Abstract
Systolic blood pressure (SBP) treatment targets for adults with diabetes remain unclear. SBP levels among 12,275 adults with diabetes, prior cardiovascular disease, and treated hypertension were evaluated in the TECOS randomized trial of sitagliptin vs. placebo. The association between baseline SBP and recurrent cardiovascular disease was evaluated using multivariable Cox proportional hazards modeling with restricted cubic splines, adjusting for clinical characteristics. Kaplan-Meier curves by baseline SBP were created to assess time to cardiovascular disease and two potential hypotension-related adverse events: worsening kidney function and fractures. The association between time-updated SBP and outcomes was examined using multivariable Cox proportional hazards models. Overall, 42.2% of adults with diabetes, cardiovascular disease, and hypertension had an SBP ≥140 mmHg. The association between SBP and cardiovascular disease risk was U-shaped, with a nadir around 130 mmHg. When the analysis was restricted to those with baseline SBP of 110–150 mmHg, the adjusted association between SBP and cardiovascular disease risk was flat (HR per 10-mmHg increase, 0.96; 95% CI, 0.91–1.02). There was no association between SBP and risk of fracture. Above 150 mmHg, higher SBP was associated with increasing risk of worsening kidney function (HR per 10-mmHg increase, 1.10; 95% CI, 1.02–1.18). Many patients with diabetes have uncontrolled hypertension. The U-shaped association between SBP and cardiovascular disease events was largely driven by those with very high or low SBP, with no difference in cardiovascular disease risk between 110 and 150 mmHg. Lower SBP was not associated with higher risks of fractures or worsening kidney function.
Keywords: cardiovascular disease, diabetes, fractures, hypertension, kidney function, systolic blood pressure
Worldwide, nearly one in 10 adults has diabetes, and the global prevalence of diabetes continues to rise.1 Diabetes is a potent risk factor for cardiovascular disease (CVD), doubling the risk of both coronary heart disease and ischemic stroke.2,3 In the United States, 22% of adults with diabetes have coronary heart disease and 9% have prior stroke.4 This group is at particularly high risk of recurrent CVD events and should be targeted for aggressive risk factor modification for secondary prevention.
One of the most prevalent and modifiable cardiac risk factors in adults with diabetes is hypertension.5 While the prevalence of hypertension among those with diabetes is high, systolic blood pressure (SBP) treatment targets remain unclear. Prior guidelines from the Seventh Joint National Committee recommended that adults with diabetes be tightly controlled with a goal of <130/80 mmHg. However, the authors noted that “available data are somewhat sparse to justify the low target level.”6 Subsequent guidance from the panel members appointed to the Eighth Joint National Committee raised the threshold to 140/90 mmHg in patients with diabetes, citing lack of randomized controlled trial evidence for more stringent goals.7 The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial found patients with diabetes had similar cardiovascular (CV) outcomes when randomized to SBP targets of 140 vs 120 mmHg.8 Epidemiological studies have actually suggested a possible U-shape to the association between SBP and CVD events, with a possible increase in hazard among those in patients with lower on-treatment SBPs.9 In contrast, the Systolic Blood Pressure Intervention Trial (SPRINT) found in a non-diabetic but high CV risk population that aggressive SBP lowering (<120 mmHg) significantly reduced the risk of CVD and overall mortality.10 The American Diabetes Association recently recommended that an optional target for hypertension management was <130 mmHg, based on data that lower SBP targets in adults with diabetes may reduce risk of stroke and albuminuria.11
Our study goals were to use longitudinal data from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS)12 to 1) describe current patterns in SBP control among patients with diabetes and hypertension on a global scale; 2) determine the unadjusted and risk-adjusted association between on-treatment SBP and CV outcomes; and 3) determine if lower on-treatment SBP was associated with adverse clinical events including bone fractures and renal insufficiency (both overall and among older individuals).
Methods
TECOS was a double-blind, randomized clinical trial of sitagliptin or placebo added to usual care in adults age ≥50 years with diabetes and prevalent CVD (prior coronary artery disease, ischemic cerebrovascular disease, or atherosclerotic peripheral arterial disease) between December 2008 and July 2012. Overall, 14,671 patients were randomized and included in the intention-to-treat (ITT) population. Of these, we included adults with a clinical diagnosis of hypertension and confirmed CVD at baseline, and who were on at least one antihypertensive medication at trial enrollment. All TECOS trial participants provided informed consent to participate, and the protocol was approved by the ethics committee at each participating trial site. Blood pressures were collected in clinic at baseline and at study follow-up visits at enrolling sites per local clinic protocols.
