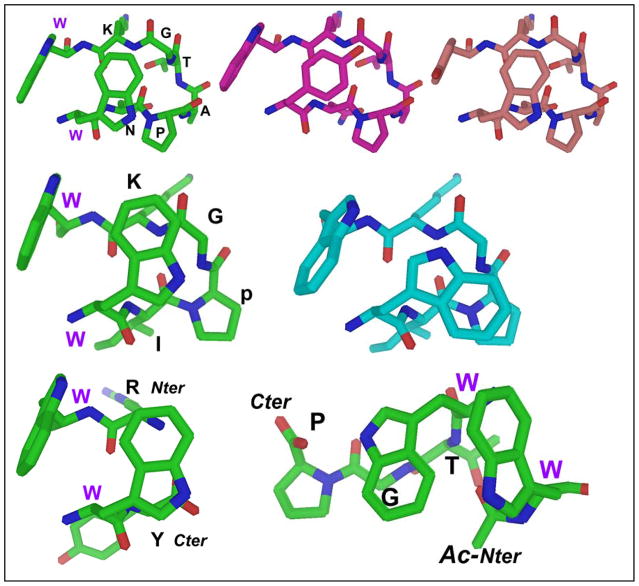
Figure 10.
Top panel -- views of the Trp/Trp interaction in an NPATGK-turn-flanking context. From left to right: Trp/Trp, TrpF/TyrE, TyrF/TrpE. The rationale for more highly shifted Hβ3 ArylE protons for face = Trp is apparent: for phenyl rings the Hβ3 position on the cross-strand partner is not as close and not in the middle of the shielding cone. Middle panel -- two different Trp/Trp conformers available to WIpGKW-hairpins: the standard and inverted χ2 rotamers. The alternate conformer found flanking 2:4 turns (especially XpGX turns, or in the presence of fluoroalcohol cosolvent) is on the right. For the typical conformer, TrpE presents its Hε3 proton to TrpF, but in the alternate conformer Hδ1 is presented to TrpF. Major CSD changes are observed for the turn (e.g., the Gly HN CSD shifts from −1.0 ppm to −3.5 ppm) as its position changes relative to the TrpE indole. Bottom – the Coulombic +XW/WX- (left) versus the hydrophobic β-cap (Ac-Ar/ArTG). In all cases, extrapolated CSD variations (Table 4) for 100% folded constructs are consistent with the aromatic ring orientations shown.