [44] and the absence of LXRα expression in B and T cells [3]. Chronic inflammation makes a significant contribution to atherosclerosis [65] and as described in section 5 LXRs interface with a number of pathways that limit inflammation in myeloid cells.
5. Anti-inflammatory Activity
5a. In vitro and in vivo activity
Like many nuclear receptors including glucocorticoid receptors, and retinoic acid receptors, LXRs exhibit anti-inflammatory activity [81]. Studies in cell culture systems indicate that pre-treatment with LXR agonists can blunt the response to a subsequent pro-inflammatory challenge; for instance treatment with lipopolysaccharide (LPS). Gene expression analysis indicates that LXR agonist treatment can limit the transcriptional up-regulation of inflammatory genes such as tumor necrosis factor alpha (TNFα), cyclooxygenase 2 (COX2), inducible nitric oxide synthase (NOS2) and matrix metalloprotease 9 (MMP9) mediated by nuclear factor kappa beta (NFκβ) and activating protein 1 (AP1) [62, 63, 82, 83]. Conversely activating toll like receptors 3 and 4 (TLR3 and TLR4) via viral or bacterial infection acutely represses LXR transcriptional activity leading to decreased expression of LXR target genes [84]. TLR-dependent LXR inhibition requires the activity of interferon regulatory factor 3 (IRF3), an additional pro-inflammatory transcription factor. The mechanistic basis for the IRF3 inhibition of LXR activity, however, has not yet been defined. Taken together the in vitro studies have defined counter regulatory interactions or a ying and yang between LXR activity and pro-inflammatory signals with each acting to limit the other. Interestingly, lipidomic studies have shown that bacterial or viral infection of macrophages significantly increases the levels of the endogenous LXR agonist 25-hydroxycholesterol. Treatment of macrophages with 25-hydroxycholesterol also reduces inflammation, however, the anti-inflammatory activity of 25-hydroxycholesterol has been reported to be LXR independent [85–87].
In vivo LXR knockout mice are more susceptible to challenge with LPS or to infection with agents such as Listeria monocytogenes and Salmonella typhimurium. Pre-treatment of mice with LXR agonists blunts the response to these agents as well [88]. Similarly, treatment with LXR agonists impairs infection by hepatitis C virus [89, 90] and by human immunodeficiency virus [91–93]. Agonists are also effective in murine models of dermatitis [63, 94, 95]. Finally, as discussed in section 4b, there is reason to suggest that anti-inflammatory activity makes a major contribution to the ability of LXRs to limit diet-induced atherosclerosis in animal models.
5b. Anti-inflammatory mechanisms
A number of mechanisms (described below) have been put forward to account for LXR anti-inflammatory activity and it is likely that multiple pathways converge to broadly limit inflammatory signaling.
5b.i. Transrepression
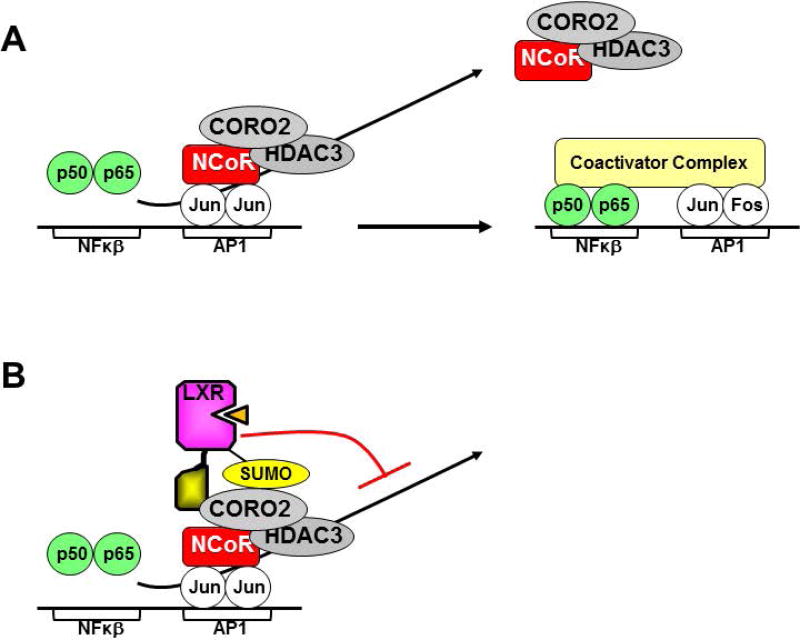
Ghisletti et al. [62] have shown that pro-inflammatory gene expression is repressed in the absence of inflammatory signals by corepressor complexes containing NCOR1 and NCOR2 (SMRT), the same corepressors that interact with nuclear receptors (section 1). Pro-inflammatory signals lead to the release of corepressors via an actin-dependent mechanism that requires coronin 2A (CORO2A), an additional component of the corepressor complexes, binding of positively acting transcription factor such as NFκβ and recruitment of coactivators (Figure 3A) [96]. Treatment with LXR agonists results in the SUMOylation of LXRs and recruitment of SUMO-LXR to the repressor complexes present on pro-inflammatory gene promoters by a SUMO-dependent interaction between LXR and CORO2A [83, 96]. The LXR-CORO2A interaction impairs the inflammatory signal-dependent release of repressor complexes leading to decreased pro-inflammatory gene expression (Figure 3B). Since direct binding to DNA is not required in order to recruit LXRs to pro-inflammatory gene promoters this mechanism has been referred to as transrepression. Interestingly, in these studies LXR agonist-dependent transrepression is essentially lost in fetal liver derived macrophages prepared from Ncor1 knockout mice [62].
Figure 3. Transrepression.
A) In the absence of inflammatory signals transcription of pro-inflammatory genes is inhibited by the recruitment of corepressor complexes containing CORO2. Inflammation results in the binding of NFκβ to the promoters of pro-inflammatory genes, release of corepressor complexes in a CORO2-dependent manner and recruitment of coactivator complexes. B) Agonist bound LXRs make SUMO-dependent interactions with CORO2 that block corepressor release upon inflammatory signaling inhibiting pro-inflammatory gene expression.