Our primary effectiveness outcome was the composite CVD endpoint including CV death, stroke, myocardial infarction, hospitalization for unstable angina, or hospitalization for heart failure. This composite was also assessed excluding hospitalization for heart failure (non-heart failure CVD).
Two safety outcomes, potential adverse events associated with too aggressive blood pressure lowering, were also assessed: bone fractures (potentially associated with falls) and worsening kidney function. Kidney function was defined using site-reported estimated glomerular filtration rate (eGFR) estimated with the Modification of Diet in Renal Disease method.13 Worsening kidney function was defined in two ways: 1) for those with baseline chronic kidney disease (site-reported eGFR < 90 mL/min/1.73m2) as a decrease in site-reported eGFR ≥50% or development of end-stage renal disease requiring dialysis or transplantation), and 2) for those without chronic kidney disease as development of end-stage renal disease or a decrease in eGFR of ≥30% to a value of less than 60 mL/min/1.73 m2.
Statistical Analysis
The study population was divided into five groups based on their baseline SBP: <120 mmHg, 120 to <130 mmHg, 130 to <140 mmHg, 140 to <160 mmHg, and ≥160 mmHg. Categorical and continuous baseline characteristics for these groups were described.
Unadjusted Kaplan-Meier curves were used to determine associations between baseline SBP and the effectiveness/safety endpoints of interest. The shape of the association between SBP at baseline and effectiveness/safety endpoints was evaluated using multivariable adjusted Cox proportional hazards models to generate a plot of the predicted event rates at 48 months by baseline SBP. We then evaluated the association between SBP as a time-updated variable using all SBP measurements obtained during follow-up study visits and CVD events. If the test for nonlinearity for baseline SBP was significant based on the Wald chi-square test, the risk for SBP was approximated using a piecewise linear spline, with clinical input and visual inspection of the shape of the adjusted association between SBP and endpoint of interest used to identify the cut points. Cox models were stratified by region and adjusted for the following risk factors: age, sex, race, prior stroke or transient ischemic attack, prior congestive heart failure, prior coronary disease, prior peripheral arterial disease, eGFR, hemoglobin A1c, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, smoking status, prior chronic obstructive pulmonary disease, prior atrial fibrillation or atrial flutter, albumin:creatinine ratio, creatinine, body mass index, hemoglobin, statin use, and treatment arm (sitagliptin or placebo).
Missing data for risk factors utilized in multivariable modeling were imputed using the fully conditional specification method,14 and missing SBP measurements at follow-up were imputed using the last observation carried forward for measurements obtained no more than 1 year prior to the missing observation. Missing SBP at baseline was not imputed.
Subgroup and Sensitivity Analyses
To further evaluate the shape of the association between baseline SBP and CVD events, multivariable spline and Cox models were repeated for adults with baseline SBP between 110 and 150 mmHg. These ranges were selected because they eliminated the potential effects of those with ‘outlier’ low or high SBP and were more representative of SBP ranges recommended by hypertension guidelines.
To evaluate the impact of pre-existing heart failure on the results, the association between SBP and non-heart failure CVD events was evaluated excluding adults with heart failure at baseline. To evaluate whether the association between SBP and CVD endpoints varied with age, we looked at the association between baseline SBP and CVD outcomes, stratified by age group (>70 years and ≤70 years), and tested the interaction between treated SBP and age (continuous) in Cox proportional hazards modeling utilized to determine association between time-updated SBP and CVD.
Safety Analyses
Safety endpoints were analyzed as described above; however, the multivariable analyses included the risk factors mentioned above in addition to diuretic usage for Cox proportional hazards models utilizing time to development of bone fracture as the outcome. For worsening kidney failure, the multivariable model included use of an angiotensin receptor blocker or angiotensin-converting enzyme inhibitor.
Results
Of the 14,671 patients in the ITT population for TECOS, 12,648 (86.2%) had a clinical diagnosis of hypertension and were on at least one blood pressure lowering medication at baseline; 373 of these adults were excluded due to lack of SBP values at baseline. The average age of adults was 66.0 years, 30.7% were female, and 30.0% were non-White. Coronary artery disease was the most common type of CVD (present in 74.8%), and 19.5% of patients had prior congestive heart failure.
