Abstract
Background
By the time patients with a failed shoulder arthroplasty require revision surgery, a substantial number are older than 80 years. The risk of complications of revision arthroplasty in this elderly population is largely unknown and needs to be considered when contemplating whether these patients are too frail for revision surgery.
Questions/purposes
(1) What are the 90-day medical and surgical complications after revision to reverse shoulder arthroplasty (RSA) in patients older than 80 years? (2) What are the 2- and 5-year survival rates after revision? (3) Was there an improvement in pain at rest or with activity, range of motion (ROM), and strength after revision surgery?
Methods
Between 2004 and 2013, 38 patients who were older than 80 years (84 ± 3 years) underwent revision surgery to a RSA. Of those, five were lost to followup before 2 years, and two had died within 2 years of revision surgery, leaving 31 for analysis of our survivorship, pain, ROM, and strength endpoints at a minimum of 2 years or until revision surgery had occurred (mean, 28 months; range, 1–77 months); all 38 patients were included for purposes of evaluating medical and surgical complications at 90 days. During the period in question, our general indication for using RSA included failure of previous shoulder arthroplasty because of instability, glenoid loosening with bone loss, or rotator cuff insufficiency. The indication for revision to RSA did not change during the study period. The index procedure (revision to RSA at the age of 80 years or older) was the first revision arthroplasty in 33 (87%) patients and the second in five (13%) patients. We tallied 90-day medical and surgical complications by performing a retrospective chart and institutional joint registry review. The cumulative incidence of implant loosening (implant migration or tilting, or complete radiolucent lines present) and revision surgery was calculated at 2 and 5 years using competing risk of death method. Pain levels at rest or with activity (rated in a 1 to 5 Likert-type scale) were collected through a retrospective chart review and values before and after surgery were compared.
Results
Medical complications occurred in three of 38 (8%) patients and surgical complications occurred in five of 38 (13%) patients. The 90-day mortality was 3% (one of 38 patients), and the total mortality was 26% (10 of 38 patients). The cumulative incidence of revision was 11% (95% CI, 0%–20%) at 2 years and 16% (95% CI, 1%–30%) at 5 years; the cumulative incidence of loosening was 8% (95% CI, 0%–20%) at 2 years and 16% (95% CI, 1%–30%) at 5 years. Pain at rest or with activity improved from pre- to postoperation (preoperative: median, 4 [range, 2–5]; postoperative: median, 1 [range, 1–4]; median difference: -2, 95% CI -3 to 0; p < 0.000). The active ROM improved during the preoperative compared with postoperative periods: mean ± SD forward flexion of 52° ± 40° to 109° ± 44°, respectively (mean difference: 56; 95% CI, 40–72; p < 0.000), and mean ± SD external rotation of 15° ± 22° to 31° ± 21°, respectively (mean difference: 16; 95% CI, 8–25; p < 0.000).
Conclusions
Age should not be used as a reason to not consider revision surgery to RSA in patients older than 80 years. Further studies with a prospective design, larger sample size, investigating risk factors for complications or poor outcome, and incorporation of functional scores are required.
Level of Evidence
Level IV, therapeutic study.
Introduction
Owing to an increase in life expectancy in developed countries along with the increased prevalence of shoulder conditions with aging, shoulder arthroplasty in elderly patients is becoming increasingly common [12, 20]. By the time patients with a failed shoulder arthroplasty require revision surgery, a substantial number are older than 80 years. Concerns regarding the outcomes of revision to reverse shoulder arthroplasty (RSA) in patients older than 80 years are the result of the potential effect of surgery on patients’ general health, the possibility of substantial bone wear and poor bone quality, lesser functional demands, and postoperative instability secondary to poor soft tissues [7]. Therefore, consideration of revision to RSA in the older population is a topic of current interest.
Total shoulder arthroplasty (TSA) in older patients (80 years and older) provides good pain relief and restoration of function with an acceptable rate of complications and mortality [3, 7, 19, 20]. Interestingly, the results of RSA in patients 80 years or older have not been reported, to our knowledge. Cazeneuve and Cristofari [2] reported disappointing long-term functional and radiographic outcomes of RSA in elderly patients. However, they included only procedures performed for fracture or fracture-dislocations, and the age range of their patients was 58 to 92 years.
