Abstract
Purpose
Aldosterone-producing adenomas with concurrent subclinical cortisol hypersecretion are reported in an increasing number of patients. Five aldosterone-producing adenomas from patients with primary aldosteronism and subclinical hypercortisolism were examined. The aims of our study were: (1) to analyze pathological features and immunohistochemical expression of CYP11B1 (11β-hydroxylase) and CYP11B2 (aldosterone synthase) in these tumors; (2) to investigate somatic mutations involved in adrenal steroid hypersecretion and/or tumor growth.
Methods
Archival micro-dissected paraffin-embedded slides from tumor specimens were used for histological and molecular studies. Immunohistochemistry was performed using monoclonal anti-CYP11B1 and anti-CYP11B2 antibodies. Cellular composition was determined by examining for known features of zona fasciculata and zona glomerulosa, and immunoreactivity for CYP11B1 and CYP11B2 by McCarty H-score. Spot regions for mutations in KCNJ5, ATP1A1, ATP2B3, CACNA1D, PRKACA, and CTNNB1 gene sequences were evaluated.
Results
Four APAs showed a predominant (≥50%) zona fasciculata-like cell pattern: one tumor had CYP11B1 H-score = 150, no detectable CYP11B2 expression, and harbored a PRKACA p.Leu206Arg mutation (that we have reported previously elsewhere), one had no CYP11B1 expression, CYP11B2 H-score = 40, and no mutations; the remaining two adenomas had high CYP11B1 H-score (160 and 240, respectively) and low CYP11B2 H-score (30 and 15, respectively), with the latter harboring a CTNNB1 p. Ser45Phe activating mutation. One of five aldosterone-producing adenomas had a predominant zona glomerulosa-like pattern, CYP11B1 H-score = 15, CYP11B2 H-score = 180, and no mutations.
Conclusions
The majority of aldosterone-producing adenomas with concurrent subclinical cortisol hypersecretion were composed mainly of zona fasciculata-like cells, while CYP11B1 and CYP11B2 immunostaining demonstrated clear heterogeneity. In a subset of cases, different somatic mutations may be involved in hormone excess and tumor formation.
Keywords: Aldosterone-producing adenoma, Subclinical hypercortisolism, Histopathology
Introduction
Hogan et al. first reported a patient with primary aldosteronism and Cushing’s syndrome due to an aldosterone-producing and cortisol-producing adenoma in 1977 [1]. Subsequently, several cases of aldosterone-producing adenomas (APAs) with Cushing’s syndrome have been published [2–4]. APAs with concurrent subclinical cortisol hypersecretion (SCH) are also reported in an increasing number of patients [2, 5–9] characterized by a high cardiovascular risk [10], which reflects the combined damaging effects of the two steroid excess [11, 12]. In Japan, the prevalence of unilateral primary aldosteronism with concurrent subclinical hypercortisolism has been found to be 15 ~ 30% [6, 10]. Moreover, subclinical hypercortisolism in unilateral primary aldosteronism may generally be under-diagnosed due to incomplete screening, i.e., many of these patients will not undergo 1 mg dexamethasone overnight administration. Using this screening test, Piaditis et al. have reported in fact that primary aldosteronism with subtle hypercortisolism was observed in 12.1% of 83 unilateral adrenal incidentalomas [13].
There are scarce data on the histopathological features of surgically removed APAs co-secreting cortisol at subclinical level. Furthermore, recent discovery of somatic mutations in genes regulating Ca2+ homeostasis (KCN5J, ATP1A1, ATP2B3, CACNA1D) [14] or beta-catenin (CTNNB1) in APA [15–17], or in genes involved in protein kinase A (PKA) catalytic subunit (PRKACA) in cortisol-producing adenomas [18, 19], have opened new insights on the molecular mechanisms that control autonomous corticosteroid synthesis. Few reports are available on the presence these mutations in tumors co-secreting aldosterone and cortisol [20].
The aims of our study were: (1) to analyze pathological features and immunohistochemical expression of CYP11B1 (11β-hydroxylase) and CYP11B2 (aldosterone synthase) in a retrospective series of adenomas removed from patients with concurrent unilateral primary aldosteronism and subclinical hypercortisolism; (2) to investigate somatic mutations involved in adrenal steroid hypersecretion and/or tumor growth. This study includes two patients previously reported by Fallo et al. [5] and by Rhayem et al. [21].
Methods
Patients and clinical annotations
Adrenal glands included in the study were removed from 5 patients (3 females and 2 males, age range 43–72 years) affected by unilateral primary aldosteronism without any signs or symptoms of cortisol excess, all studied and diagnosed at our institutions (case 1, 2, and 3 at the University Hospital of Munich, Germany, case 4 at the University Hospital of Bologna, Italy, and case 5 at University Hospital of Padova, Italy, respectively). Informed consent was obtained from all individual participants to the study, which was approved by local Ethics Committees. Patients were diagnosed with primary aldosteronism according to institutional and Endocrine Society clinical practice guidelines [5, 21, 22]. In Italy, patients for primary aldosteronism were screened using the aldosterone/plasma renin activity (PRA) ratio: the cutoff level for a positive ratio was 1000 (aldosterone in pmol/L and PRA in 1.5–5.2 ng/ml per h) together with a aldosterone levels greater than 416.6 pmol/L. In Germany, patients were screened using the aldosterone/direct renin concentration (DRC) ratio: the cutoff level for a positive ratio was 27 (aldosterone in pmol/L and DRC in mU/L). The confirmatory test was an intravenous saline load (2 L of 0.9% NaCl infused over 4 h) that was considered positive if post-test aldosterone levels were >138.8 pmol/L. All patients with confirmed primary aldosteronism underwent an adrenal computed tomography scan with fine cuts (2.5–3 mm). In three of the 5 patients, an adrenal venous sampling (AVS) was performed to differentiate between unilateral and bilateral aldosterone hypersecretion. AVS was performed with each adrenal vein cannulated sequentially (left before right) and without adrenocorticotropic hormone (ACTH) stimulation. Since unilateral cortisol overproduction and contralateral suppression coupled with a higher peripheral cortisol level would limit its usefulness for assessing cannulation success in patients with concomitant APA and SCH (selectivity index) [23, 24], successful cannulation of the adrenal veins was assessed by an adrenal/peripheral venous aldosterone gradient Similarly, since lateralization ratio (lateralization index) would not be accurate, the absolute value of plasma aldosterone levels was employed for diagnostic criteria of laterality for aldosterone hypersecretion [8]. The presence of the syndrome of glucocorticoid-remediable aldosteronism was excluded by the long polymerase chain reaction test [25].