Rates of Blood Pressure Control
At baseline, 42.2% of individuals had an SBP ≥ 140 mmHg, and 9.6% an SBP ≥ 160 mmHg, while 31.8% had an SBP < 130 mmHg and 13.1% an SBP < 120 mmHg. Characteristics of the overall population by baseline SBP are described in Table 1. Patients with higher SBP levels were older, more often female, more likely to have prior stroke, and less likely to have coronary artery disease. The median number of blood pressure medications used was the same (2.0) across all groups. Of those with SBP < 120 mmHg (n=1613) at baseline, 69.3% (n=1118) had SBP ≥ 110 and < 120 mmHg, 24.2% (n=390) had SBP ≥ 100 and < 110 mmHg, and 6.5% (n=105) had SBP < 100 mmHg.
Table 1.
Characteristics of Adults in the TECOS Trial With Treated Hypertension and Prior Cardiovascular Disease by Baseline SBP
| Characteristic | SBP (mmHg) | All patients (N=12275) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| < 120 (N=1613) | 120–129 (N=2287) | 130–139 (N=3192) | 140–159 (N=4000) | >= 160 (N=1183) | ||
| Age | 65.0 (60.0, 71.0) | 65.0 (59.0, 71.0) | 65.0 (60.0, 71.0) | 66.0 (60.0, 72.0) | 67.0 (61.0, 73.0) | 66.0 (60.0, 71.0) |
| Female | 25.2% | 29.1%) | 30.5% | 32.5% | 36.3% | 30.7% |
| Race | ||||||
| White | 67.3% | 68.6% | 69.7% | 73.2% | 66.7% | 70.0% |
| Black | 4.0% | 3.7% | 2.6% | 3.0% | 5.1% | 3.3% |
| Asian | 22.6% | 21.2% | 21.0% | 17.5% | 18.9% | 19.9% |
| Other | 6.2% | 6.4% | 6.7% | 6.4% | 9.3% | 6.7% |
| Coronary artery disease | 84.3% | 77.8% | 74.0% | 71.4% | 69.1% | 74.8% |
| Cerebrovascular disease | 22.1% | 24.6% | 25.4% | 27.9% | 28.5% | 25.9% |
| Peripheral arterial disease | 12.7% | 13.1% | 16.7% | 17.6% | 20.1% | 16.1% |
| Prior myocardial infarction | 48.7% | 44.8% | 42.8% | 42.3% | 36.9% | 43.2% |
| Prior congestive heart failure | 18.6% | 18.4% | 18.9% | 21.3% | 18.8% | 19.5% |
| Prior stroke | 15.7% | 17.0 | 18.1% | 20.1% | 20.3% | 18.4% |
| Atrial fibrillation/atrial flutter | 11.5% | 9.1% | 7.7%% | 8.4% | 5.7% | 8.5% |
| eGFR (mL/min/1.73 m2) | 71.0 (59.0, 86.0) | 72.0 (60.0, 87.0) | 73.0 (60.0, 88.0) | 72.0 (60.0, 87.5) | 70.0 (57.0, 86.8) | 72.0 (60.0, 87.0) |
| Hemoglobin (g/L) | 136.0 (125.0, 146.0) | 136.0 (125.0, 146.0) | 137.0 (127.0, 148.0) | 138.0 (127.3, 148.0) | 135.0 (125.0, 145.0) | 137.0 (126.0, 147.0) |
| Diastolic blood pressure (mmHg) | 68.0 (60.0, 72.0) | 75.0 (69.0, 80.0) | 80.0 (70.0, 82.0) | 80.0 (76.0, 90.0) | 87.0 (80.0, 94.0) | 80.0 (70.0, 84.0) |
| BMI (kg/m2) | 29.2 (26.1, 33.4) | 29.8 (26.4, 33.6) | 29.8 (26.6, 33.3) | 30.2 (27.0, 33.9) | 30.2 (26.9, 34.0) | 29.9 (26.7, 33.7) |
| Number of BP medications | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) |
| Total cholesterol | 3.8 (3.3, 4.5) | 4.0 (3.4, 4.7) | 4.1 (3.5, 5.0) | 4.3 (3.6, 5.2) | 4.3 (3.6, 5.3) | 4.1 (3.5, 4.9) |