5b.ii. Linking Lipid Metabolism and Inflammation
The transrepression model (Figure 3) proposes a unique ligand-dependent mode of action for LXRs that is distinct from the classical mechanism of ligand-dependent gene regulation described in Figure 1. A number of studies exploring LXR anti-inflammatory activity, however, are not consistent with transrepression model. First, LXR anti-inflammatory can be reconstituted in LXR null macrophages using LXRs carrying mutations in the LXR SUMOylation sites demonstrating that modification with SUMOylation is not necessary for LXR anti-inflammatory activity [97]. Second, the transrepression studies described above suggest that NCOR1 plays a critical role in LXR-dependent anti-inflammatory activity [62]. Nevertheless, Li et al. found that macrophage-specific knockout of NCOR1 paradoxically decreases inflammation [41]. In Ncor1−/− macrophages increased expression of LXR target genes (via relief of repression; Figure 1) including ABCA1 and genes involved in fatty acid synthesis is observed. Furthermore Li et al. suggest up-regulation of LXR regulated genes encoding enzymes involved in the synthesis of long chain PUFAs with known anti-inflammatory activity including palmitoleic acid, eicospentaenoic acid (EPA) and docosahexaenoic acid (DHA) is essential for the anti-inflammatory phenotype observed in Ncor1 knockout mice [41]. The observation that treatment of macrophages with LPS in vitro transiently inhibits fatty acid synthesis [5] further supports the hypothesis that fatty acids may play important roles in limiting inflammation. The reestablishment of fatty acid synthesis observed at later stages after LPS administration has been proposed to play a role in the subsequent resolution of the inflammatory response [5]. Along with regulating fatty acid synthesis, LXRs also modulate the composition of phospholipids by directly regulating expression of LPCAT3 an enzyme that remodels phospholipids by exchanging fatty acids at the sn-2 position [50–52]. Thus the ability of LXRs to coordinately regulate fatty acid synthesis and LPCAT3 leads to increased incorporation of long chain PUFAs into phospholipids. Rong et al. have shown that LPCAT3-dependent changes in phospholipid composition can reduce inflammation both by regulating inflammatory kinase activation through changes in membrane composition and by affecting substrate availability for inflammatory mediator production [51]. Taken together the analysis of SUMO-defective LXR mutants, Ncor1−/− mice, and LXR-dependent regulation of LPCAT3 suggest that positive regulation of fatty acid synthesis contributes to LXR-dependent anti-inflammatory activity.
Along with regulation of fatty acid synthesis, increased expression of ABCA1 reduces inflammation in part by removing excess free cholesterol which can be toxic to cells [98, 99]. Like LPCAT3-dependent membrane remodeling, ABCA1-dependent cholesterol efflux has been shown to alter plasma membrane organization and disrupt inflammatory signaling [97]. Indeed Ito et al. have demonstrated that LXR-dependent up-regulation of ABCA1 is necessary for LXR agonists to decrease inflammation [97]. In contrast, genetic deletion of ABCA1 in macrophages has been shown to increase pro-inflammatory gene expression [99, 100]. These studies defining cross-talk between ABCA1 and inflammatory signaling directly link the ability of LXRs to defend cholesterol homeostasis to the regulation of inflammation.
5b.iii. Macrophage phenotype
Macrophages can assume a number of different phenotypes or polarization states in response to environmental ques with inflammatory M1 (classically activated) and anti-inflammatory M2 (alternatively activated) being two extremes [101]. Interestingly, over expression of ABCA1 induces expression of the anti-inflammatory cytokine IL10, a marker of the M2 macrophage phenotype, via a protein kinase A-dependent pathway [98]. Activation of LXRs has also been shown to increase expression of arginase 1 and arginase 2, two additional markers of the M2 phenotype [102–104]. These studies support the hypothesis that LXRs function to counter inflammation and favor resolution of the inflammatory response. Importantly, the ability of LXRs to influence macrophage polarization in mouse models may be sensitive to the genetic background [105].
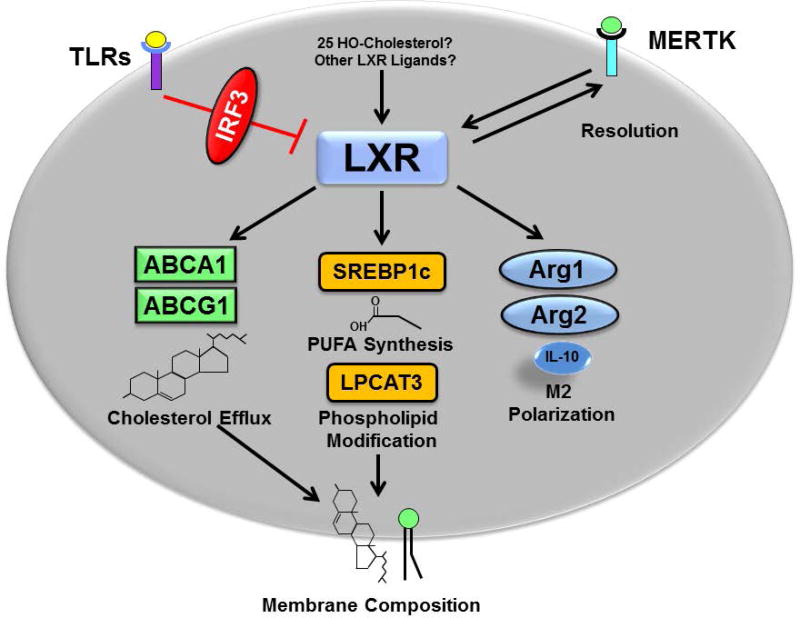
A model unifying these studies (Figure 4) includes an initial decrease in LXR-dependent gene regulation mediated at least in part by IRF3 at early times after an inflammatory signal. Reactivation of LXRs at later times, perhaps in response to increased levels of 25 hydroxycholesterol or other unidentified ligands, promotes the synthesis of anti-inflammatory fatty acids, stimulates the efflux of free cholesterol and reorganizes membranes (via ABCA1 and LPCAT3) to decrease inflammation. Increased LXR activity may also alter macrophage polarization towards a more anti-inflammatory, pro-resolving phenotype. LXRs have also been shown to directly regulate the expression of Mer proto-oncogene tyrosine kinase (MERTK), a cell surface receptor with known roles in the resolution of inflammation [106, 107]. Interestingly Choi et al have found that MERTK can reciprocally regulate LXR expression [106] identifying a potential feedback loop that may function to tip the balance from inflammation to resolution and tissue repair.