Special consideration must be given to revision arthroplasty in elderly patients. By the time patients with a failed shoulder arthroplasty require revision surgery, many are older than 80 years. Nonetheless, medical and surgical complications, pain relief, restoration of function, and implant survival after revision to a RSA in these elderly patients have not been reported, to our knowledge. This topic needs to be considered when contemplating whether these patients are too frail for revision surgery.
We therefore asked (1) What are the 90-day medical and surgical complications after revision to RSA in patients older than 80 years? (2) What are the 2- and 5-year survival rates after revision? (3) Was there an improvement in pain at rest or with activity, range of motion (ROM), and strength after revision surgery?
Patients and Methods
A retrospective study was conducted to respond to the research questions. After institutional review board approval (Protocol ID 16-001464), our institutional total joint registry database was used to identify all patients undergoing RSA between January 2004 and December 2013 by any of the three senior surgeons (JSS, RHC, and JWS). Inclusion criteria were age 80 years or older, revision arthroplasty (conversion of TSA, hemiarthroplasty, or RSA to a RSA, which was considered the index procedure), minimum clinical followup of 2 years (or less in patients who needed a revision surgery or died), and availability of preoperative and recent postoperative AP and axillary radiographs. Inclusion of patients was not restricted to specific indications or type of previous implants.
During the period in question, our general indications for use of RSA in the revision setting included pain and shoulder dysfunction secondary to failure of previous shoulder arthroplasty because of instability, glenoid loosening with bone loss, or rotator cuff insufficiency. The indication for revision to RSA did not change during the study period. The index procedure (revision to RSA in patients 80 years or older) was the first revision arthroplasty in 33 (87%) patients and the second in five (13%) patients.
A total of 1289 RSAs were performed during the study period by the three senior surgeons participating in this study. Of these, 276 were performed in patients 80 years or older, and 38 of them were for revision arthroplasty (Table 1). The mean age and BMI were 84 years (SD, ± 3 years) and 28 kg/m2 (SD, ± 6 kg/m2), respectively. Of the 38 patients, the indications for revision arthroplasty included: rotator cuff tear after anatomic TSA or hemiarthroplasty in 12 (32%), glenoid wear after hemiarthroplasty in 10 (26%), glenoid loosening in seven (18%), instability in four (11%), painful arthroplasty in two (5%), implant loosening or fracture in two (5%), and infected TSA in one (3%). Revision RSA was performed for failed hemiarthroplasty in 18 (47%) patients, failed TSA in 18 (47%) patients, and failed RSA in two (6%) patients.
Table 1.
Demographic characteristics and comorbidities
| Parameter | Number (%)* |
|---|---|
| Gender | |
| Male | 14 (37) |
| Female | 24 (63) |
| Side | |
| Right | 25 (66) |
| Left | 13 (34) |
| Dominant side surgically treated | 25 (66) |
| Tobacco use | 14 (37) |
| ASA grade | |
| I | 0 (0) |
| II | 14 (37) |
| III | 23 (60) |
| IV | 1 (3) |
| Comorbidities | |
| Diabetes mellitus | 7 (18) |
| Hypertension | 30 (79) |
| Coronary artery disease | 19 (50) |
| Myocardial infarction | 10 (26) |
| Congestive heart failure | 9 (24) |
| Peripheral vascular disease | 10 (26) |
| Dementia | 5 (13) |
| Chronic obstructive pulmonary disease | 5 (13) |
| Peptic ulcer disease | 13 (34) |
| Renal failure | 6 (16) |
ASA = American Society of Anesthesiologists; *percentage of patients with respect to the entire sample (38 patients).
The mean clinical and radiographic followups were 28 months (range, 1–77 months) and 21 months (range, 1–73 months). At most recent followup, five of the 38 patients were alive but were lost to followup and two patients had died within the first 2 years after their revision surgery. All 38 patients were included for the analysis of mortality and morbidity, but only 31 (82%) patients with a minimum followup of 2 years (or until revision surgery) were included in the analysis of surgical complications, reoperations, clinical outcomes, and radiographic outcomes. All patients returned to the clinic for followup at some time after surgery. Their most recent followup was a physical examination for 18 (47%) patients and a validated questionnaire that our institutional total joint registry sends to patients unable to return for followup for 20 (53%) patients [23]. The questionnaire includes functional scores (American Shoulder and Elbow Surgeons and Simple Shoulder Test scores), but because no preoperative data were available for these tests, they were not included in our study.