| LDL cholesterol (mmol/L) | 1.9 (1.5, 2.5) | 2.1 (1.6, 2.7) | 2.1 (1.7, 2.8) | 2.3 (1.8, 3.0) | 2.3 (1.8, 3.1) | 2.2 (1.7, 2.8) |
| HDL cholesterol (mmol/L) | 1.0 (0.9, 1.2) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) |
| Aspirin use | 81.1% | 80.9%) | 78.6% | 77.5% | 76.8% | (78.8%) |
| ACE inhibitor or ARB | 83.6% | 84.9% | 83.4% | 86.0% | 86.9% | 84.9% |
| Beta blocker use | 72.2% | 68.3% | 66.6% | 66.3% | 65.7% | 67.5% |
| Thiazide diuretic | 22.9% | 24.9% | 26.6% | 28.6% | 29.3% | 26.7% |
| Calcium channel blockers | 29.1% | 35.0% | 38.1% | 40.7% | 46.1% | 37.9% |
| Nitrates | 26.2% | 20.8% | 19.2% | 18.4% | 15.9% | 19.8% |
| Alpha 1 blockers | 10.6% | 6.7% | 7.2% | 8.1% | 9.0% | 8.0% |
| Aldosterone antagonists | 9.5% | 7.1% | 5.3% | 5.3% | 5.2% | 6.2% |
| Hydralazine | 0.7% | 0.5% | 0.8% | 0.8% | 1.4% | 0.8% |
| Renin inhibitor (e.g., aliskerin) | 0.3% | 0.4% | 0.6% | 0.6% | 0.8% | 0.6% |
| Other anti-hypertensive | 5.0% | 6.0% | 5.2% | 6.8% | 8.4% | 6.1% |
ACE indicates angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SBP, systolic blood pressure.
eGFR by site-reported Modification of Diet in Renal Disease equation. Continuous variables presented as median (IQR). Categorical variables presented as %.
The characteristics displayed in this table are not imputed but rather the original values.
Association of Blood Pressure Control and CV Outcomes
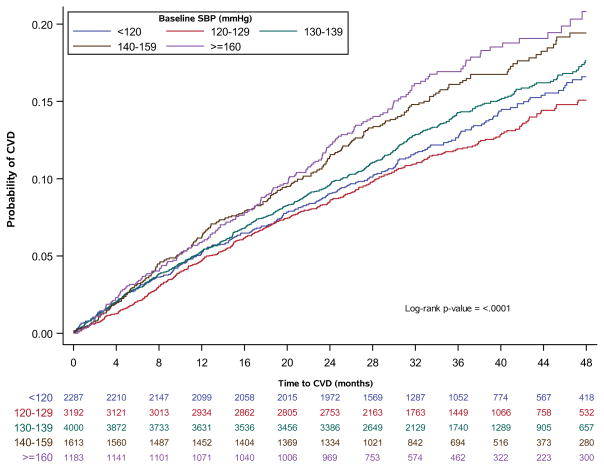
The median duration of follow-up was 3.0 years. Overall, the composite CV event rate was 13.4%, or 4.9% per 100 person-years. In unadjusted analyses, time to development of CVD differed by baseline SBP (p<0.0001). The risk of development of CVD appeared to be higher for those with baseline SBP ≥ 140 mmHg when compared to the lower SBP groups (Figure 1).
Figure 1.
SBP at baseline and time to CVD in adults with diabetes, treated hypertension, and prior CVD in TECOS. Unadjusted Kaplan-Meier CVD event rates for adults with prior CVD, hypertension, and on blood pressure treatment. Numbers below the x-axis are the number at risk at the time point, stratified by SBP group of interest.
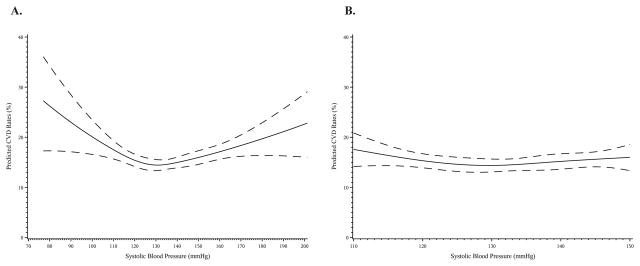
Restricted cubic spline analysis revealed that the association between SBP at baseline and predicted CVD risk was nonlinear, with a U-shaped association observed in both univariable (Figure S1a) and multivariable analysis (Figure 2a). Based on the shape of this curve and clinical input, the association between time-updated SBP and CVD events was modeled with a piecewise linear spline: Above 130 mmHg, every 10 mmHg increase in SBP was associated with a 7% increase in the hazard of CVD (HR, 1.07; 95% confidence interval [CI], 1.02–1.11). Below 130 mmHg, every further 10 mmHg decrease was associated with a 12% increase in hazard of CVD (HR, 1.12; 95% CI, 1.05–1.20).