Several days before testing for differential diagnosis of primary aldosteronism subtype, all patients were given 1 mg overnight dexamethasone as a screening suppression test for subclinical hypercortisolism [26]. In the case of failure to suppress plasma cortisol to less than 50 nmol/L, plasma ACTH and urinary cortisol were measured. Subclinical hypercortisolism was defined as the combination of postdex cortisol equal to or more than 50 nmol/L and at least one of two other abnormal hormonal parameters, that is, ACTH less than 2 pmol/L and urinary cortisol more than 694 nmol/24 h.
Baseline clinical and biochemical characteristics of the patients included in the study are summarized in Table 1. Supplemental Table 1 details adrenal vein and peripheral aldosterone and cortisol data of the 3 patients who undergo AVS.
Table 1.
Baseline clinical and biochemical characteristics of patients with APAs and concurrent SCH
| Case n° | Age /sex | SBP/DBP (mmHg) |
No. of drugs before AVS |
Serum K mmol/L) |
Plasma aldosterone (pmol/L) |
PRA (ng/ ml per h) |
DRC (mU/L) |
ARR | ACTH (pmol/L) |
Urinary Cortisol (nmol/24 h) |
Serum cortisol (nmol/L) |
Serum cortisol by 1 mg DST (nmol/L) |
CT scan | Laterality at AVS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/F | 149/90 | 2 | 2.5 | 1387.2 | 9.4 | 147.5 | 1.32 | 456 | 278.3 | 187.6 | Right nodule | Right APA Right SCH | |
| 2 | 63/F | 144/98 | 2 | 2.8 | 1409.1 | 7.9 | 178.3 | 0.44 | 339 | 303.1 | 190.1 | Right nodule | Right APA Right SCH | |
| 3 | 64/M | 187/106 | 4 | 2.8 | 1687.9 | 10.9 | 154.8 | 0.88 | 554 | 942.5 | 96.4 | Left nodule | Left APA Left SCH | |
| 4 | 43/F | 170/102 | 3 | 1.9 | 1054.2 | 0.2 | 5271 | 2.86 | 937 | 355.5 | 104.7 | Left nodule | NA | |
| 5 | 72/M | 180/100 | 3 | 2.9 | 1095.7 | 0.1 | 10957 | 1.54 | 885 | 509.8 | 77.1 | Right nodule | NA |
Serum K refers to the lowest measured concentration
SBP systolic blood pressure, DBP diastolic blood pressure, PRA plasma renin activity, DRC direct renin concentration, ARR aldosterone-to-renin ratio, DST dexamethasone suppression test, CT computed tomography, AVS adrenal venous sampling, SCH subclinical cortisol hypersecretion, NA not available
Hormone assays
Blood samples were taken at 08:00–09:00 h, after overnight fasting. Plasma aldosterone concentrations were measured using commercial RIAs (in Italy from Sorin Biomedical Diagnostics, Saluggia [27] and in Germany from Siemens (Coat-a-count), Los Angeles, CA, USA [21]; normal range (upright) is 138–969 pmol/L. In the two patients form Italy, PRA was determined by radioimmunoassay with kits purchased from Sorin Biomedical Diagnostics, Saluggia, Italy: [27]: normal range (upright) is 1.5–5.2 ng/mL per h. In the three patients from Germany, direct plasma DRC levels were measured with a chemiluminescent immunometric method (LIASON Direct Renin, DiaSorin, Dietzenbach, Germany) applied to a fully automated analyzer [28]; normal range (upright) is 4.4–46 mU/L. Plasma ACTH was measured by competitive chemiluminescent enzyme immunoassay (IMMULITE 2000 systems; Siemens Healthcare Diagnostics Inc., Muenchen, Germany); normal range at 08:00 h, 2–10 pmol/L. Plasma and 24-h urinary cortisol were measured by an automated chemiluminescence assay (IMMULITE 2000, Liaison, Diasorin, Italy). Normal range for plasma cortisol at 08:00 h is 138–690 nmol/L and normal range for urine cortisol is 90–694 nmol/ 24 h (detection limit = 5.5 nmol/L). For hormone measurements, intra-assay and interassay coefficients of variation were less than 10%.
Adrenal tissue samples
Archival microdissected paraffin-embedded slides of from the patients were used for histological examinations and molecular studies. All adrenal tissue specimens have been handled according to the standards of the Royal College of Pathologists [29].
Pathological analysis
Histological examination of APA tissues was performed by an experienced pathologist (I.C.). All adrenal glands included in the study were paraffin embedded, cut into 3 μm thick slices and stained with H&E.
Cellular composition was determined by examining for known features of zona fasciculata (ZF), i.e., large, lipid-laden clear cells, with round to oval vesicular nuclei, zona glomerulosa (ZG), i.e., small, compact cells, with high nuclear/cytoplasmic ratio and moderate amount of lipid, and zona reticularis, i.e., lipid-sparse cytoplasm, compact cells [30, 31]. The tumors were classified as ZF-like when the percentage of large vacuolated cells was greater than 50%, and ZG-type when the percentage of ZF-like cells was <50% and ZG-like cells were the most prominent cell type.
Microscopical examination of tissue adjacent to the tumor in cases 1–4 was not available.
Immunohistochemical procedure
Immunohistochemistry was performed using the following primary antibodies: rat monoclonal anti-human CYP11B1-80-7 (11β-hydroxylase) and mouse monoclonal anti-human CYP11B2-41-17 (aldosterone synthase) [32]. For both protocols, sections of 3 μm thickness from paraffin-embedded adrenal tissue were incubated with H2O2, and pre-treated with EDTA 0.1 mM (pH 8) for 40 minutes at 98 °C for antigen retrieval. Subsequently, to detect CYP11B1 expression, after endogenous biotin blocking by sequential avidin–biotin treatment, the slides were incubated overnight at 4 °C with the primary antibody diluted 1:100. After rinsing in PBS, slides were treated with biotinylated secondary antibody goat anti-rat (STARD131, AbD Serotec, diluted 1:300) for 30 min, followed by the incubation with Streptavidin-HRP (Millipore) for 15 min. To detect aldosterone synthase, after antigen retrieval, slides were incubated with anti-CYP11B2 (1:1000) over-night at 4 °C. After rinsing in PBS, the EnVision reagent (Dako, Carpinteria, CA, USA) coupled with peroxidase-labeled polymer was incubated as secondary antibody for 30 min. The proteins were visualized with 3,3′-diaminobenzidine tetra-hydrochloride and counterstained with hematoxylin. Immunoreactivity for CYP11B and CYP11B2 was assessed semiquantitatively by the McCarty H-score (ranging from 0 to 300), with all tumors examined under a ×20 objective. In each field the percentage of immunopositive cells was assessed and then multiplied by a factor (from 0 to 3) according to the intensity of the immunopositivity [33]. The relative immunointensity of specific immunoreactivity was characterized as not present (0), weak but detectable above control (1 +); distinct (2 +); very strong (3 +) [34].