After the retrospective chart review was completed, a radiographic assessment was done in which all preoperative (before index procedure), early followup, and last followup radiographs were evaluated by two senior surgeons (JSS and JWS) blinded to the clinical outcomes. The final conclusion on each radiographic reading was achieved by consensus between both surgeons (JSS and JWS). The analysis of data included mortality, morbidity (medical complications), surgical complications, reoperations, clinical outcomes, and radiographic outcomes.
Surgical Procedure
All surgical procedures were performed through a deltopectoral approach. The mean operative time was 108 minutes (SD, ± 35 minutes). There were 31 (82%) Comprehensive® Reverse Shoulder System prostheses (Biomet, Warsaw, IN, USA), four (10%) Encore Reverse® shoulder prostheses (DJO Global, Vista, CA, USA), and three (8%) Delta® Reverse shoulder systems (DePuy, Warsaw, IN, USA). Bone graft was used in 12 (32%) patients: two (5%) on the humeral side and 10 (26%) on the glenoid side. The humeral component was cemented in 14 (37%) patients.
Outcome Measurement
Data collection was performed by researchers (EAG and NJC) not involved in the care of the patients and included mortality, major and minor medical complications, surgical complications, reoperations, clinical outcomes, and radiographic outcomes. A medical complication was considered any unexpected medical event that was directly or indirectly related to the surgical procedure or general anesthesia, including, but not limited to, postoperative anemia, acute pneumonia, urinary tract infection, stroke, acute myocardial infarction, acute renal failure, atrial fibrillation, or venous or artery thrombosis). A major complication was considered a medical complication that increased the hospital stay. Surgical complications included, but were not limited to, deep or superficial infection, wound dehiscence, postoperative hematoma, nerve palsy, component dissociation, implant loosening, complex regional pain syndrome, shoulder stiffness, or continued pain without an identifiable cause. Time to death and cause of death were extracted along with any medical complication that occurred during the hospital stay or within 90 days of discharge.
The clinical and radiographic outcomes included (1) preoperative and postoperative pain; (2) preoperative and postoperative active ROM for forward flexion, external rotation, and internal rotation; (3) preoperative and postoperative strength for shoulder elevation, abduction, external rotation, and internal rotation; (4) preoperative radiograph characteristics; (5) postoperative radiograph characteristics during the early (within 6 weeks after surgery) and final followups; and (6) complications and reoperations. The postoperative pain, ROM, and strength were collected at the last followup. Pain was graded using a 1 to 5 Likert-type scale, where 1 was no pain, 2 mild pain, 3 pain after usual activities, 4 moderate pain, and 5 severe pain. ROM was measured by the senior surgeons (JSS and JWS) during followups or reported by the patients using the validated questionnaires [23]. Strength was graded as 0 if no movement was observed, 1 if there was visible contraction but no segment movement, 2 if there was active movement on resistance without gravity, 3 if there was active movement against gravity, 4 if there was active movement against gravity and examiners’ resistance, and 5 if there was normal strength. A clinical failure was considered as the need for revision surgery regardless of the etiology.
The radiographic information collected during the radiograph reading session included humeral head superior and AP subluxation during all three periods (preoperative, immediate postoperative, and late postoperative), preoperative glenoid erosion degree and location, and presence of humeral or glenoid component loosening (presence of subsidence, shifting, migration, or tilt) at the immediate and late postoperative periods. The degree of subluxation was categorized as none, mild (< 25%), moderate (25%-50%), and severe (> 50%). Scapular notching was classified according to Sirveaux et al. [22].
Preoperative radiographs showed no superior subluxation in seven (21%) patients, mild in two (6%), moderate in six (18%), and severe in 18 (55%). There was no AP subluxation in 19 (58%) patients, moderate posterior subluxation in five (15%), posterior dislocation in two (6%), mild anterior subluxation in one (3%), moderate anterior subluxation in two (6%), severe anterior subluxation in three (9%), and anterior dislocation in one (3%). Nineteen (58%) patients had mild or moderate glenoid erosion, which was superior in eight (24%), superior-central in six (18%), medial in three (9), superomedial in one (3%), and central in one (3%). Radiographic evidence of glenoid and humeral loosening was observed in eight (24%) and one (3%) shoulders, respectively.