Figure 4. LXR anti-inflammatory activities.
Activation of TLRs inhibits LXRs via an IRF3-dependent mechanism during the early stages of the immune response. At later times activation of LXRs, perhaps due to increases in 25-hydroxycholesterol or other unidentified LXR ligands, leads to increases in cholesterol efflux, polyunsaturated fatty acid synthesis (PUFA), modification of phospholipids with PUFAs and M2 polarization all of which contribute to anti-inflammatory activity. LXR activation also induces expression of MERTK a receptor that recognizes apoptotic cells and plays a role resolution of the inflammatory response. MERTK activation has also been shown to increase the level of LXRs.
6. Conclusions and Perspectives
The LXRs have been well described as important transcriptional regulators of lipid metabolism. The ability of these receptors to coordinately limit inflammation, however, has important implications for understanding the cross-talk between lipid metabolism and inflammation and for drug discovery. The role of LXRs in the pathogenesis of atherosclerosis is perhaps still the most thoroughly explored therapeutic indication for synthetic LXR ligands. Studies utilizing tissue-specific knockouts indicate that LXR function in hematopoietic cells is necessary for the ability of LXR agonists to limit atherosclerosis in mouse models while LXR function in the liver, the major site for the regulation of reverse cholesterol transport and cholesterol excretion, is not [36, 38, 68]. Liver LXRα activity, however, is necessary for the hyperlipidemic effects of LXR agonists [38, 48]. This dissociation between the sites of action for limiting atherosclerosis and for elevating lipid levels suggests that limiting or inhibiting the activity of LXR agonists in the liver could avoid a major on-target negative side effect of LXR-based therapeutics. Currently two approaches have been explored to tissue-restrict the activity of LXR agonists. First, since LXRα mediates the hyperlipidemic activity of LXR agonists [38, 44, 48] the discovery of LXRβ-selective ligands have been pursued. Identifying LXRβ-selective ligands has proven to be challenging due to the high level of protein sequence homology between the ligand binding pockets of the two subtypes. While small molecules with LXRβ selectivity in vitro have been described [71, 72, 108, 109] we have tested several of these compounds in vivo and have been unable to identify a small molecule LXR ligand that does not regulate LXR target genes in LXRβ knockout mice (i.e. can activate LXRα. I. Schulman unpublished observations). It is likely that the reported ability of LXRβ-selective ligands to reduce atherosclerosis in rodent models without promoting hyperlipidemia [47, 110, 111] results from a combination of modest LXRβ selectivity and pharmacokinetic profiles that limit drug levels in the liver. Nevertheless, the most advanced of such compounds was shown to still increase plasma lipid levels upon multiple dosing humans [47]. A second approach that has shown some promise is the use of nanoparticles to specifically target LXR agonists to myeloid cells [75].
Analysis of mice with myeloid-specific knockout of the major LXR-regulated cholesterol efflux pumps, ABCA1 and ABCG1, as well as blocking LXR agonist stimulated reverse cholesterol transport indicates that stimulating the efflux of cholesterol out of macrophages to HDL particles is not required for the anti-atherogenic activity of LXR agonists [38, 76]. These studies point to the therapeutic potential of other LXR-regulated pathways, particularly the control of inflammation. As described in section 5 a number of anti-inflammatory mechanisms have been proposed for LXRs including direct recruitment of LXRs to pro-inflammatory gene promoters (transrepression), alteration of macrophage phenotype, increased cholesterol efflux, changes in plasma membrane signaling systems via modulation of membrane lipid composition and increased synthesis of fatty acids with anti-inflammatory activity. The finding that treatment of macrophages with LPS results in an acute inhibition of fatty acid synthesis and that the subsequent restoration of lipogenesis facilitates resolution of the immune response [5] supports a role for the positive regulation of lipogenesis by LXRs in inhibiting inflammation.
The ability of LXRs to coordinately regulate lipid metabolism and inhibit inflammation raises questions as to why these processes are linked. The engulfment of dead and dying cells by macrophages and other phagocytes (efferocytosis) is associated with acute changes in lipid levels that phagocytes must respond to. For instance increases in intracellular free cholesterol derived from engulfed cells can be toxic. Activation of LXRs during efferocytosis can promote the efflux of free cholesterol by activation of ABCA1 and ABCG1. Interestingly apoptotic cells induce macrophage expression of ABCA1 by both LXR-dependent [107] and LXR independent [112] pathways highlighting an important role for ABCA1 mediated cholesterol efflux in efferocytosis. Simultaneous LXR-dependent inhibition of the immune response may help to insure that efferocytosis does not lead to inappropriate activation of the immune system. Finally, inhibiting LXR activity until later stages of an inflammatory response may function to restrict the synthesis of fatty acid-derived signaling molecules to times when resolution of immune responses and tissue repair dominate.
Acknowledgments
Work in the author’s laboratory has been supported by the American Heart Association (15GRNT25560038) and the National Institutes of Health (1R56HL131779-01).