CYP17A1 (17α-hydroxylase) immunohistochemistry was also performed in all cases. The details of the rabbit polyclonal antibody against cytochrome P450 17A1 have been previously reported [35]. Briefly, sections were antigen-retrieved with an autoclave (5 min in citric acid buffer, pH 6.0), and treated with a blocking reagent (Histofine, Nichirei, Tokyo, Japan) for 30 min at room temperature. Sections were incubated with αCYP17A1 (1:500) overnight at 4 °C. Immunoreactivity was visualized with 3,3′-diaminobenzidine (DAB; brown staining) with a peroxidase-based Histofine Simple Stain Kit (Nichirei, Tokyo, Japan) and counterstained with hematoxylin.
Adrenal tissue of case 5 was stained with Melan-A/MART-1 mouse monoclonal antibody (Ab-4, clone A103, Thermo Scientific, Monza, Italy, diluted 1:200) and with Synaptophysin monoclonal mouse antibody (clone DAK-SYNAP, Dako, Carpinteria, CA, USA, diluted 1:400).
DNA sequencing of KCNJ5, ATP1A1, ATP2B3, CACNA1D, PRKACA, and CTNNB1
DNA fragments from the tumors were sequenced for KCNJ5, ATP1A1, ATP2B3, CACNA1D, PRKACA, and CTNNB1 by PCR amplification, using primers reported previously [21, 36–38]. Hot spot regions for mutations in KCNJ5, ATP1A1, ATP2B3, CACNA1D, PRKACA, and CTNNB1 were sequenced in all five APAs included in the study.
Results
Study cases
All patients showed improvement in their hypertensive status, based on lowered blood pressure after surgery and decreased number of antihypertensive medications, as well as normalization of hormonal and biochemical parameters at 3–5 years follow-up (median 32 months) after unilateral adrenalectomy (Table 2).
Table 2.
Post-operative clinical and biochemical parameters of patients with APAs and concurrent SCH
| Case n° | Follow-up (months) | SBP/DBP (mmHg) | No. of drugs after ADX | Serum K (mmo l/L) | Plasma Aldosterone (pmol/L) | PRA (ng/ml per h) | DRC (mU/ L) | ARR | ACTH (pmol/L) | Urinary cortisol, (nmol/24 h) | Serum Cortisol (nml/L) | Serum cortisol by 1 mg DST (nmol/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 | 124/80 | 2 | 4.2 | 887.6 | 38 | 23.3 | 2.6 | 598.1 | 331.0 | 66.2 | |
| 2 | 18 | 116/69 | 1 | 5.0 | 574.1 | 32 | 17.9 | 3.1 | 328.2 | 284.1 | 52.4 | |
| 3 | 25 | 146/93 | 2 | 4.5 | 399.4 | 40 | 9.9 | 4.4 | 554.4 | 871.8 | 38.6 | |
| 4 | 24 | 130/80 | 1 | 4.7 | 502.0 | 3.4 | 147.6 | 2.8 | 135.2 | 278.6 | 33.1 | |
| 5 | 48 | 150/80 | 1 | 4.4 | 418.8 | 2.6 | 161.0 | 4.0 | 463.0 | 386.2 | 38.6 |
SBP systolic blood pressure, DBP diastolic blood pressure, ADX adrenalectomy, PRA plasma renin activity, DRC direct renin concentration, DST dexamethasone suppression test
Pathological, immunohistochemical and genetic findings
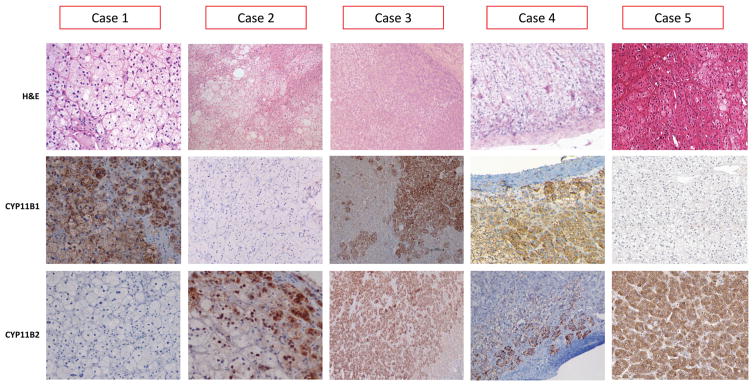
Histopathological and genetic findings in the adrenal tissue of our 5 patients with APAs and concurrent SCH are summarized in Table 3. The cut surface was yellowish in all tumors, with no necrosis or hemorrhagic areas. Microscopically, all criteria for adrenocortical adenoma were fulfilled (Fig. 1). Four adenomas showed a predominant (≥50%) ZF-like cell pattern: one tumor (case 1) had CYP11B1 H-score = 150, no detectable CYP11B2 expression, and harbored a PRKACA p.Leu206Arg mutation, one (case 2) had no CYP11B1 expression, CYP11B2 H-score = 40, and no mutations; the remaining two adenomas (case 4 and 5) had high CYP11B1 H-score (160 and 240, respectively) and low CYP11B2 H-score (30 and 15, respectively), with the latter harboring a CTNNB1 (β-catenin) p.Ser45Phe activating mutation. One of the five APAs had a predominant ZG-like pattern (case 3), CYP11B1 H-score = 15, CYP11B2 H-score = 180, and no mutations. Immunohistochemical expression of CYP17A1 showed strong positivity in 4 out of five examined, and a weak positivity in one case (case 3) (Supplemental Fig. 1).
Table 3.
Histopathological and genetic findings of APAs with concurrent SCH
| Case n° | Tumor size (mm) | Microscopic evaluation | Predominant cell pattern | CYP11B1H-score | CYP11B2H-score | Mutational status |
|---|---|---|---|---|---|---|
| 1 | 12 | Single nodule | ZF-like cells | 150 | 0 | PRKACA pLeu206Arg |
| 2 | 32 | Single nodule | ZF-like cells | 0 | 40 | WT |
| 3 | 11 | Single nodule | ZG-like cells | 15 | 180 | WT |
| 4 | 50 | Single nodule | ZF-like cells | 160 | 30 | WT |
| 5 | 40 | Single nodule with adjacent tissue containing multiple micronodules | ZF-like cells | 240 | 15 | CTNNB1 pSer45Phe |
ZF zona fasciculata, ZG zona glomerulosa, WT wild type
Fig. 1.