Figure 2.
Multivariable adjusted predicted CVD event rates at 48 months in adults with diabetes, hypertension, and prior CVD by SBP at baseline: (a) SBP 70–200 mmHg, (b) SBP 110–150 mmHg. Predicted CVD event rates by baseline SBP adjusting for sex, age, race (white, black, Asian, other), history of coronary disease, history of stroke/TIA, history of peripheral artery disease, history of COPD, history of congestive heart failure, history of atrial fibrillation/flutter, eGFR, baseline hemoglobin A1c, albumin:creatinine ratio, creatinine, hemoglobin, HDL-cholesterol, LDL-cholesterol, smoking status (never, current, former), statin use, BMI, eGFR, and randomized treatment. Predicted event rates represent predicted rates from an average subject and are calculated from the Cox model based on an individual with mean values for each variable in the equation.
However, further examination of the association between baseline SBP and CVD events restricted to those in the SBP range of 110–150 mmHg eliminated the U-shaped association (unadjusted curve Figure S1a, adjusted curve Figure 2b, p-value for nonlinearity=0.18). When formally tested in the time-updated multivariable model, there was no association between SBP and CVD in adults with an SBP of 110–150 mmHg (p-value = 0.20, HR per 10 mmHg increase, 0.96; 95% CI, 0.91–1.02, Table 2).
Table 2.
Hazard Ratios for the Association Between Time-Updated SBP and CVD Endpoints
| Endpoint | Population | HR ≤ 130 mmHg Per 10 mmHg decrease | HR >130 mmHg Per 10 mmHg increase |
|---|---|---|---|
| Overall | |||
| Composite CVD | All adults with hypertension | 1.12 (1.05–1.20) | 1.07 (1.02–1.11) |
| Composite CVD | Excluding baseline HF | 1.12 (1.03–1.21) | 1.08 (1.03–1.13) |
| Non-HF CVD | All adults with hypertension | 1.12 (1.02–1.22) | 1.10 (1.04–1.15) |
| Non-HF CVD | Excluding baseline HF | 1.10 (1.02–1.22) | 1.07 (1.04–1.15) |
|
| |||
| SBP 110–150 mmHg | HR per 10 mmHg increase | ||
| Composite CVD | All adults with hypertension | 0.96 (0.91–1.02), p-value = 0.20 | |
| Composite CVD | Excluding baseline HF | 0.96 (0.90–1.02), p-value = 0.20 | |
| Non-HF CVD | All adults with hypertension | 0.97 (0.92–1.03), p-value = 0.32 | |
| Non-HF CVD | Excluding baseline HF | 0.96 (0.90–1.04), p=value = 0.32 | |
CVD indicates cardiovascular disease; HF, heart failure; SBP, systolic blood pressure.
Sensitivity Analysis
The U-shape of the association between baseline SBP and CVD was similar when applied to non-heart failure CVD events after excluding adults with heart failure at baseline (Figure S2). Specifically, in the overall population, there was a U-shaped association, but this was not apparent when limited to adults with an SBP of 110–150 mmHg.
The shapes of the associations between baseline SBP and CVD were also similar in both younger (age ≤70 years) and older (age >70 years) adults (Figures S3 and S4). In multivariable modeling, the association between time-varying SBP and CVD did not differ by age (interaction p-value 0.11).