Abbreviations
- ABC
ATP binding cassette
- APOA1
Apolipoprotein A1
- APOE
Apolipoprotein E
- CORO2A
Coronin 2A
- CYP7A1
Cytochrome p450 A1
- DHA
Docosahexaenoic acid
- DR
Direct repeat
- EPA
Eicospentaenoic acid
- FASN
Fatty acid synthase
- HDL
High density lipoprotein
- IR
Inverted repeat
- IRF
Interferon regulatory factor
- LBD
Ligand binding domain
- LDL
Low density lipoprotein
- LPCAT3
Lysophosphatidylcholine acyl transferase
- LPS
Lipopolysaccharide
- LXR
Liver X receptor
- MERTK
Mer proto-oncogene tyrosine kinase
- NCOA
Nuclear receptor coactivator
- NCOR
Nuclear receptor corepressor
- PEPCK
phosphoenolpyruvate carboxykinase
- RCT
Reverse cholesterol transport
- RXR
Retinoid X receptor
- SMRT
Silencing mediator of retinoid and thyroid receptors
- SREBP
Sterol regulatory element binding protein
- VLDL
Very low density lipoprotein
References
- 1.Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995;9:1033–1045. doi: 10.1101/gad.9.9.1033. [DOI] [PubMed] [Google Scholar]
- 2.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–99. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diehl CJ, Barish GD, Downes M, Chou MY, Heinz S, Glass CK, Evans RM, Witztum JL. Research resource: Comparative nuclear receptor atlas: basal and activated peritoneal B-1 and B-2 cells. Mol Endocrinol. 2011;25:529–45. doi: 10.1210/me.2010-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boergesen M, Pedersen TA, Gross B, van Heeringen SJ, Hagenbeek D, Bindesboll C, Caron S, Lalloyer F, Steffensen KR, Nebb HI, Gustafsson JA, Stunnenberg HG, Staels B, Mandrup S. Genome-wide profiling of liver X receptor, retinoid X receptor, and peroxisome proliferator-activated receptor alpha in mouse liver reveals extensive sharing of binding sites. Mol Cell Biol. 2012;32:852–67. doi: 10.1128/MCB.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oishi Y, Spann NJ, Link VM, Muse ED, Strid T, Edillor C, Kolar MJ, Matsuzaka T, Hayakawa S, Tao J, Kaikkonen MU, Carlin AF, Lam MT, Manabe I, Shimano H, Saghatelian A, Glass CK. SREBP1 Contributes to Resolution of Pro-inflammatory TLR4 Signaling by Reprogramming Fatty Acid Metabolism. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr, Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–52. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A. 1999;96:266–71. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXRa. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 9.Yang C, McDonald JG, Patel A, Zhang Y, Umetani M, Xu F, Westover EJ, Covey DF, Mangelsdorf DJ, Cohen JC, Hobbs HH. Sterol intermediates from cholesterol biosynthetic pathway as liver X receptor ligands. J Biol Chem. 2006;281:27816–26. doi: 10.1074/jbc.M603781200. [DOI] [PubMed] [Google Scholar]
- 10.Malini N, Rajesh H, Berwal P, Phukan S, Balaji VN. Analysis of crystal structures of LXRbeta in relation to plasticity of the ligand-binding domain upon ligand binding. Chem Biol Drug Des. 2008;71:140–54. doi: 10.1111/j.1747-0285.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 11.Svensson S, Ostberg T, Jacobsson M, Norstrom C, Stefansson K, Hallen D, Johansson IC, Zachrisson K, Ogg D, Jendeberg L. Crystal structure of the heterodimeric complex of LXRalpha and RXRbeta ligand-binding domains in a fully agonistic conformation. EMBO J. 2003;22:4625–33. doi: 10.1093/emboj/cdg456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu X, Li S, Wu J, Xia C, Lala DS. Liver X receptors interact with corepressors to regulate gene expression. Mol Endocrinol. 2003;17:1019–26. doi: 10.1210/me.2002-0399. [DOI] [PubMed] [Google Scholar]
- 13.Son YL, Lee YC. Molecular determinants of the interactions between SRC-1 and LXR/RXR heterodimers. FEBS Lett. 2010;584:3862–6. doi: 10.1016/j.febslet.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Oberkofler H, Schraml E, Krempler F, Patsch W. Potentiation of liver X receptor transcriptional activity by peroxisome-proliferator-activated receptor gamma co-activator 1 alpha. Biochem J. 2003;371:89–96. doi: 10.1042/BJ20021665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim GH, Park K, Yeom SY, Lee KJ, Kim G, Ko J, Rhee DK, Kim YH, Lee HK, Kim HW, Oh GT, Lee KU, Lee JW, Kim SW. Characterization of ASC-2 as an Anti-Atherogenic Transcriptional Coactivator of Liver X Receptors in Macrophages. Mol Endocrinol. 2009 doi: 10.1210/me.2008-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SW, Park K, Kwak E, Choi E, Lee S, Ham J, Kang H, Kim JM, Hwang SY, Kong YY, Lee K, Lee JW. Activating signal cointegrator 2 required for liver lipid metabolism mediated by liver X receptors in mice. Mol Cell Biol. 2003;23:3583–92. doi: 10.1128/MCB.23.10.3583-3592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–9. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rong S, Cortes VA, Rashid S, Anderson NN, McDonald JG, Liang G, Moon YA, Hammer RE, Horton JD. Expression of SREBP-1c requires SREBP-2-mediated generation of a sterol ligand for LXR in livers of mice. eLife. 2017;6 doi: 10.7554/eLife.25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedi S, Hines GV, Lozada-Fernandez VV, de Jesus Piva C, Kaliappan A, Rider SD, Jr, Hostetler HA. Fatty acid binding profile of the liver X receptor alpha. J Lipid Res. 2017;58:393–402. doi: 10.1194/jlr.M072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou J, Tu H, Shan B, Luk A, DeBose-Boyd RA, Bashmakov Y, Goldstein JL, Brown MS. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc Natl Acad Sci U S A. 2001;98:6027–32. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci U S A. 2001;98:507–12. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 23.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of Absorption and ABC1-Mediated Efflux of Cholesterol by RXR Heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 24.Singaraja RR, Van Eck M, Bissada N, Zimetti F, Collins HL, Hildebrand RB, Hayden A, Brunham LR, Kang MH, Fruchart JC, Van Berkel TJ, Parks JS, Staels B, Rothblat GH, Fievet C, Hayden MR. Both hepatic and extrahepatic ABCA1 have discrete and essential functions in the maintenance of plasma high-density lipoprotein cholesterol levels in vivo. Circulation. 2006;114:1301–9. doi: 10.1161/CIRCULATIONAHA.106.621433. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–24. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–8. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CY, Duan H, Mazzone T. Apolipoprotein E-dependent cholesterol efflux from macrophages: kinetic study and divergent mechanisms for endogenous versus exogenous apolipoprotein E. J Lipid Res. 1999;40:1618–27. [PubMed] [Google Scholar]