Histopathological findings in APAs of the 5 patients with SCH (original magnification ×20). Upper panel: Hematoxylin–eosin (H&E) staining of APAs, with prevalent ZF-like cells in case 1, 2, 4, 5) and by ZG-like cell in case 3; middle panel: CYP11B1 immunostaining of APAs, with no immunopositivity in case 2; lower panel: CYP11B2 immunostaining of APAs, with no immunopositivity in case 1. Case 1 harbored a p.Leu206Arg mutation of PRKACA gene, and case 5 harbored a p.Ser45Phe mutation of CTNNB1 gene
Case 5 showed a main nodule with multiple adjacent micronodules. Melan-A staining was negative in adenoma and in peri-adenoma adrenocortical tissue; synaptophysin staining was weakly positive in adenoma and negative in peri-adenoma tissue (Supplemental Fig. 2).
Discussion
Functional zonation of the human adrenal cortex, i.e., the ability of each zone to differentially produce aldosterone and cortisol, relies on the zone-specific expression of CYP11B1 and CYP11B2 isozymes, and zonal expression of the isozymes results from transcriptional regulation of their coding genes. In this regard, APA tissue is able to make cortisol [39]. Several histopathological studies have been performed to characterize histopathology of APA in patients with clinical and biochemical features of overt cortisol secretion. Early reports using antibodies against CYP17A showed positive staining in these tumors at least focally in ZG-compact cells, and staining with antibodies against CYP11B1 and CYP11B2 were all positive [2]. Very few data are available on the histopathological features of APAs characterized by a concurrent subclinical autonomous cortisol hypersecretion. Fujii et al. [40] described a positive immunoreaction of CYP11B1 predominantly in ZG compact cells and of CYP11B2 mainly in the predominant ZF clear cells. Hiraishi et al. [6] reported a histopathological study and CYP11B2 mRNA level in 8 APAs with subclinical Cushing syndrome, showing no difference with pure APAs. Yamada et al. [4] did not report any difference between CYP11B1 and CYP11B2 mRNA levels from two cases of APAs in patients with subclinical hypercortisolism and those pure APAs. Recent availability of specific antibodies that selective detect CYP11B1 and CYP11B2 has allowed their more precise localization in normal and pathological adrenals, with the existence of variable patterns [32]. Furthermore, a simple semiquantitative system such as the McCarty H-score, although described in a different setting, has been validated as the best method currently available to allow the estimation of steroidogenic enzyme activity in the objective fashion in APA tissues [35, 41, 42]. Nakamura et al. [41] reported in fact that APAs contained not only a mix of CYP11B2 and CYP11B1-positive cells but also cells expressing both CYP11B enzymes. Four of our cases of APAs with SCH were composed mainly of ZF-like cells, while CYP11B1/CYP11B2 immunostaining demonstrated clear heterogeneity. In one of our cases (case 1) with predominant ZF-like cells, CYP11B2 was not expressed in several histological sections. The same case, already reported by Rhayem at al. by using different histological sections, showed CYP11B2 expression only in very few adenoma cells [21]. Furthermore, we found high CYP11B1 expression and low CYP11B2 expression on case 5, confirming our previous findings on the same case using in-situ hybridization [5], and in case 4. It might appear surprising that some APAs do not show or show very low CYP11B2 expression. This has been previously reported by Lenzini et al. [43], who found 37.6% of APA with a CYP11B2 gene expression inferior to normal adrenals, by Dekkers et al. [44], who found 9.6% APA that were CYP11B2-negative, and by Monticone et al. [42], who found 8/71 APAs with negative CYP11B2 immunostaining. However, as APA is composed of a much larger number of aldosterone-secreting cells than the normal ZG, a high secretion of aldosterone and a negative or a low CYP11B2 expression may occur: even if the production of aldosterone per single APA cell is small, the overall aldosterone synthesis can in fact be several folds higher in an APA than in a normal adrenal gland. This concept could also be applied for the lack or low CYP11B1 expression in two of our APA cases with subclinical hypercortisolism characterized by highly predominant ZF-cells and prominent ZG-like cells, i.e., case 2 and 3, respectively. Moreover, in the same APAs the intensity of CYP11B2 expression was somewhat different. Due to lack of available pathological material allowing to perform double staining of CYP11B1 and CYP11B2 and/or to use laser capture microdissection of ZF-like/ZG-like cells [45], it was not possible to clarify whether co-secretion of cortisol and aldosterone originated from hybrid cells or from a cell subpopulation with specific phenotype. CYP17A1 intensively stained in all but one of our cases of APAs with mild cortisol hypersecretion, confirming the active involvement of 17α-hydroxylase in early steroidogenic pathway leading to cortisol formation, as also observed in the common form of APA [39]. Interestingly, CYP17A1 staining was weak in the only APA with subclinical hypercortisolism (case 3) having predominant ZG-like cell population.
Recently, exome sequencing analyses demonstrated that 50 to 80% of APAs harbor somatic mutations in KCNJ5, ATP1A1, ATP2B3, CACNA1D, leading to an increased intracellular Ca2+ concentration, an activation of Ca2+ signaling, and an increase in CYP11B2 transcription [46]. Interestingly, APAs with KCNJ5 mutations have more ZF-like or mixed ZG–ZF phenotype, and heterologous expression of a KCNJ5 variant in HAC15 cells causes not only upregulation of CYP11B2 expression but also increased expression of CYP11B1 and synthesis of hybrid steroids 18-hydroxycortisol and 18-oxocortisol, as well as corticosterone [47], raising the question whether they can produce clinically relevant amounts of glucocorticoids. Yamada et al. reported 2 female patients with APA co-secreting cortisol, without clinical signs of Cushing’s syndrome, which harbored mutations in KCNJ5 [4]. Tong et al. described a patient with familial PA associated with overt Cushing’s syndrome, harboring a KNCJ5 germline mutations, who had a massive bilateral hyperplasia [48]. We could not confirm the presence of KCNJ5 mutations, as well as of mutations in other genes (i.e., ATP1A1, ATP2B3, CACNA1D) involved in calcium signaling, in our series of cases. Since a higher frequency of KCNJ5 mutations in APAs (65%) as well a higher prevalence of APAs associated with mild cortisol secretion are reported in Japan, ethnicity-related difference may explain our findings. Moreover, a larger series of this type of tumors should be examined to draw any conclusion on the frequency of these mutations.
The recent discovery in cortisol-producing adenomas of somatic mutations in the gene (PRKACA) encoding the catalytic subunit of PKA, have opened a new scenario on the molecular mechanisms that control autonomous cortisol synthesis and cell proliferation, despite they remain largely unknown [49]. Beuschlein et al. identified in fact the p. Leu206Arg PRKACA variant as the most frequent somatic mutation to be found in cortisol-producing adenomas, associated with severe forms of adrenal Cushing’s syndrome [50]. No PRKACA variants were found in cortisol-producing adenomas associated with subclinical Cushing’s syndrome or in inactive adenomas [19, 50]. Although subclinical Cushing’s syndrome has been observed in up to 21% of APA cases in some series [6], no PRKACA mutations have been identified in APAs in two recent reports [50, 51]. We found a PRKACA p.Leu206Arg somatic mutation in one of our cases (case 1), which was the one of the two cases over a series of 122 APAs previously reported by Rhayem et al. [21].