Association Between SBP and Safety Outcomes
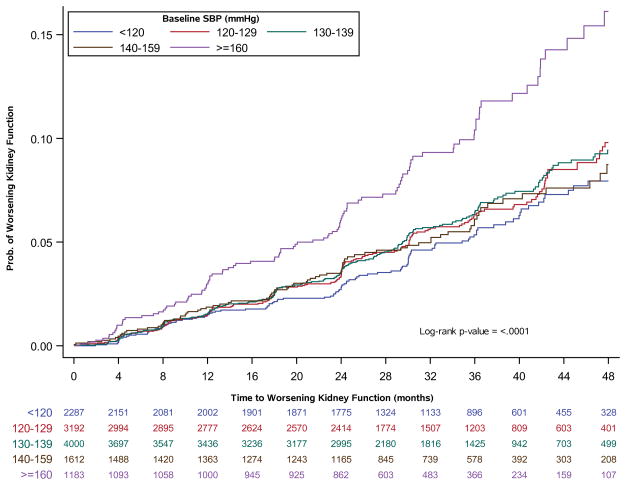
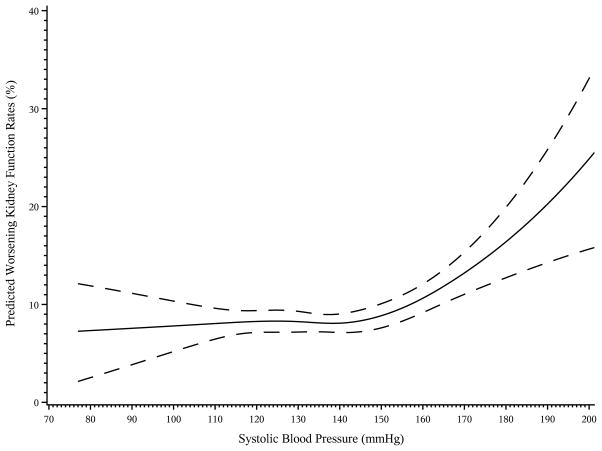
Kaplan-Meier curves showed a significant association between baseline SBP and development of worsening kidney function (p<0.001, Figure 3). Adults with a baseline SBP ≥ 160 mmHg appeared to be at higher risk of development of worsening kidney failure when compared to the other four SBP groups at baseline. In multivariable modeling, the shape of the association between baseline SBP and worsening kidney function appeared flat until baseline SBP was ≥ 150 mmHg, at which point the risk of worsening kidney function increased (multivariable adjusted spline shown in Figure 4, unadjusted curve shown in Figure S5). Therefore, SBP of 150 mmHg was used as the cut point used for the linear splines of SBP included in the multi-variable modeling. After adjustment for baseline factors, there was an association between time-varying SBP and time to development of worsening kidney function for SBP over 150 mmHg (HR per 10 mmHg decrease for SBP ≤ 150 mmHg, 1.04; 95% CI, 0.996–1.08), HR per 10 mmHg increase for SBP > 150 mmHg, 1.10; 95% CI, 1.02–1.18).
Figure 3.
SBP at baseline and time to worsening kidney function in adults with diabetes, treated hypertension, and prior CVD in TECOS. Unadjusted Kaplan-Meier event rates of worsening kidney function by baseline SBP category. Numbers below the x-axis are the number at risk at the time point, stratified by SBP group of interest.
Figure 4.
Multivariable adjusted predicted rates of worsening kidney function at 48 months in adults with diabetes, hypertension, and prior CVD by SBP at baseline: (a) SBP 70–200 mmHg, (b) SBP 110–150 mmHg. Predicted event rates for worsening kidney function at 48 months by baseline SBP adjusting for sex, age, race (white, black, Asian, other), history of coronary disease, history of stroke/TIA, history of peripheral artery disease, history of COPD, history of congestive heart failure, history of atrial fibrillation/flutter, eGFR, baseline hemoglobin A1c, albumin:creatinine ratio, creatinine, hemoglobin, HDL-cholesterol, LDL-cholesterol, smoking status (never, current, former), statin use, BMI, and randomized treatment. Predicted event rates represent predicted rates from an average subject and are calculated from the Cox model based on an individual with mean values for each variable in the equation.
No relationship could be detected between SBP at baseline and time to development of bone fractures using the unadjusted Kaplan-Meier event rates (log-rank p-value 0.23, Figure S6) or in multivariable analysis evaluating time-updated SBP (HR per 10 mmHg increase, 0.95; 95% CI, 0.89–1.02, p=0.15).
Discussion
Adults with diabetes and hypertension have high longitudinal risks for CV and non-CV adverse outcomes. Our study of TECOS data has several important findings. First, we found that SBP control in patients with diabetes from a global perspective is suboptimal. Up to 40% of these individuals with high CVD risk had an SBP ≥ 140 mmHg and nearly 10% had an SBP ≥ 160 mmHg. Second, while there appeared to be a U-shaped association between on-treatment SBP at baseline and CVD events in the overall TECOS population, this apparent association was being driven by the extremes of SBP. Within the SBP range targeted by many guidelines (110 to 150 mmHg), there was no apparent difference in risk of CVD events by SBP level. Finally, we found no evidence that lower SBP (<130 mmHg) was associated with increased rates of bone fractures or worsening kidney function.