Statistical Analysis
Descriptive statistics were used to summarize outcomes. Data were reported as number of patients (n), percentage, mean, median, SD, and range. The comparison between preoperative and postoperative outcomes was performed with a paired t-test (for quantitative variables) or Bowker’s test of symmetry (for qualitative variables), which is an extension of McNemar’s test for paired comparison of categorical data. The 2- and 5-year cumulative probability of revision or radiographic loosening (survival analysis) was calculated using the competing risk of death method of Gooley et al. [8]. Rates were reported with 95% CIs. The α level was set at 0.05. All the statistical analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC) and SPSS, Version 21.0 (IBM Corporation, Armonk, NY, USA).
Results
The risk of medical complications was 8% (three of 38 patients), risk of surgical complications was 13% (five of 38 patients), 90-day mortality was 3% (one of 38 patients), 1-year mortality was 5% (two of 38 patients), and total mortality was 26% (10 of 38 patients). Medical complications included pneumonia in 3% (one of 38) of patients, urinary tract infection in 3% (one of 38), and stroke in 3% (one of 38). Surgical complications included glenoid loosening in 8% (three of 38) of patients, deep infection with humeral component loosening in 3% (one of 38), and component dissociation in 3% (one of 38). The reoperation rate was 13% (five of 38 patients). Reoperations because of glenoid loosening occurred at 2, 9, and 15 months after the index procedure. Reoperations because of deep infection and component dissociation occurred at 35 months and at 1 month after the index procedure, respectively. There were no cases of scapular notching. Median time to death was 46 months (range, 1–93 months). Causes of death included myocardial infarction in 8% (three of 38, at 12, 61, and 93 months after surgery) of patients, pneumonia in 5% (two of 38, at 26 and 65 months after surgery), stroke in 3% (one of 38, at 1 month after surgery), and undetermined in 10% (four of 38, at 25, 53, 40, and 73 months after surgery).
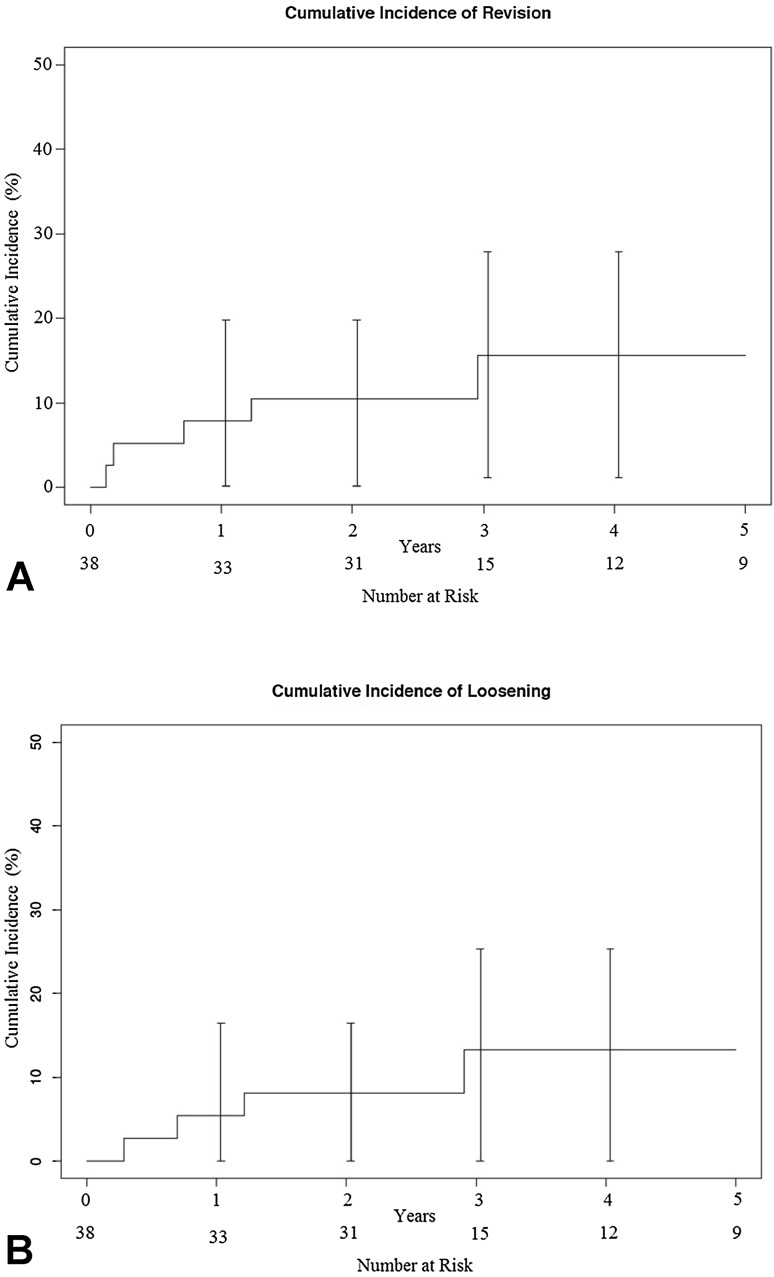
The cumulative incidence of revision was 11% (95% CI, 0%–20%) at 2 years and 16% (95% CI, 1%–30%) at 5 years; the cumulative incidence of loosening was 8% (95% CI, 0%–20%) at 2 years and 16% (95% CI, 1%–28%) at 5 years (Fig. 1).
