Abstract
When mammalian cells and animals face a variety of internal or external stresses, they need to make homeostatic changes so as to cope with various stresses. To this end, mammalian cells are equipped with two critical stress responses, autophagy and cellular senescence. Autophagy and cellular senescence share a number of stimuli including telomere shortening, DNA damage, oncogenic stress and oxidative stress, suggesting their intimate relationship. Autophagy is originally thought to suppress cellular senescence by removing damaged macromolecules or organelles, yet recent studies also indicated that autophagy promotes cellular senescence by facilitating the synthesis of senescence-associated secretory proteins. These seemingly opposite roles of autophagy may reflect a complex picture of autophagic regulation on cellular senescence, including different types of autophagy or a unique spatiotemporal activation of autophagy. Thus, a better understanding of autophagy process will lead us to not only elucidate the conundrum how autophagy plays dual roles in the regulation of cellular senescence but also helps the development of new therapeutic strategies for many human diseases associated with cellular senescence. We address the pro-senescence and anti-senescence roles of autophagy while focusing on the potential mechanistic aspects of this complex relationship between autophagy and cellular senescence.
Keywords: aging, autophagy, cellular senescence, selective autophagy, senescence-associated secretory phenotype
INTRODUCTION
Cellular senescence is a stress-activated genetic program that permanently prevents damaged cells from further proliferation, acting as a potent tumor suppressive mechanism (Childs et al., 2015; Kuilman et al., 2010; Lopez-Otin et al., 2013; Munoz-Espin and Serrano, 2014). It is originally recognized as a response to telomere shortening caused by replicative exhaustion, yet since then many stresses including DNA damage, oxidative stress, and oncogenic stress have been also shown to activate cellular senescence. In addition to cell cycle arrest, senescent cells have additional effector programs such as an enlarged flattened morphology, an altered chromatin structure called senescence-associated heterochromatin formation (SAHF), and a massive secretion of several factors called senescence-associated secretory phenotype (SASP). Critical among these is the SASP that includes many cytokines, chemokines, proteases, growth factors, and extra cellular matrix (ECM), as it affects not only senescent cells themselves in an autocrine manner but also nearby cells and the tissue microenvironment in a non-cell-autonomous manner. For example, SASP factors can reinforce cell cycle arrest of senescent cells and mobilize the immune system to suppress tumorigenesis and promote an optimal tissue repair. When dysregulated, however, SASP factors can stimulate adjacent premalignant and malignant cells to promote tumorigenesis and may cause chronic inflammation, contributing to many age-associated diseases including aging itself (Childs et al., 2015; Coppe et al., 2010; He and Sharpless, 2017; Soto-Gamez and Demaria, 2017; Tchkonia et al., 2013).
Macroautophagy (hereinafter referred to as autophagy) is another critical effector program of cellular senescence. Autophagy, a major lysosomal degradation pathway, was originally recognized as a response to starvation, recycling cellular components to maintain energy homeostasis (Boya et al., 2013; Choi et al., 2013; Kang and Avery, 2008; 2009a; 2009b; Kang et al., 2007; Klionsky and Codogno, 2013; Kroemer et al., 2010). As in the case of cellular senescence, however, it has been shown that autophagy can be activated by a variety of stresses including those that cause cellular senescence. In fact, autophagy is found to increase in senescent cells but its roles in the regulation of cellular senescence are still under debate (Gewirtz, 2013; Kang and Elledge, 2016; Kuilman et al., 2010). On the one hand, this phenotype can be viewed as ‘cellular senescence by autophagy’ (pro-senescence). Given the well-known homeostatic function of autophagy, on the other hand, it can be viewed as ‘cellular senescence with autophagy’ - a failed attempt to prevent cellular senescence by autophagy (anti-senescence). In this review, we focus on the complex relationship between autophagy and cellular senescence. We address each mode of action of autophagy on cellular senescence and provide an intriguing hypothesis of how autophagy may differentially modulate cellular senescence depending on its types or its unique spatiotemporal activation.