Activating somatic mutations of CTNNB1 gene seem also implicated in benign aldosterone-secreting and cortisol-secreting tumor growth. CTNNB1 encodes β-catenin of the Wnt/β–catenin pathway, which is known to play an important role in adrenocortical development and cancer in other organs [52]. Such mutations prevent β-catenin degradation and cause proliferation. Even though such events have been shown to trigger benign aldosterone-secreting and cortisol-secreting tumor development as well as malignancy in a mouse model and human tissue samples, the exact mechanisms underlying hormone secretion in CTNNB1 mutated tumors remain to be determined [46]. CTNNB1 mutations are able to stabilize β-catenin and increase the activity of the finely tuned Wnt signaling pathway, leading to tumor formation [53]. In fact, in APAs harboring CTNNB1 mutations, the nuclear and/or cytoplasmic accumulation of active β-catenin protein has been shown to be increased especially for female patients and he accumulation of β-catenin protein could be involved in APA proliferation and anti-apoptotic process [54]. We did observe a CTNNB1 mutation in one of our APAs with SCH (case 5) where, at variance with high CYP11B2 expression reported in APA with somatic CTNNB1 mutations [17], the CYP11B2 expression was relatively low and CYP11B1 expression was high. Exon 3 of the CTNNB1 gene contains specific serine and threonine residues that, when phosphorylated, mark β-catenin for degradation [55]. Specifically, we found a somatic p.Ser45Phe CTNNB1 mutation, previously analyzed by Akerstrom et al. [17], which preclude phosphorylation of β-catenin, leading to aberrant activation of Wnt signaling [56]. In this context, a common pathway of PRKACA, CTNNB1, and GNAS has been also suggested [57], and mutations in the GNAS gene (p. Arg201Cys) in 2 of 33 APAs, both of which showing autonomous cortisol secretion, have been in fact reported [58].
Unfortunately, no sufficient somatic and germinal DNA was available to allow sequencing of GNAS or PRKAR1A, coding for the regulatory type 1 R1A subunit of PKA, in our patients. Germline heterozygous inactivating mutations of PRKAR1A have been reported in about 45% of patients with Carney complex, and up to 80% of Carney complex patients with Cushing’s syndrome due to primary pigmented nodular adrenal disease (PPNAD). Somatic inactivating mutations of PRKAR1A have been also observed in macronodules of PPNAD and in sporadic cortisol-secreting adrenal adenomas, from PPNAD to adrenocortical adenomas and cancer [59, 60]. A PRKACA copy number gain was recently found in the germline of several patients with cortisol-producing bilateral adrenal hyperplasia, whereas the somatic Leu206Arg recurrent PRKACA mutation was found in as many as half of all adrenocortical adenomas associated with ACTH-independent Cushing’s syndrome [60, 61]. This could have hypothetically occurred in case 5, who presented a predominant nodule and multiple adjacent micronodules associated with somatic β-catenin mutation that may reflect a germline PRKAR1A or PRKACA defect. A PRKACA amplification within the dominant tumor and/or in surrounding micronodules, leading to cortisol oversecretion, cannot also be excluded [62, 63]. Case 5 displayed a negative staining for melan-A in both adenoma and peri-adenoma tissue, and a weakly positive staining for synaptophysin in adenoma and a negative staining for synaptophysin in peri-adenoma tissue, respectively. This immunostaining pattern was not consistent with that found in adrenals of patients with Cushing’s syndrome due to PPNAD [64, 65], while it has been reported in cortisol-secreting tumors or in APAs [66, 67].
In conclusion, the majority of our APAs with concurrent SCH were composed mainly of ZF-like cells, while CYP11B1 and CYP11B2 immunostaining demonstrated clear heterogeneity. In a subset of cases, different somatic mutations may be involved in hormone excess and tumor formation.
Supplementary Material
adrenal vein sampling results in case 1, 2 and 3
Acknowledgments
The authors thank Dr. Vincenza Guzzardo for technical assistance.
Funding This study was supported by Fondo Investimenti Ricerca di Base (FIRB) Accordi di Programma 2011, RBAP1153LS-02 from the Ministry of Education, University, and Research- Rome, Italy. Dr. C. E.G.S was supported by National Heart, Lung and Blood Institute grant R01 HL27255 and the National Institute of General Medical Sciences of the National Institutes of Health, USA, under Award Number 1U54GM115428.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12020-017-1295-4) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent Informed consent was obtained from all individual participants included in the study.