Fig. 1A–B.
The cumulative incidence of (A) revision and (B) implant loosening using the competing risk of death method are shown.
Pain (at rest or with activity) improved from pre- to postoperation (preoperative: 4 [range, 2–5]; postoperative: 1 [range, 1–4]; median difference, −2; 95% CI, −3–0; p < 0.000) (Table 2). The active ROM improved during the preoperative compared with postoperative period: mean ± SD forward flexion of 52° ± 40° to 109° ± 44°, respectively (mean difference, 56; 95% CI, 40–72; p < 0.000), and mean ± SD external rotation of 15° ± 22° to 31° ± 21°, respectively (mean difference, 16; 95% CI, 8–25; p < 0.000) (Table 2). The strength for abduction improved during the preoperative compared with postoperative period: median ± range of 3 (2–4) to 4 (2–5), p = 0.039 (Table 2). With the numbers available, there were no differences in active internal rotation ROM and strength (forward flexion, external rotation, and internal rotation) between the preoperative and postoperative periods (Table 2).
Table 2.
Preoperative and postoperative pain, ROM, and strength
| Parameter | Preoperative | Postoperative* | Mean/median difference (95% CI) | p value |
|---|---|---|---|---|
| Forward flexion ROM (°)(mean ± SD)* | 52 ± 40 | 109 ± 44 | 56 (40–72) | < 0.000 |
| External rotation ROM (º)(mean ± SD)* | 15 ± 22 | 31 ± 21 | 16 (8–25) | < 0.000 |
| Pain (median/range) | 4 (2–5) | 1 (1–4) | −2 (−3 to 0) | < 0.000 |
| Internal rotation ROM (median/range) | Sacrum (T10-abdomen) | Buttock (T6-abdomen) | 0.296 | |
| Forward flexion strength (median/range) | 4 (2–5) | 4 (2–5) | 0 (−1 to 2) | 0.68 |
| Abduction strength (median/range) | 3 (2–4) | 4 (2–5) | 1 (−1 to 2) | 0.039 |
| External rotation strength (median/range) | 3 (2–4) | 4 (2–5) | 1 (−1 to 2) | 0.069 |
| Internal rotation strength (mean/range) | 3 (2–4) | 4 (2–5) | 1 (−1 to 2) | 0.065 |
*One missing value of the 38 patients during the postoperative period.
Discussion
Despite shoulder replacement being a successful procedure [4, 12, 13, 16, 18, 21, 24, 26], failed arthroplasty does occur, mainly owing to implant loosening, instability, or cuff tear with time. Failed arthroplasties have a substantial negative effect on patients’ function and quality of life. By the time a revision arthroplasty is needed, many patients are older than 80 years. Elderly patients may have an increased risk of mortality, medical and surgical complications, and reoperations, along with potentially worse outcomes compared with younger patients. RSA is being increasingly used in the revision setting. Currently, there is no information available, to our knowledge, regarding the risk of medical or surgical complications, survivorship, pain relief, or functional improvement with revision to RSA in patients older than 80 years.