Regardless of the specific target used, SBP was a major undertreated but highly modifiable risk factor in this high-risk population. Nearly half of TECOS participants had elevated SBP ≥ 140 mmHg, an area where there is little controversy regarding the benefits of blood pressure lowering in high-risk adults with diabetes. This highlights the important difference between what is recommended and what is achieved in a population. Given that 100% goal attainment is unlikely, one unintended consequence of lower SBP recommended targets may be to shift the entire distribution of SBPs downward, thus decreasing the numbers of adults with extremely high SBPs.
The U-shaped association seen between SBP and CVD outcomes has raised concern about the risks of potentially over-treating SBP. For example, a recently reported analysis of adults with coronary artery disease in a large multi-country registry reported that on-treatment SBP < 120 mmHg was associated with increased risks of CVD events for both diabetic and non-diabetic adults.9 However, all adults with SBP < 120 mmHg were analyzed together; the influence of outliers in this analysis may have been substantial. While our analysis also demonstrated this association, we also showed that this U-shape was largely influenced by adults with extremely low SBP.
Some of our findings are consistent with what has been observed previously. We found no strong association between macrovascular CV outcomes among those with on-treatment SBPs at baseline ranging from 110 to 150 mmHg. While trials have shown benefit in treatment of hypertension in adults with diabetes,15 there have been limited data supporting improved macrovascular outcomes < 140 mmHg. In ACCORD, the intensive treatment group achieving a mean SBP of 119.3 mmHg had non-significantly lower CV event rates relative to the standard treatment arm who achieved a mean SBP of 133.5 mmHg.8 In this analysis, we chose to focus on the association between SBP and CV events given the moving target for SBP for adults with diabetes. Other studies have shown a U-shaped association for DBP and CVD events,16 which was also seen in our sample (Figure S7). SBP and DBP are correlated; novel modeling approaches are needed to simultaneously evaluate the effect of SBP, DBP, and pulse pressure on CVD risk.
In addition to the focus on SBP, our analysis has several limitations. First, this is an observational analysis of trial data, and treatment biases may not have been fully accounted for by adjustment variables at baseline. It is possible that adults with lower on-treatment SBP had more comorbidities and therefore had more aggressive approaches to their blood pressure. In addition, blood pressure measurements were taken at outpatient clinic visits, which cannot detect white coat hypertension or masked hypertension, and can be affected by measurement error.17 However, this can also be interpreted as a strength, as it reflects blood pressures that clinicians use in practice. This differs from blood pressures in the SPRINT trial, which used methods that led to systematically lower blood pressure readings than are seen in clinical practice.18 Given the observational nature of this study, our findings should be interpreted with caution. The association between SBP and CVD may not imply that changes in treatment would change outcomes. Although we were unable to account for dosing, the number of blood pressure medication classes used did not substantially vary by baseline blood pressure. Thus, it is possible that the differences seen reflect more of the patients’ vascular biology and response to blood pressure medications than their actual achieved blood pressure. Next, the TECOS population includes adults with relatively well controlled diabetes, and thus these results may not be generalizable to all adults with diabetes. Finally, this analysis only included adults with prior CVD, thus showing the relationship between treated SBP and recurrent CVD events.
Perspectives
Globally, hypertension control remains suboptimal in adults with diabetes and cardiovascular disease, demonstrating the need for further efforts to treat elevated blood pressure to prevent recurrent cardiovascular disease events. The U-shaped curve seen between SBP and CVD events was largely driven by increased risk at extremes of SBP and indicates a wide margin for safety for treatment of SBP below 140 mmHg. Among those with SBP between 110 and 150 mmHg, we could detect no increased risk of CVD events. We also found no risks for CVD, fractures, or worsening kidney function among those with on-treatment SBP down to 110 mmHg. Given the potential microvascular improvements and lack of observed increase in macrovascular complications in both randomized trials and observational data, targeting SBP under 130 mmHg as currently optionally recommended by the ADA guidelines appears safe. Our data heighten the need for improved hypertension treatment in high risk adults with diabetes and CVD in community practice. Regardless of the target used, there remains substantial room for improvement in blood pressure control in adults with diabetes to reduce the risk of CVD.
Supplementary Material
Novelty and Significance.
What Is New?
While other studies have shown the U-shaped relationship between SBP and CV events, this analysis demonstrates how the shape of that curve is largely driven by extremely low SBPs. Between 110–150 mmHg, the risk of CVD events in patients with diabetes and cardiovascular disease was similar.
What Is Relevant?
Hypertension is a leading modifiable cause of events in adults with diabetes and CVD, yet remains suboptimal globally.