AUTOPHAGY AS A PRO-SENESCENCE MECHANISM
Autophagy was simply presumed to suppress cellular senescence via its homeostatic action such as removing damaged cellular components until an elegant study from the Narita’s group described the first causal relationship between autophagy and cellular senescence (Levine and Kroemer, 2008; Rubinsztein et al., 2011; Young et al., 2009). The authors found that autophagy is activated during oncogene-induced senescence and knockdown of several key autophagy regulators delays oncogene-induced senescence, especially the SASP. The fact that autophagy mainly modulates the SASP at the post-transcriptional level led the authors to the intriguing hypothesis that autophagy may provide building blocks for the protein synthesis of the SASP (Young et al., 2009). Later, the same group identified a very specialized type of autophagy called the TOR-autophagy spatial coupling compartment (TASCC), which is in fact responsible for the protein synthesis of some SASP factors (Narita et al., 2011). At the TASCC, autophagy provides amino acids to activate mTOR, which in turn stimulates the local synthesis of IL6 and IL8 proteins. Thus, the TASCC connects the cell’s catabolic (autophagy) process to its anabolic (protein synthesis) process for efficiently coordinating the massive synthesis of the SASP proteins during oncogene-induced senescence.
Soon after these seminal findings, several groups reported that autophagy promotes cellular senescence under several different conditions. For example, overexpression of CDK inhibitors such as p16, p19, and p21 activates autophagy and cellular senescence in both human fibroblasts and breast cancer cells (Capparelli et al., 2012). Inhibition of the cyclin-dependent kinases CDK4 and CDK6 has been shown to restore PML-induced cellular senescence in tumor cells and this is well correlated with an increase in autophagy that may reestablish senescence specific epigenetic markers through the regulation of DNMT1. In addition, activation of autophagy by prolonged mTOR inhibition triggers premature senescence induced by irradiation in cancer cells (Nam et al., 2013). All these studies, however, rather provide a correlated relationship between autophagy and cellular senescence, not a causal relationship.
Additional evidence showing the causal relationship came from senescence studies in cancer cells. Liu H et al. found that in melanoma, autophagic activity decreased as assessed by levels of ATG5 and LC3B, two key autophagy related genes (Liu et al., 2014). Furthermore, they showed that knockdown of ATG5 bypasses BRAF oncogene-induced senescence, suggesting that autophagy positively regulates cellular senescence. Studies by Huang et al. (2014) also showed that inhibition of autophagy suppresses irradiation-induced senescence in cancer cells. In this case, however, it rather changes the cells’ fate to apoptosis, making it hard to rule out the possibility that autophagy simply inhibits apoptosis, not directly causes cellular senescence. This situation can be referred to as ‘non-senescence addiction’, analogous to the well-known ‘non-oncogene addiction’ where cancer cells rely on certain cellular processes or genes that are not oncogenic by themselves: autophagy could be such a process that acts in cellular senescence but is not pro-senescence by themselves (Galluzzi et al., 2013; Luo et al., 2009). The massive synthesis of the SASP factors may cause endoplasmic reticulum (ER) stress and an unfolded protein response, leading to proteotoxic stress in senescent cells that can be managed by autophagy. In fact, it has been shown that therapy-induced senescent lymphomas that display a high level of the SASP are sensitive to blocking autophagy (Amaravadi et al., 2007).