References
- 1.Hogan MJ, Schambelan M, Biglieri EG. Concurrent hypercortisolism and hypermineralocorticoidism. Am J Med. 1977;62:777–782. doi: 10.1016/0002-9343(77)90883-x. [DOI] [PubMed] [Google Scholar]
- 2.Späth M, Korovkin S, Antke C, Anlauf M, Willenberg HS. Aldosterone- and cortisol-co-secreting adrenal tumors: the lost subtype of primary aldosteronism. Eur J Endocrinol. 2011;164:447–455. doi: 10.1530/EJE-10-1070. [DOI] [PubMed] [Google Scholar]
- 3.Vicennati V, Repaci A, di Dalmazi G, Rinaldi E, Golfieri R, Giampalma E, Minni F, Marrano N, Santini D, Pasquali R. Combined aldosterone and cortisol secretion by adrenal incidentaloma. Int J Surg Pathol. 2012;20:316–319. doi: 10.1177/1066896911427036. [DOI] [PubMed] [Google Scholar]
- 4.Yamada M, Nakajima Y, Taguchi R, Okamura T, Ishii S, Tomaru T, Ozawa A, Shibusawa N, Yoshino S, Toki A, Ishida E, Hashimoto K, Satoh T, Mori M. KCNJ5 mutations in aldosterone- and cortisol-co-secreting adrenal adenomas. Endocr J. 2012;59:735–774. doi: 10.1507/endocrj.ej12-0247. [DOI] [PubMed] [Google Scholar]
- 5.Fallo F, Bertello C, Tizzani D, Fassina A, Boulkroun S, Sonino N, Monticone S, Viola A, Veglio F, Mulatero P. Concurrent primary aldosteronism and subclinical cortisol hypersecretion: a prospective study. J Hypertens. 2011;29:1773–1777. doi: 10.1097/HJH.0b013e32834937f3. [DOI] [PubMed] [Google Scholar]
- 6.Hiraishi K, Yoshimoto T, Tsuchiya K, Minami I, Doi M, Izumiyama H, Sasano H, Hirata Y. Clinicopathological features of primary aldosteronism associated with subclinical Cushing’s syndrome. Endocr J. 2011;58:543–551. doi: 10.1507/endocrj.k10e-402. [DOI] [PubMed] [Google Scholar]
- 7.Yoon V, Heyliger A, Maekawa T, Sasano H, Carrick K, Woodruff S, Rabaglia J, Auchus RJ, Ghayee HK. Benign adrenal adenomas secreting excess mineralocorticoids and glucocorticoids. Endocrinol Diabetes Metab Case Rep. 2013;2013:130042. doi: 10.1530/EDM-13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto K, Honjo S, Tatsuoka H, Hamamoto Y, Kawasaki Y, Matsuoka A, Ikeda H, Wada Y, Sasano H, Koshiyama H. Primary aldosteronism associated with subclinical Cushing syndrome. J Endocrinol Invest. 2013;36:564–567. doi: 10.3275/8818. [DOI] [PubMed] [Google Scholar]
- 9.Chang KY, Ryu S, Cho JY, Kim HW. Aldosterone- and cortisol-co-producing adrenal adenoma without clinical features of Cushing syndrome. Korean J Intern Med. 2014;29:679–682. doi: 10.3904/kjim.2014.29.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakajima Y, Yamada M, Taguchi R, Satoh T, Hashimoto K, Ozawa A, Shibusawa N, Okada S, Monden T, Mori M. Cardiovascular complications of patients with aldosteronism associated with autonomous cortisol secretion. J Clin Endocrinol Metab. 2011;96:2512–2518. doi: 10.1210/jc.2010-2743. [DOI] [PubMed] [Google Scholar]
- 11.Prejbisz A, Warchoł-Celińska E, Lenders JW, Januszewicz A. Cardiovascular risk in rimary hyperaldosteronism. Horm Metab Res. 2015;47:973–980. doi: 10.1055/s-0035-1565124. [DOI] [PubMed] [Google Scholar]
- 12.Di Dalmazi G, Pasquali R, Beuschlein F, Reincke M. Subclinical hypercortisolism: a state, a syndrome, or a disease? Eur J Endocrinol. 2015;173:M61–M71. doi: 10.1530/EJE-15-0272. [DOI] [PubMed] [Google Scholar]
- 13.Piaditis GP, Kaltsas GA, Androulakis II, Gouli A, Makras P, Papadogias D, Dimitriou K, Ragkou D, Markou A, Vamvakidis K, Zografos G, Chrousos G. High prevalence of autonomous cortisol and aldosterone secretion from adrenal adenomas. Clin Endocrinol (Oxf) 2009;71:772–778. doi: 10.1111/j.1365-2265.2009.03551.x. [DOI] [PubMed] [Google Scholar]
- 14.Monticone S, Else T, Mulatero P, Williams TA, Rainey WE. Understanding primary aldosteronism: impact of next generation sequencing and expression profiling. Mol Cell Endocrinol. 2015;399:311–320. doi: 10.1016/j.mce.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scholl UI, Healy JM, Thiel A, Fonseca AL, Brown TC, Kunstman JW, Horne MJ, Dietrich D, Riemer J, Kücükköylü S, Reimer EN, Reis AC, Goh G, Kristiansen G, Mahajan A, Korah R, Lifton RP, Prasad ML, Carling T. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin Endocrinol (Oxf) 2015;83:779–789. doi: 10.1111/cen.12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teo AE, Garg S, Shaikh LH, Zhou J, Karet Frankl FE, Gurnell M, Happerfield L, Marker A, Bienz M, Azizan EA, Brown MJ. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N Engl J Med. 2015;373:1429–1436. doi: 10.1056/NEJMoa1504869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Åkerström T, Maharjan R, Sven Willenberg H, Cupisti K, Ip J, Moser A, Stålberg P, Robinson B, Alexander Iwen K, Dralle H, Walz MK, Lehnert H, Sidhu S, Gomez-Sanchez C, Hellman P, Björklund P. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci Rep. 2016;6:19546. doi: 10.1038/srep19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Dalmazi G, Kisker C, Calebiro D, Mannelli M, Canu L, Arnaldi G, Quinkler M, Rayes N, Tabarin A, Laure Jullié M, Mantero F, Rubin B, Waldmann J, Bartsch DK, Pasquali R, Lohse M, Allolio B, Fassnacht M, Beuschlein F, Reincke M. Novel somatic mutations in the catalytic subunit of the protein kinase A as a cause of adrenal Cushing’s syndrome: a European multicentric study. J Clin Endocrinol Metab. 2014;99:E2093–E2100. doi: 10.1210/jc.2014-2152. [DOI] [PubMed] [Google Scholar]
- 19.Thiel A, Reis AC, Haase M, Goh G, Schott M, Willenberg HS, Scholl UI. PRKACA mutations in cortisol-producing adenomas and adrenal hyperplasia: a single-center study of 60 cases. Eur J Endocrinol. 2015;172:677–685. doi: 10.1530/EJE-14-1113. [DOI] [PubMed] [Google Scholar]
- 20.Yamada M, Nakajima Y, Taguchi R, Okamura T, Ishii S, Tomaru T, Ozawa A, Shibusawa N, Yoshino S, Toki A, Ishida E, Hashimoto K, Satoh T, Mori M. KCNJ5 mutations in aldosterone- and cortisol-co-secreting adrenal adenomas. Endocr J. 2012;59:735–741. doi: 10.1507/endocrj.ej12-0247. [DOI] [PubMed] [Google Scholar]
- 21.Rhayem Y, Perez-Rivas LG, Dietz A, Bathon K, Gebhard C, Riester A, Mauracher B, Gomez-Sanchez C, Eisenhofer G, Schwarzmayr T, Calebiro D, Strom TM, Reincke M, Beuschlein F. PRKACA somatic mutations are rare findings in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2016;101:3010–3017. doi: 10.1210/jc.2016-1700. [DOI] [PubMed] [Google Scholar]
- 22.Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF., Jr The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. doi: 10.1210/jc.2015-4061. [DOI] [PubMed] [Google Scholar]
- 23.Goupil R, Wolley M, Ahmed AH, Gordon RD, Stowasser M. Does concomitant autonomous adrenal cortisol overproduction have the potential to confound the interpretation of adrenal venous sampling in primary aldosteronism ? Clin Endocrinol (Oxf) 2015;83:456–461. doi: 10.1111/cen.12750. [DOI] [PubMed] [Google Scholar]
- 24.Kishino M, Yoshimoto T, Nakadate M, Katada Y, Kanda E, Nakaminato S, Saida Y, Ogawa Y, Tateishi U. Optimization of left adrenal vein sampling in primary aldosteronism: coping with asymmetrical cortisol secretion. Endocr J. 2017 doi: 10.1507/endocrj.EJ16-0433. [DOI] [PubMed] [Google Scholar]
- 25.Mulatero P, Veglio F, Pilon C, Rabbia F, Zocchi C, Limone P, Boscaro M, Sonino N, Fallo F. Diagnosis of glucocorticoid-remediable aldosteronism in primary aldosteronism: aldosterone response to dexamethasone and long polymerase chain reaction for chimeric gene. J Clin Endocrinol Metab. 1998;83:2573–2575. doi: 10.1210/jcem.83.7.4946. [DOI] [PubMed] [Google Scholar]
- 26.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mulatero P, Milan A, Fallo F, Regolisti G, Pizzolo F, Fardella C, Mosso L, Marafetti L, Veglio F, Maccario M. Comparison of confirmatory tests for the diagnosis of primary aldosteronism. J Clin Endocrinol Metab. 2006;91:2618–2623. doi: 10.1210/jc.2006-0078. [DOI] [PubMed] [Google Scholar]
- 28.Manolopoulou J, Fischer E, Dietz A, Diederich S, Holmes D, Junnila R, Grimminger P, Reincke M, Morganti A, Bidlingmaier M. Clinical validation for the aldosterone-to-renin ratio and aldosterone suppression testing using simultaneous fully automated chemiluminescence immunoassays. J Hypertens. 2015;33:2500–2511. doi: 10.1097/HJH.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 29.Moonim MT, Johnson SJ, McNicol AM. Cancer dataset for the histological reporting of adrenal cortical carcinoma and phaeochromocytoma/paraganglioma. (2) 2012 https://www.rcpath.org/resourceLibrary/g109_adrenaldataset_jan12-pdf.html.