Our study has some limitations. First, this was a retrospective case series without a comparison group (similar-aged group undergoing a different treatment or patients younger than 80 years). The retrospective case series nature of the study entails no possibility to establish a causal-effect relationship between the surgical procedure and the outcomes evaluated. In retrospective studies, there is a risk of selection bias by having changed the indication of RSA with time as experience with this implant increased. However, the indications did not change with time in the current sample and initial lack of experience with this implant should not have been an issue given that the first patient older than 80 years undergoing revision to RSA had the operation 2 years after the implant was being used in our institution and the majority had the operation at least 6 years after the first RSA was used. Retrospective studies also have the risk of transfer bias related to completeness of followup. Patients lost at followup did not affect the analysis of medical or surgical complications as all patients were included. However, only 31 patients (82%) had a followup greater than 2 years, but we believe this is an acceptable rate of patients lost considering the sample characteristics and we believe this has had no substantial effect on the results. Second, this study has a risk of assessment bias because radiographic parameters were evaluated by the actual shoulder surgeons operating on the patients included in this study. The final decision was made by consensus as opposed to blinded decisions, so there is no possibility to evaluate the reproducibility of the radiographic measurements. We tried to limit the effect of this issue by blinding the observers to the clinical outcomes. Third, there is a certain risk of having missed minor complications in patients who had them develop postoperatively but which were not reported. Nonetheless, it is unlikely that this affected major complications. Fourth, all surgical procedures were conducted by subspecialty-trained, high-volume, experienced shoulder surgeons. Therefore, the generalizability of the results should be considered with caution, because complications and mortality depend on the surgeon’s experience [9–11, 14].
The 30-day and 90-day mortality of shoulder arthroplasty in the general population is 0.4% and 0.58%, respectively [6, 25]. In the current study, only one (3%) patient died during the first 90 days. The overall mortality rate in our patients was 26%, which is not surprising considering the age of the patients (mean, 84 years) and current life expectancy in the United States (76 years for men and 81 years for women) [1]. Perioperative medical complications of shoulder arthroplasty in the general population range between 1.2% and 8% [5, 6, 10]. In patients older than 80 years, the rate of perioperative complications ranges between 0% and 9% (Table 3). In our study, three (8%) patients experienced major perioperative medical complications, including pneumonia and stroke in one patient each. Ricchetti et al. [19] compared the 90-day mortality and complication rates between patients older and younger than 80 years undergoing TSA. Although their mortality rate was 0%, the rate of complications in each group was 7% and 2%, respectively. They also found that older patients had an increased rate of transfusion (16% compared with 2%) and a decreased number of direct home discharges (67% compared with 98%). Revision to RSA was not related to serious medical complications, but patients should be advised that their risk of perioperative complications is likely increased compared with their younger counterparts. Regarding surgical complications and reoperation rate, the 13% chance observed in our study does not seem exceedingly high considering the sample characteristics. Overall, the reoperation rate in our patients is slightly higher compared with those reported in other studies including primary anatomic replacement and RSA (Table 3) [3, 7, 15, 17].
Table 3.
Summary of studies involving shoulder arthroplasty in patients 80 years or older
| Study | Age (years) | Followup | Type of surgery | Type of implant | Total mortality | 90-day mortality | Medical complications | Surgical complications/revisions | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Mullett et al. [17] | 84 (81–93) | 4.5 (2–9) years | Primary | HA (22) TSA (7) |
14% | 0% | 0% | 1 (5%) cuff failure (revised to RSA) | Constant score 77 (adjusted) FF 88-106º |
| Churchill [3] | 93 (91–97) | 2.2 (1–4) years | Primary | TSA | 16% | 0% | 0% | NR | FF 137° ER 50° SST 7.4 |
| Foruria et al. [7] | 82 (80–89) | 5.5 (2–12) years | Primary | TSA | 88% | 0% | 9% | Cuff failure 10% Glenoid loosening 8% Revision 7% |
FF 138º ER 48º 68% excellent |
| Ricchetti et al. [19] | 82 (80-90) | 90-day mortality | Primary | TSA | 0% | 0% | Major 7% Minor 23% |
Periprosthetic fracture 5% | NR |
| Mangano et al. [15] | 82 (79–88) | 59 (28–82) months | Primary | RSA | 13% | 0% | 0% | Complications 10% Glenoid loosening 0% |
SF-12 PCS 41 SF-12 MCS 46 ASES 78 |
| Current study | 84 (80–89) | 28 (1–77) months | Revision | RSA | 26% | 2.6% | 8% | Infection 3% Glenoid loosening 8% Reoperations 13% |
FF 109° ER 31° |
HA = hemiarthroplasty; TSA = total shoulder arthroplasty; RSA = reverse shoulder arthroplasty; NR = not reported; FF = forward flexion ROM; ER = external rotation ROM; SST = simple shoulder test; PCS = Physical Component Summary; MCS = Mental Component Summary; ASES = American Shoulder and Elbow Surgeons.