- 28.Zanotti I, Pedrelli M, Poti F, Stomeo G, Gomaraschi M, Calabresi L, Bernini F. Macrophage, but not systemic, apolipoprotein E is necessary for macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2011;31:74–80. doi: 10.1161/ATVBAHA.110.213892. [DOI] [PubMed] [Google Scholar]
- 29.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–4. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilund KR, Yu L, Xu F, Hobbs HH, Cohen JC. High-level expression of ABCG5 and ABCG8 attenuates diet-induced hypercholesterolemia and atherosclerosis in Ldlr−/− mice. J Lipid Res. 2004;45:1429–36. doi: 10.1194/jlr.M400167-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Yu L, York J, von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Stimulation of cholesterol excretion by the liver X receptor agonist requires ATP-binding cassette transporters G5 and G8. J Biol Chem. 2003;278:15565–70. doi: 10.1074/jbc.M301311200. [DOI] [PubMed] [Google Scholar]
- 32.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro J-MA, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRa. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 33.Menke JG, Macnaul KL, Hayes NS, Baffic J, Chao YS, Elbrecht A, Kelly LJ, Lam MH, Schmidt A, Sahoo S, Wang J, Wright SD, Xin P, Zhou G, Moller DE, Sparrow CP. A Novel Liver X Receptor Agonist Establishes Species Differences in the Regulation of Cholesterol 7alpha-Hydroxylase (CYP7a) Endocrinology. 2002;143:2548–2558. doi: 10.1210/endo.143.7.8907. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Mitsche MA, Lutjohann D, Cohen JC, Xie XS, Hobbs HH. Relative roles of ABCG5/ABCG8 in liver and intestine. J Lipid Res. 2015;56:319–30. doi: 10.1194/jlr.M054544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–80. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breevoort SR, Angdisen J, Schulman IG. Macrophage-Independent Regulation of Reverse Cholesterol Transport by Liver X Receptors. Arterioscler Thromb Vasc Biol. 2014;34:1650–1660. doi: 10.1161/ATVBAHA.114.303383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naik SU, Wang X, Da Silva JS, Jaye M, Macphee CH, Reilly MP, Billheimer JT, Rothblat GH, Rader DJ. Pharmacological activation of liver X receptors promotes reverse cholesterol transport in vivo. Circulation. 2006;113:90–7. doi: 10.1161/CIRCULATIONAHA.105.560177. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Breevoort SR, Angdisen J, Fu M, Schmidt DR, Holmstrom SR, Kliewer SA, Mangelsdorf DJ, Schulman IG. Liver LXRalpha expression is crucial for whole body cholesterol homeostasis and reverse cholesterol transport in mice. J Clin Invest. 2012;122:1688–1699. doi: 10.1172/JCI59817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, Neeland IJ, Yuhanna IS, Rader DR, de Lemos JA, Shaul PW. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–93. doi: 10.1056/NEJMoa1409065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu K, Miyazaki M, Man WC, Ntambi JM. Stearoyl-coenzyme A desaturase 1 deficiency protects against hypertriglyceridemia and increases plasma high-density lipoprotein cholesterol induced by liver X receptor activation. Mol Cell Biol. 2006;26:6786–98. doi: 10.1128/MCB.00077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li P, Spann NJ, Kaikkonen MU, Lu M, Oh da Y, Fox JN, Bandyopadhyay G, Talukdar S, Xu J, Lagakos WS, Patsouris D, Armando A, Quehenberger O, Dennis EA, Watkins SM, Auwerx J, Glass CK, Olefsky JM. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell. 2013;155:200–14. doi: 10.1016/j.cell.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–30. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Lustig KD, Shan B. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–8. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bischoff ED, Daige CL, Petrowski M, Dedman H, Pattison J, Juliano J, Li AC, Schulman IG. Non-redundant roles for LXRalpha and LXRbeta in atherosclerosis susceptibility in low density lipoprotein receptor knockout mice. J Lipid Res. 2010;51:900–6. doi: 10.1194/jlr.M900096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okazaki H, Goldstein JL, Brown MS, Liang G. LXR-SREBP-1c–phospholipid transfer protein axis controls very low density lipoprotein (VLDL) particle size. J Biol Chem. 2010;285:6801–10. doi: 10.1074/jbc.M109.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Groot PH, Pearce NJ, Yates JW, Stocker C, Sauermelch C, Doe CP, Willette RN, Olzinski A, Peters T, d'Epagnier D, Morasco KO, Krawiec JA, Webb CL, Aravindhan K, Jucker B, Burgert M, Ma C, Marino JP, Collins JL, Macphee CH, Thompson SK, Jaye M. Synthetic LXR agonists increase LDL in CETP species. J Lipid Res. 2005;46:2182–91. doi: 10.1194/jlr.M500116-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Kirchgessner TG, Sleph P, Ostrowski J, Lupisella J, Ryan CS, Liu X, Fernando G, Grimm D, Shipkova P, Zhang R, Garcia R, Zhu J, He A, Malone H, Martin R, Behnia K, Wang Z, Barrett YC, Garmise RJ, Yuan L, Zhang J, Gandhi MD, Wastall P, Li T, Du S, Salvador L, Mohan R, Cantor GH, Kick E, Lee J, Frost RJ. Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell Metab. 2016;24:223–33. doi: 10.1016/j.cmet.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Lund EG, Peterson LB, Adams AD, Lam MH, Burton CA, Chin J, Guo Q, Huang S, Latham M, Lopez JC, Menke JG, Milot DP, Mitnaul LJ, Rex-Rabe SE, Rosa RL, Tian JY, Wright SD, Sparrow CP. Different roles of liver X receptor alpha and beta in lipid metabolism: effects of an alpha-selective and a dual agonist in mice deficient in each subtype. Biochem Pharmacol. 2006;71:453–63. doi: 10.1016/j.bcp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Quinet EM, Savio DA, Halpern AR, Chen L, Schuster GU, Gustafsson JA, Basso MD, Nambi P. Liver X receptor (LXR)-beta regulation in LXRalpha-deficient mice: implications for therapeutic targeting. Mol Pharmacol. 2006;70:1340–9. doi: 10.1124/mol.106.022608. [DOI] [PubMed] [Google Scholar]
- 50.Hashidate-Yoshida T, Harayama T, Hishikawa D, Morimoto R, Hamano F, Tokuoka SM, Eto M, Tamura-Nakano M, Yanobu-Takanashi R, Mukumoto Y, Kiyonari H, Okamura T, Kita Y, Shindou H, Shimizu T. Fatty acyl-chain remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. eLife. 2015;4 doi: 10.7554/eLife.06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rong X, Albert CJ, Hong C, Duerr MA, Chamberlain BT, Tarling EJ, Ito A, Gao J, Wang B, Edwards PA, Jung ME, Ford DA, Tontonoz P. LXRs Regulate ER Stress and Inflammation through Dynamic Modulation of Membrane Phospholipid Composition. Cell Metab. 2013;18:685–97. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rong X, Wang B, Dunham MM, Hedde PN, Wong JS, Gratton E, Young SG, Ford DA, Tontonoz P. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. eLife. 2015;4 doi: 10.7554/eLife.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ignatova ID, Angdisen J, Moran E, Schulman IG. Differential Regulation of Gene Expression by LXRs in Response to Macrophage Cholesterol Loading. Mol Endocrinol. 2013;27:1036–1047. doi: 10.1210/me.2013-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao G, Liang Y, Broderick CL, Oldham BA, Beyer TP, Schmidt RJ, Zhang Y, Stayrook KR, Suen C, Otto KA, Miller AR, Dai J, Foxworthy P, Gao H, Ryan TP, Jiang XC, Burris TP, Eacho PI, Etgen GJ. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J Biol Chem. 2003;278:1131–6. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- 55.Commerford SR, Vargas L, Dorfman SE, Mitro N, Rocheford EC, Mak PA, Li X, Kennedy P, Mullarkey TL, Saez E. Dissection of the insulin-sensitizing effect of liver X receptor ligands. Mol Endocrinol. 2007;21:3002–12. doi: 10.1210/me.2007-0156. [DOI] [PubMed] [Google Scholar]
- 56.Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, Saez E, Tontonoz P. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci U S A. 2003;100:5419–24. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitro N, Mak PA, Vargas L, Godio C, Hampton E, Molteni V, Kreusch A, Saez E. The nuclear receptor LXR is a glucose sensor. Nature. 2007;445:219–23. doi: 10.1038/nature05449. [DOI] [PubMed] [Google Scholar]
- 58.Korach-Andre M, Archer A, Barros RP, Parini P, Gustafsson JA. Both liver-X receptor (LXR) isoforms control energy expenditure by regulating brown adipose tissue activity. Proc Natl Acad Sci U S A. 2011;108:403–8. doi: 10.1073/pnas.1017884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogihara T, Chuang JC, Vestermark GL, Garmey JC, Ketchum RJ, Huang X, Brayman KL, Thorner MO, Repa JJ, Mirmira RG, Evans-Molina C. Liver X receptor agonists augment human islet function through activation of anaplerotic pathways and glycerolipid/free fatty acid cycling. J Biol Chem. 2010;285:5392–404. doi: 10.1074/jbc.M109.064659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen G, Liang G, Ou J, Goldstein JL, Brown MS. Central role for liver X receptor in insulin-mediated activation of Srebp-1c transcription and stimulation of fatty acid synthesis in liver. Proc Natl Acad Sci U S A. 2004;101:11245–50. doi: 10.1073/pnas.0404297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 62.Ghisletti S, Huang W, Jepsen K, Benner C, Hardiman G, Rosenfeld MG, Glass CK. Cooperative NCoR/SMRT interactions establish a corepressor-based strategy for integration of inflammatory and anti-inflammatory signaling pathways. Genes Dev. 2009;23:681–93. doi: 10.1101/gad.1773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–9. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- 64.Zelcer N, Khanlou N, Clare R, Jiang Q, Reed-Geaghan EG, Landreth GE, Vinters HV, Tontonoz P. Attenuation of neuroinflammation and Alzheimer's disease pathology by liver x receptors. Proc Natl Acad Sci U S A. 2007;104:10601–6. doi: 10.1073/pnas.0701096104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–55. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290:2292–300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 67.Joseph SB, McKilligin E, Pei L, Watson MA, Collins AR, Laffitte BA, Chen M, Noh G, Goodman J, Hagger GN, Tran J, Tippin TK, Wang X, Lusis AJ, Hsueh WA, Law RE, Collins JL, Willson TM, Tontonoz P. Synthetic LXR ligand inhibits the development of atherosclerosis in mice. Proc Natl Acad Sci U S A. 2002;99:7604–9. doi: 10.1073/pnas.112059299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Levin N, Bischoff ED, Daige CL, Thomas D, Vu CT, Heyman RA, Tangirala RK, Schulman IG. Macrophage liver X receptor is required for antiatherogenic activity of LXR agonists. Arterioscler Thromb Vasc Biol. 2005;25:135–42. doi: 10.1161/01.ATV.0000150044.84012.68. [DOI] [PubMed] [Google Scholar]
- 69.Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, Inaba T. T-0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor-deficient mice. FEBS Lett. 2003;536:6–11. doi: 10.1016/s0014-5793(02)03578-0. [DOI] [PubMed] [Google Scholar]