Summary
Many patients with diabetes, CVD, and hypertension have uncontrolled blood pressure. While there was a U-shaped association between baseline SBP and CVD events in the overall population, this was largely driven by those with very high or low baseline SBPs. There was a wide safety margin for on-treatment SBPs; between 110 and 150 mmHg, we observed no difference in CVD risk.
Acknowledgments
Source of Funding
The TECOS trial was funded by Merck & Co., Inc., Kenilworth, NJ. Dr. Navar has received research funding (to her institution) from Regeneron Pharmaceuticals, Sanofi Pharmaceuticals, and Amgen, and consulting fees from Sanofi and Amgen. Dr. Navar is supported by the National Institutes of Health (1K01HLL133416).
Footnotes
Disclosures
Dr. Lokhnygina has received grants from Merck, Janssen Research & Development, AstraZeneca, GlaxoSmithKline, and Bayer HealthCare AG. Dr. Green has received grants from Merck Sharp & Dohme, AstraZeneca, and GlaxoSmithKline, personal fees from Merck Sharp & Dohme, other support from Boehringer-Ingelheim, and personal fees from Bioscientifica and The Endocrine Society. Dr. McGuire has received personal fees from Boehringer-Ingelheim, Janssen Research and Development LLC, Sanofi-Aventis Group, Merck Sharp and Dohme, Daiichi Sankyo, Inc., Lilly USA, Novo Nordisk, GlaxoSmithKline, Takeda Pharmaceuticals North America, AstraZeneca, Lexicon, Regeneron, University of Oxford, Duke Clinical Research Institute, Partners Healthcare, and the Cleveland Clinic Foundation. Dr. Armstrong has received grants from Merck and AstraZeneca, and personal fees and non-financial support from Merck. Dr. Buse has received consulting fees from PhaseBio; holds stocks/shares in PhaseBio; is an advisor under contract with his employer for AstraZeneca, Dance Biopharm, Eli Lilly, Elycylex, GI Dynamics, Lexicon, Merck, Metavention, Novo Nordisk, Orexigen, vTv Therapeutics; and received research support from AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, GI Dynamics, J&J, Lexicon, Novo Nordisk, and Orexigen. Dr. Engel is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. Dr. Lachin has received personal fees from Merck, Boehringer-Ingelheim, Gilead Pharmaceuticals, Janssen Pharmaceuticals, Eli Lilly, Novartis, and GlaxoSmithKline. Dr. Standl has received personal fees from Oxford Diabetes Trials Unit, AstraZeneca, Bayer, Boehringer-Ingelheim, Merck Serono, Excemed, Novartis, NovoNordisk, and Sanofi. Dr. Van de Werf has received grants from Merck and Boehringer-Ingelheim and personal fees from Merck, Boehringer-Ingelheim and Astra Zeneca. Dr. Holman has received grants from Merck, Bayer, AstraZeneca, and Bristol-Myers Squib, personal fees from AMGEN, Bayer, Intarcia, Merck, Novartis, Novo Nordisk, and other support from GlaxoSmithKline, Janssen, and Takeda. Dr. Peterson has received grants from Janssen and Eli Lilly, and personal fees from AstraZeneca, Bayer, Janssen, and Sanofi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.World Health Organization. [Accessed September 9, 2016];Global report on diabetes. 2016 Available at http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1.
- 2.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report. [Accessed February 23, 2017];Estimates of Diabetes and Its Burden in the United States. 2014 Available at: https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf.
- 6.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 7.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 8.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, Tendera M, Tavazzi L, Bhatt DL, Steg PG. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–2152. doi: 10.1016/S0140-6736(16)31326-5. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of Medical Care in Diabetes-2016. Diabetes Care. 2016;39(Suppl 1):S1–S106. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 12.Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 15.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–615. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan NM. The diastolic J curve: alive and threatening. Hypertension. 2011;58:751–753. doi: 10.1161/HYPERTENSIONAHA.111.177741. [DOI] [PubMed] [Google Scholar]
- 17.Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation. 2016;134:904–905. doi: 10.1161/CIRCULATIONAHA.116.022536. [DOI] [PubMed] [Google Scholar]
- 18.Kjeldsen SE, Lund-Johansen P, Nilsson PM, Mancia G. Unattended blood pressure measurements in the systolic blood pressure intervention trial: implications for entry and achieved blood pressure values compared with other trials. Hypertension. 2016;67:808–812. doi: 10.1161/HYPERTENSIONAHA.116.07257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.