Patients older than 80 years who need revision to RSA may expect a survival revision-free and implant loosening-free of 16% at 5 years. At a median followup of 5.5 years, Foruria et al. [7] reported glenoid loosening and revision arthroplasty rates of 8% and 7% in primary anatomic shoulder arthroplasty performed in octogenarians, respectively (Table 3). Using primary RSA in patients older than 80 years, Mangano et al. [15] reported no glenoid component loosening at a mean followup of 59 months (Table 3). Patients older than 80 years should be advised that survival after revision to RSA is lower than after primary anatomic replacement or RSA. However, when pain and functional impairment are substantial after an attempt with conservative treatment, a 16% chance of failure at 5 years would not justify recommending against revision to RSA in these patients.
The pain relief and ROM outcomes are somewhat variable across studies with a similar aged population (Table 3). Variability of results can be explained by differences in the type of procedure (primary versus revision), type of implant (hemiarthroplasty, TSA, or RSA), and length of followup (Table 3). None of the previous studies included revision arthroplasty in patients older than 80 years (Table 3). In general, shoulder arthroplasty provides substantial pain relief and increase in ROM [3, 7, 15, 17]. Forward flexion and external rotation in our study patients were slightly lower [3, 7] compared with those reported in other studies (Table 3). That our study was limited to revision arthroplasty is the most likely explanation for the differences in ROM between our study and others.
Given the low risk of serious medical and surgical complications, the 16% chance of revision failure (need for another revision surgery or implant loosening developing) at 5 years, and the improvement in pain, forward flexion ROM, external rotation ROM, and abduction strength age should not be used as a reason to not consider revision to RSA in patients older than 80 years. Further studies with a preoperative and postoperative, prospective design, larger sample size, investigating risk factors for complications or poor outcome, and incorporation of functional scores are required.
Acknowledgments
We thank Youlonda A. Loechler, Donna L. Riemersma, and all the staff of the total joint registry and manuscript office (all from Mayo Clinic, Rochester, MN, USA) for their support and excellent work in this project and throughout the years. We also thank Kristin C. Mara MSc and Dirk R. Larson PhD (both from the Department of Health Science Research, Division of Biostatistics, Mayo Clinic College of Medicine, Rochester, MN, USA) for expert contributions in the statistical analysis.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, have funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Arias E. Changes in life expectancy by race and Hispanic origin in the United States, 2013–2014. NCHS Data Brief. 2016;244. Available at: https://www.cdc.gov/nchs/data/databriefs/db244.pdf. Accessed May 11, 2017. [PubMed]
- 2.Cazeneuve JF, Cristofari DJ. Long term functional outcome following reverse shoulder arthroplasty in the elderly. Orthop Traumatol Surg Res. 2011;97:583–589. doi: 10.1016/j.otsr.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Churchill RS. Elective shoulder arthroplasty in patients older than ninety years of age. J Shoulder Elbow Surg. 2008;17:376–379. doi: 10.1016/j.jse.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 4.Cofield RH. Total shoulder arthroplasty with the Neer prosthesis. J Bone Joint Surg Am. 1984;66:899–906. doi: 10.2106/00004623-198466060-00010. [DOI] [PubMed] [Google Scholar]
- 5.Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;455:183–189. doi: 10.1097/01.blo.0000238839.26423.8d. [DOI] [PubMed] [Google Scholar]
- 6.Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O’Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468:717–722. doi: 10.1007/s11999-009-0996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foruria AM, Sperling JW, Ankem HK, Oh LS, Cofield RH. Total shoulder replacement for osteoarthritis in patients 80 years of age and older. J Bone Joint Surg Br. 2010;92:970–974. doi: 10.1302/0301-620X.92B7.23671. [DOI] [PubMed] [Google Scholar]