- 30.Neville AM, O’Hare MJ. Histopathology of the human adrenal cortex. Clin Endocrinol Metab. 1985;14:791–820. doi: 10.1016/s0300-595x(85)80078-5. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura Y, Felizola SJ, Satoh F, Konosu-Fukaya S, Sasano H. Dissecting the molecular pathways of primary aldosteronism. Pathol Int. 2014;64:482–489. doi: 10.1111/pin.12200. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 34.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers IL, McCarty KS., Jr Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- 35.Ono Y, Nakamura Y, Maekawa T, Felizola SJ, Morimoto R, Iwakura Y, Kudo M, Seiji K, Takase K, Arai Y, Gomez-Sanchez CE, Ito S, Sasano H, Satoh F. Different expression of 11β-hydroxylase and aldosterone synthase between aldosterone-producing microadenomas and macroadenomas. Hypertension. 2014;64:438–444. doi: 10.1161/HYPERTENSIONAHA.113.02944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes-Rosa FL, Williams TA, Riester A, Steichen O, Beuschlein F, Boulkroun S, Strom TM, Monticone S, Amar L, Meatchi Y, Mantero F, Cicala MV, Quinkler M, Fallo F, Allolio B, Bernini G, Maccario M, Giacchetti G, Jeunemaitre X, Mulatero P, Reincke M, Zennaro MC. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension. 2014;64:354–361. doi: 10.1161/HYPERTENSIONAHA.114.03419. [DOI] [PubMed] [Google Scholar]
- 37.Williams TA, Monticone S, Schack VR, Stindl J, Burrello J, Buffolo F, Annaratone L, Castellano I, Beuschlein F, Reincke M, Lucatello B, Ronconi V, Fallo F, Bernini G, Maccario M, Giacchetti G, Veglio F, Warth R, Vilsen B, Mulatero P. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014;63:188–195. doi: 10.1161/HYPERTENSIONAHA.113.01733. [DOI] [PubMed] [Google Scholar]
- 38.Bonnet S, Gaujoux S, Launay P, Baudry C, Chokri I, Ragazzon B, Libé R, René-Corail F, Audebourg A, Vacher-Lavenu MC, Groussin L, Bertagna X, Dousset B, Bertherat J, Tissier F. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96:E419–E426. doi: 10.1210/jc.2010-1885. [DOI] [PubMed] [Google Scholar]
- 39.Fallo F, Pezzi V, Barzon L, Mulatero P, Veglio F, Sonino N, Mathis JM. Quantitative assessment of CYP11B1 and CYP11B2 expression in aldosterone-producing adenomas. Eur J Endocrinol. 2002;147:795–802. doi: 10.1530/eje.0.1470795. [DOI] [PubMed] [Google Scholar]
- 40.Fujii H, Kamide K, Miyake O, Abe T, Nagai M, Nakahama H, Horio T, Takiuchi S, Okuyama A, Yutani C, Kawano Y. Primary aldosteronism combined with preclinical Cushing’s syndrome in an elderly patient. Circ J. 2005;69:1425–1427. doi: 10.1253/circj.69.1425. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura Y, Maekawa T, Felizola SJ, Satoh F, Qi X, Velarde-Miranda C, Plonczynski MW, Ise K, Kikuchi K, Rainey WE, Gomez-Sanchez EP, Gomez-Sanchez CE, Sasano H. Adrenal CYP11B1/2 expression in primary aldosteronism: immunohistochemical analysis using novel monoclonal antibodies. Mol Cell Endocrinol. 2014;392:73–79. doi: 10.1016/j.mce.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monticone S, Castellano I, Versace K, Lucatello B, Veglio F, Gomez-Sanchez CE, Williams TA, Mulatero P. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol Cell Endocrinol. 2015;411:146–154. doi: 10.1016/j.mce.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenzini L, Seccia TM, Aldighieri E, Belloni AS, Bernante P, Giuliani L, Nussdorfer GG, Pessina AC, Rossi GP. Heterogeneity of aldosterone-producing adenomas revealed by a whole transcriptome analysis. Hypertension. 2007;50:1106–1113. doi: 10.1161/HYPERTENSIONAHA.107.100438. [DOI] [PubMed] [Google Scholar]
- 44.Dekkers T, ter Meer M, Lenders JW, Hermus AR, Kool LS, Langenhuijsen JF, Nishimoto K, Ogishima T, Mukai K, Azizan EA, Tops B, Deinum J, Küsters B. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J Clin Endocrinol Metab. 2014;99:E1341–E1351. doi: 10.1210/jc.2013-4255. [DOI] [PubMed] [Google Scholar]
- 45.Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 46.Seidel E, Scholl UI. Intracellular molecular differences in aldosterone-compared to cortisol-secreting adrenal cortical adenomas. Front Endocrinol (Lausanne) 2016;7:75. doi: 10.3389/fendo.2016.00075. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hattangady NG, Karashima S, Yuan L, Ponce-Balbuena D, Jalife J, Gomez-Sanchez CE, Auchus RJ, Rainey WE, Else T. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J Mol Endocrinol. 2016;57:1–11. doi: 10.1530/JME-15-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tong A, Liu G, Wang F, Jiang J, Yan Z, Zhang D, Zhang Y, Cai J. A novel phenotype of familial hyperaldosteronism Type III: concurrence of aldosteronism and Cushing’s syndrome. J Clin Endocrinol Metab. 2016;101:4290–4297. doi: 10.1210/jc.2016-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirschner LS. Medicine A unified cause of adrenal Cushing’s syndrome. Science. 2014;344:804–805. doi: 10.1126/science.1254901. [DOI] [PubMed] [Google Scholar]