- 70.Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, Tangirala RK, Tontonoz P. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–46. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kick EK, Busch BB, Martin R, Stevens WC, Bollu V, Xie Y, Boren BC, Nyman MC, Nanao MH, Nguyen L, Plonowski A, Schulman IG, Yan G, Zhang H, Hou X, Valente MN, Narayanan R, Behnia K, Rodrigues AD, Brock B, Smalley J, Cantor GH, Lupisella J, Sleph P, Grimm D, Ostrowski J, Wexler RR, Kirchgessner T, Mohan R. Discovery of Highly Potent Liver X Receptor beta Agonists. ACS medicinal chemistry letters. 2016;7:1207–1212. doi: 10.1021/acsmedchemlett.6b00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kick E, Martin R, Xie Y, Flatt B, Schweiger E, Wang TL, Busch B, Nyman M, Gu XH, Yan G, Wagner B, Nanao M, Nguyen L, Stout T, Plonowski A, Schulman I, Ostrowski J, Kirchgessner T, Wexler R, Mohan R. Liver X receptor (LXR) partial agonists: biaryl pyrazoles and imidazoles displaying a preference for LXRbeta. Bioorg Med Chem Lett. 2015;25:372–7. doi: 10.1016/j.bmcl.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 73.Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, Wang X, Lusis AJ, Tontonoz P, Schulman IG. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Teupser D, Kretzschmar D, Tennert C, Burkhardt R, Wilfert W, Fengler D, Naumann R, Sippel AE, Thiery J. Effect of macrophage overexpression of murine liver X receptor-alpha (LXR-alpha) on atherosclerosis in LDL-receptor deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:2009–15. doi: 10.1161/ATVBAHA.108.175257. [DOI] [PubMed] [Google Scholar]
- 75.Zhang XQ, Even-Or O, Xu X, van Rosmalen M, Lim L, Gadde S, Farokhzad OC, Fisher EA. Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis. Advanced healthcare materials. 2015;4:228–36. doi: 10.1002/adhm.201400337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Activation of Liver X Receptor Decreases Atherosclerosis in Ldlr−/− mice in the Absence of ABCA1 and ABCG1 in Myeloid Cells. Arterioscler Thromb Vasc Biol. 2013;34:279–284. doi: 10.1161/ATVBAHA.113.302781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tolani S, Pagler TA, Murphy AJ, Bochem AE, Abramowicz S, Welch C, Nagareddy PR, Holleran S, Hovingh GK, Kuivenhoven JA, Tall AR. Hypercholesterolemia and reduced HDL-C promote hematopoietic stem cell proliferation and monocytosis: studies in mice and FH children. Atherosclerosis. 2013;229:79–85. doi: 10.1016/j.atherosclerosis.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–93. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zelcer N, Tontonoz P. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest. 2006;116:607–14. doi: 10.1172/JCI27883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castrillo A, Joseph SB, Marathe C, Mangelsdorf DJ, Tontonoz P. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J Biol Chem. 2003;278:10443–9. doi: 10.1074/jbc.M213071200. [DOI] [PubMed] [Google Scholar]
- 83.Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–16. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 85.Blanc M, Hsieh WY, Robertson KA, Kropp KA, Forster T, Shui G, Lacaze P, Watterson S, Griffiths SJ, Spann NJ, Meljon A, Talbot S, Krishnan K, Covey DF, Wenk MR, Craigon M, Ruzsics Z, Haas J, Angulo A, Griffiths WJ, Glass CK, Wang Y, Ghazal P. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity. 2013;38:106–18. doi: 10.1016/j.immuni.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu SY, Aliyari R, Chikere K, Li G, Marsden MD, Smith JK, Pernet O, Guo H, Nusbaum R, Zack JA, Freiberg AN, Su L, Lee B, Cheng G. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity. 2013;38:92–105. doi: 10.1016/j.immuni.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reboldi A, Dang EV, McDonald JG, Liang G, Russell DW, Cyster JG. Inflammation. 25-Hydroxycholesterol suppresses interleukin-1-driven inflammation downstream of type I interferon. Science. 2014;345:679–84. doi: 10.1126/science.1254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O'Connell R M, Cheng G, Saez E, Miller JF, Tontonoz P. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 89.Bocchetta S, Maillard P, Yamamoto M, Gondeau C, Douam F, Lebreton S, Lagaye S, Pol S, Helle F, Plengpanich W, Guerin M, Bourgine M, Michel ML, Lavillette D, Roingeard P, le Goff W, Budkowska A. Up-regulation of the ATP-binding cassette transporter A1 inhibits hepatitis C virus infection. PLoS One. 2014;9:e92140. doi: 10.1371/journal.pone.0092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zeng J, Wu Y, Liao Q, Li L, Chen X, Chen X. Liver X receptors agonists impede hepatitis C virus infection in an Idol-dependent manner. Antiviral research. 2012;95:245–56. doi: 10.1016/j.antiviral.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 91.Dubrovsky L, Van Duyne R, Senina S, Guendel I, Pushkarsky T, Sviridov D, Kashanchi F, Bukrinsky M. Liver X receptor agonist inhibits HIV-1 replication and prevents HIV-induced reduction of plasma HDL in humanized mouse model of HIV infection. Biochem Biophys Res Commun. 2012;419:95–8. doi: 10.1016/j.bbrc.2012.01.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morrow MP, Grant A, Mujawar Z, Dubrovsky L, Pushkarsky T, Kiselyeva Y, Jennelle L, Mukhamedova N, Remaley AT, Kashanchi F, Sviridov D, Bukrinsky M. Stimulation of the liver X receptor pathway inhibits HIV-1 replication via induction of ATP-binding cassette transporter A1. Mol Pharmacol. 2010;78:215–25. doi: 10.1124/mol.110.