- 8.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 9.Hammond JW, Queale WS, Kim TK, McFarland EG. Surgeon experience and clinical and economic outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2003;85:2318–2324. doi: 10.2106/00004623-200312000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Jain N, Pietrobon R, Hocker S, Guller U, Shankar A, Higgins LD. The relationship between surgeon and hospital volume and outcomes for shoulder arthroplasty. J Bone Joint Surg Am. 2004;86:496–505. doi: 10.2106/00004623-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Jain NB, Hocker S, Pietrobon R, Guller U, Bathia N, Higgins LD. Total arthroplasty versus hemiarthroplasty for glenohumeral osteoarthritis: role of provider volume. J Shoulder Elbow Surg. 2005;14:361–367. doi: 10.1016/j.jse.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93:2249–2254. doi: 10.2106/JBJS.J.01994. [DOI] [PubMed] [Google Scholar]
- 13.Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6:449–454. doi: 10.1016/S1058-2746(97)70052-1. [DOI] [PubMed] [Google Scholar]
- 14.Lyman S, Jones EC, Bach PB, Peterson MG, Marz RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132–137. doi: 10.1097/01.blo.0000150571.51381.9a. [DOI] [PubMed] [Google Scholar]
- 15.Mangano T, Cerruti P, Repetto I, Felli L, Ivaldo N, Giovale M. Reverse shoulder arthroplasty in older patients: is it worth it? A subjective functional outcome and quality of life survey. Aging Clin Exp Res. 2016;28:925–933. doi: 10.1007/s40520-015-0493-2. [DOI] [PubMed] [Google Scholar]
- 16.Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95:1297–1304. doi: 10.2106/JBJS.L.00820. [DOI] [PubMed] [Google Scholar]
- 17.Mullett H, Levy O, Raj D, Even T, Abraham R, Copeland SA. Copeland surface replacement of the shoulder: results of an hydroxyapatite-coated cementless implant in patients over 80 years of age. J Bone Joint Surg Br. 2007;89:1466–1469. doi: 10.1302/0301-620X.89B11.18850. [DOI] [PubMed] [Google Scholar]
- 18.Neer CS, 2nd, Watson KC, Stanton FJ. Recent experience in total shoulder replacement. J Bone Joint Surg Am. 1982;64:319–337. doi: 10.2106/00004623-198264030-00001. [DOI] [PubMed] [Google Scholar]
- 19.Ricchetti ET, Abboud JA, Kuntz AF, Ramsey ML, Glaser DL, Williams GR., Jr Total shoulder arthroplasty in older patients: increased perioperative morbidity? Clin Orthop Relat Res. 2011;469:1042–1049. doi: 10.1007/s11999-010-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh JA, Ramachandran R. Age-related differences in the use of total shoulder arthroplasty over time: use and outcomes. Bone Joint J. 2015;97:1385–1389. doi: 10.1302/0301-620X.97B10.35696. [DOI] [PubMed] [Google Scholar]
- 21.Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: analysis of 2588 shoulders over three decades (1976 to 2008) J Bone Joint Surg Br. 2011;93:1513–1517. doi: 10.1302/0301-620X.93B11.26938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Mole D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff: results of a multicentre study of 80 shoulders. J Bone Joint Surg Br. 2004;86:388–395. doi: 10.1302/0301-620X.86B3.14024. [DOI] [PubMed] [Google Scholar]
- 23.Smith AM, Barnes SA, Sperling JW, Farrell CM, Cummings JD, Cofield RH. Patient and physician-assessed shoulder function after arthroplasty. J Bone Joint Surg Am. 2006;88:508–513. doi: 10.2106/JBJS.E.00132. [DOI] [PubMed] [Google Scholar]
- 24.Wall B, Nove-Josserand L, O’Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. J Bone Joint Surg Am. 2007;89:1476–1485. doi: 10.2106/JBJS.F.00666. [DOI] [PubMed] [Google Scholar]
- 25.White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18:886–888. doi: 10.1016/S0883-5403(03)00269-9. [DOI] [PubMed] [Google Scholar]
- 26.Wirth MA, Loredo R, Garcia G, Rockwood CA, Jr, Southworth C, Iannotti JP. Total shoulder arthroplasty with an all-polyethylene pegged bone-ingrowth glenoid component: a clinical and radiographic outcome study. J Bone Joint Surg Am. 2012;94:260–267. doi: 10.2106/JBJS.J.01400. [DOI] [PubMed] [Google Scholar]