- 50.Beuschlein F, Fassnacht M, Assié G, Calebiro D, Stratakis CA, Osswald A, Ronchi CL, Wieland T, Sbiera S, Faucz FR, Schaak K, Schmittfull A, Schwarzmayr T, Barreau O, Vezzosi D, Rizk-Rabin M, Zabel U, Szarek E, Salpea P, Forlino A, Vetro A, Zuffardi O, Kisker C, Diener S, Meitinger T, Lohse MJ, Reincke M, Bertherat J, Strom TM, Allolio B. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med. 2014;370:1019–1028. doi: 10.1056/NEJMoa1310359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakajima Y, Okamura T, Gohko T, Satoh T, Hashimoto K, Shibusawa N, Ozawa A, Ishii S, Tomaru T, Horiguchi K, Okada S, Takata D, Rokutanda N, Horiguchi J, Tsushima Y, Oyama T, Takeyoshi I, Yamada M. Somatic mutations of the catalytic subunit of cyclic AMP-dependent protein kinase (PRKACA) gene in Japanese patients with several adrenal adenomas secreting cortisol [Rapid Communication] Endocr J. 2014;61:825–832. doi: 10.1507/endocrj.ej14-0282. [DOI] [PubMed] [Google Scholar]
- 52.El Wakil A, Lalli E. The Wnt/beta-catenin pathway in adrenocortical development and cancer. Mol Cell Endocrinol. 2011;332:32–37. doi: 10.1016/j.mce.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 54.Wu VC, Wang SM, Chueh SJ, Yang SY, Huang KH, Lin YH, Wang JJ, Connolly R, Hu YH, Gomez-Sanchez CE, Peng KY, Wu KD. The prevalence of CTNNB1 mutations in primary aldosteronism and consequences for clinical outcomes. Sci Rep. 2017;7:39121. doi: 10.1038/srep39121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 56.Hagen T, Vidal-Puig A. Characterisation of the phosphorylation of beta-catenin at the GSK-3 priming site Ser45. Biochem Biophys Res Commun. 2002;294:324–328. doi: 10.1016/S0006-291X(02)00485-0. [DOI] [PubMed] [Google Scholar]
- 57.Wilson CH, McIntyre RE, Arends MJ, Adams DJ. The activating mutation R201C in GNAS promotes intestinal tumourigenesis in Apc (Min/ +) mice through activation of Wnt ERK1/2 MAPK pathways. Oncogene. 2010;29:4567–4575. doi: 10.1038/onc.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima Y, Okamura T, Horiguchi K, Gohko T, Miyamoto T, Satoh T, Ozawa A, Ishii S, Yamada E, Hashimoto K, Okada S, Takata D, Horiguchi J, Yamada M. GNAS mutations in adrenal aldosterone-producing adenomas. EndocrJ. 2016;63:199–204. doi: 10.1507/endocrj.EJ15-0642. [DOI] [PubMed] [Google Scholar]
- 59.Cazabat L, Ragazzon B, Groussin L, Bertherat J. PRKAR1A mutations in primary pigmented nodular adrenocortical disease. Pituitary. 2006;9:211–219. doi: 10.1007/s11102-006-0266-1. [DOI] [PubMed] [Google Scholar]
- 60.Berthon AS, Szarek E, Stratakis CA. PRKACA: the catalytic subunit of protein kinase A adrenocortical tumors Front. Cell Dev Biol. 2015;3:26. doi: 10.3389/fcell.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stratakis CA. E pluribus unum? The main protein kinase A catalytic subunit (PRKACA), a likely oncogene, cortisol-producing tumors. Clin Endocrinol Metab J. 2014;99:3629–3633. doi: 10.1210/jc.2014-3295. Erratum in: J. Clin. Endocrinol. Metab. 100, 764 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carney JA, Lyssikatos C, Lodish MB, Stratakis CA. Germline PRKACA amplification leads to Cushing syndrome caused by 3 adrenocortical pathologic phenotypes. Hum Pathol. 2015;46:40–49. doi: 10.1016/j.humpath.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lodish MB, Yuan B, Levy I, Braunstein GD, Lyssikatos C, Salpea P, Szarek E, Karageorgiadis AS, Belyavskaya E, Raygada M, Faucz FR, Izatt L, Brain C, Gardner J, Quezado M, Carney JA, Lupski JR, Stratakis CA. Germline PRKACA amplification causes variable phenotypes that may depend on the extent of the genomic defect: molecular mechanisms clinical presentations. Eur Endocrinol J. 2015;172:803–811. doi: 10.1530/EJE-14-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stratakis CA, Carney JA, Kirschner LS, Willenberg HS, Brauer S, Ehrhart-Bornstein M, Bornstein SR. Synaptophysin immunoreactivity in primary pigmented nodular adrenocortical disease: neuroendocrine properties of tumors associated with Carney complex. Clin Endocrinol Metab J. 1999;84:1122–1128. doi: 10.1210/jcem.84.3.5549. [DOI] [PubMed] [Google Scholar]
- 65.Carney JA, Libé R, Bertherat J, Young WF. Primary pigmented nodular adrenocortical disease: the original 4 cases revisited after 30 years for follow-up new investigations, molecular genetic findings. Am Surg Pathol J. 2014;38:1266–1273. doi: 10.1097/PAS.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 66.Shigematsu K, Nishida N, Sakai H, Igawa T, Toriyama K, Nakatani A, Takahara O, Kawai K. Synaptophysin immunoreactivity in adrenocortical adenomas: a correlation between synaptophysin CYP17A1 expression. Eur Endocrinol J. 2009;161:939–945. doi: 10.1530/EJE-09-0596. [DOI] [PubMed] [Google Scholar]
- 67.Sangoi AR, McKenney JK. A tissue microarray-based comparative analysis of novel and traditional immunohistochemical markers in the distinction between adrenal cortical lesions and pheochromocytoma. Am J Surg Pathol. 2010;34:423–432. doi: 10.1097/PAS.0b013e3181cfb506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
adrenal vein sampling results in case 1, 2 and 3