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramezani A, Dubrovsky L, Pushkarsky T, Sviridov D, Karandish S, Raj DS, Fitzgerald ML, Bukrinsky M. Stimulation of Liver X Receptor Has Potent Anti-HIV Effects in a Humanized Mouse Model of HIV Infection. J Pharmacol Exp Ther. 2015;354:376–83. doi: 10.1124/jpet.115.224485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fowler AJ, Sheu MY, Schmuth M, Kao J, Fluhr JW, Rhein L, Collins JL, Willson TM, Mangelsdorf DJ, Elias PM, Feingold KR. Liver X receptor activators display anti-inflammatory activity in irritant and allergic contact dermatitis models: liver-X-receptor-specific inhibition of inflammation and primary cytokine production. The Journal of investigative dermatology. 2003;120:246–55. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 95.Hatano Y, Man MQ, Uchida Y, Crumrine D, Mauro TM, Feingold KR, Elias PM, Holleran WM. Murine atopic dermatitis responds to peroxisome proliferator-activated receptors alpha and beta/delta (but not gamma) and liver X receptor activators. The Journal of allergy and clinical immunology. 2010;125:160–9. doi: 10.1016/j.jaci.2009.06.049. e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang W, Ghisletti S, Saijo K, Gandhi M, Aouadi M, Tesz GJ, Zhang DX, Yao J, Czech MP, Goode BL, Rosenfeld MG, Glass CK. Coronin 2A mediates actin-dependent de-repression of inflammatory response genes. Nature. 2011;470:414–8. doi: 10.1038/nature09703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife. 2015;4:e08009. doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ma L, Dong F, Zaid M, Kumar A, Zha X. ABCA1 Protein Enhances Toll-like Receptor 4 (TLR4)-stimulated Interleukin-10 (IL-10) Secretion through Protein Kinase A (PKA) Activation. J Biol Chem. 2012;287:40502–12. doi: 10.1074/jbc.M112.413245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pradel LC, Mitchell AJ, Zarubica A, Dufort L, Chasson L, Naquet P, Broccardo C, Chimini G. ATP-binding cassette transporter hallmarks tissue macrophages and modulates cytokine-triggered polarization programs. European journal of immunology. 2009;39:2270–80. doi: 10.1002/eji.200838867. [DOI] [PubMed] [Google Scholar]
- 100.Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of ATP-Binding Cassette Transporters A1 and G1 in Macrophages Increases Inflammation and Accelerates Atherosclerosis in Mice. Circ Res. 2013;112:1456–65. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leitinger N, Schulman IG. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler Thromb Vasc Biol. 2013;33:1120–6. doi: 10.1161/ATVBAHA.112.300173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim SY, Lim EJ, Yoon YS, Ahn YH, Park EM, Kim HS, Kang JL. Liver X receptor and STAT1 cooperate downstream of Gas6/Mer to induce anti-inflammatory arginase 2 expression in macrophages. Scientific reports. 2016;6:29673. doi: 10.1038/srep29673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marathe C, Bradley MN, Hong C, Lopez F, Ruiz de Galarreta CM, Tontonoz P, Castrillo A. The arginase II gene is an anti-inflammatory target of liver X receptor in macrophages. J Biol Chem. 2006;281:32197–206. doi: 10.1074/jbc.M605237200. [DOI] [PubMed] [Google Scholar]
- 104.Pourcet B, Feig JE, Vengrenyuk Y, Hobbs AJ, Kepka-Lenhart D, Garabedian MJ, Morris SM, Jr, Fisher EA, Pineda-Torra I. LXRalpha regulates macrophage arginase 1 through PU.1 and interferon regulatory factor 8. Circ Res. 2011;109:492–501. doi: 10.1161/CIRCRESAHA.111.241810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marathe C, Bradley MN, Hong C, Chao L, Wilpitz D, Salazar J, Tontonoz P. Preserved glucose tolerance in high-fat-fed C57BL/6 mice transplanted with PPARgamma−/−, PPARdelta−/−, PPARgammadelta−/−, or LXRalphabeta−/− bone marrow. J Lipid Res. 2009;50:214–24. doi: 10.1194/jlr.M800189-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi JY, Seo JY, Yoon YS, Lee YJ, Kim HS, Kang JL. Mer signaling increases the abundance of the transcription factor LXR to promote the resolution of acute sterile inflammation. Science signaling. 2015;8:ra21. doi: 10.1126/scisignal.2005864. [DOI] [PubMed] [Google Scholar]
- 107.N AG, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–58. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu B, Bernotas R, Unwalla R, Collini M, Quinet E, Feingold I, Goos-Nilsson A, Wilhelmsson A, Nambi P, Evans M, Wrobel J. Quinoline-3-carboxamide containing sulfones as liver X receptor (LXR) agonists with binding selectivity for LXRbeta and low blood-brain penetration. Bioorg Med Chem Lett. 2010;20:689–93. doi: 10.1016/j.bmcl.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 109.Hu B, Unwalla R, Collini M, Quinet E, Feingold I, Goos-Nilsson A, Wihelmsson A, Nambi P, Wrobel J. Discovery and SAR of cinnolines/quinolines as liver X receptor (LXR) agonists with binding selectivity for LXRbeta. Bioorg Med Chem. 2009;17:3519–27. doi: 10.1016/j.bmc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Quinet EM, Basso MD, Halpern AR, Yates DW, Steffan RJ, Clerin V, Resmini C, Keith JC, Berrodin TJ, Feingold I, Zhong W, Hartman HB, Evans MJ, Gardell SJ, DiBlasio-Smith E, Mounts WM, LaVallie ER, Wrobel J, Nambi P, Vlasuk GP. LXR ligand lowers LDL cholesterol in primates, is lipid neutral in hamster, and reduces atherosclerosis in mouse. J Lipid Res. 2009;50:2358–70. doi: 10.1194/jlr.M900037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wrobel J, Steffan R, Bowen SM, Magolda R, Matelan E, Unwalla R, Basso M, Clerin V, Gardell SJ, Nambi P, Quinet E, Reminick JI, Vlasuk GP, Wang S, Feingold I, Huselton C, Bonn T, Farnegardh M, Hansson T, Nilsson AG, Wilhelmsson A, Zamaratski E, Evans MJ. Indazole-based liver X receptor (LXR) modulators with maintained atherosclerotic lesion reduction activity but diminished stimulation of hepatic triglyceride synthesis. J Med Chem. 2008;51:7161–8. doi: 10.1021/jm800799q. [DOI] [PubMed] [Google Scholar]
- 112.Fond AM, Lee CS, Schulman IG, Kiss RS, Ravichandran KS. Apoptotic cells trigger a membrane-initiated pathway to increase ABCA1. J Clin Invest. 2015;125:2748–58. doi: 10.1172/JCI80300. [DOI] [PMC free article] [PubMed] [Google Scholar]