Two recent studies not only root for the positive regulation of cellular senescence by autophagy but also provide the comprehensive mechanisms how autophagy achieves this task. Horikawa et al. (2014) examined the molecular mechanism by which Δ133p53α, a p53 isoform that inhibit full-length p53, is downregulated during replicative senescence. They found that autophagy degrades Δ133p53α through the action of STUB1, a chaperone-associated E3 ubiquitin ligase, which in turn activate p53-mediated cellular senescence. Δ133p53α is ubiquitinated for autophagic degradation and STUB1 seems to be at least partly responsible for the ubiquitination of Δ133p53α. However, STUB1 rather inhibits, not promotes, the degradation of Δ133p53α, raising the conundrum for the role of Δ133p53α ubiquitination by STUB1 during cellular senescence. STUB1 might compete with an unidentified E3 ligase to ubiquitinate Δ133p53α at the same sites but with different conjugation types (e.g. K48-linked v.s. K63-linked): STUB1 protects Δ133p53α from degradation, whereas an unidentified E3 ligase promotes its degradation. STUB1 is also downregulated during replicative senescence, yet the upstream mechanism of such a regulation is currently unclear. An interesting study from the Berger’s group described one of the first examples of autophagic degradation for nuclear components (Dou et al., 2015; 2016). The authors showed that LC3B, a ubiquitin-like protein associated with the autophagosomal membrane during autophagy induction, exists in the nucleus and specifically interacts with the nuclear lamina component lamin B. Normally, LC3B delivers autophagic substrates to the autophagosome for their degradation together with autophagic receptors such as p62, yet LC3B-lamin B interaction does not downregulate lamin B under basal conditions, nor starvation conditions. During oncogene-induced senescence, however, lamin B decreases in an autophagy dependent manner and this is achieved by nucleus-to-cytoplasm transport that delivers lamin B1 to the lysosome. Surprisingly, a portion of chromatin called Lamin-Associated Domains (LADs) is transported together with lamin B1 and degraded in the lysosome, suggesting a very interesting possibility that cellular senescence becomes ‘truly irreversible’ at this point. Why senescent cells secrete a portion of chromatin into cytoplasm for degradation remains elusive. Since secreted chromatin is decorated by DNA damage markers such as gamma-H2AX, this might be a desperate move of senescent cells to sustain their viability by removing DNA damage that is otherwise irreparable and thus causes cell death. Inhibition of autophagy suppresses lamin b1 degradation and attenuates oncogene-induced senescence, partially by maintaining nuclear membrane integrity that can cause abnormal changes in gene expression and thus lead to high levels of proteotoxic stress.
Collectively, these data support a pro-senescence mechanism of autophagy, especially during oncogene-induced senescence, as seen above.
AUTOPHAGY AS AN ANTI-SENESCENCE MECHANISM
An initial report by the Narita’s group that autophagy mediates cellular senescence shocked the world of autophagy, as autophagy was normally believed to remove stressors that can cause cellular senescence (e.g. damaged proteins or damaged mitochondria); therefore, the overriding assumption was that autophagy would be an anti-senescence mechanism (Boya et al., 2013; Kroemer et al., 2010; Rubinsztein et al., 2011). In fact, later, several studies have supported such a view about the role of autophagy in the regulation of cellular senescence. For example, prolonged inhibition of autophagy by either knockdown of ATG7 or ATG5 induces cellular senescence in primary human fibroblasts, mainly due to the defective mitochondria and increased levels of reactive oxygen species (ROS) (Kang et al., 2011). The critical difference between this study and the one from the Narita’s group is that what type of autophagy is examined: basal autophagy (in the former case) versus oncogene-induced autophagy (in the latter case) (Kang et al., 2011; Narita et al., 2011; Young et al., 2009). In consistent with this, Garcia-Prat et al. found that basal autophagy maintains stemness of satellite cells, muscle stem cells, by preventing cellular senescence (Garcia-Prat et al., 2016; Wen and Klionsky, 2016). The authors found that during aging, the regenerative capacity of muscle stem cell declines alongside autophagic activity. It occurred with a transition from a normal quiescence into an irreversible senescence state of satellite cells. When basal autophagy is genetically impaired in young satellite cells, it causes loss of proteostasis, increased mitochondrial dysfunction and oxidative stress, resulting in cellular senescence. Conversely, when basal autophagy activity is restored in old satellite cells, it prevents cellular senescence and restores the regenerative capacity of satellite cells. Thus, these studies emphasize a critical role of basal autophagy in suppressing cellular senescence.
Similarly, but in slightly different conditions, autophagy has been reported to suppress cellular senescence by maintaining homeostasis under stressful conditions. This is particularly prominent when cells are challenged with oxidative stress. Whereas oxidative stress normally activates autophagy, excessive oxidative stress has been shown to rather impair autophagic activity, eventually causing cellular senescence (Han et al., 2016; Tai et al., 2017). These observations are probably resulting from the balance act between the level of oxidative stress and the capacity of autophagy counteracting it. Enhancing or restoring autophagic activity under excessive oxidative stress by either AMPK activation or mTOR inhibition efficiently suppresses cellular senescence (Dalle Pezze et al., 2014; Han et al., 2016; Nopparat et al., 2017; Tai et al., 2017). These results are also supported by recent findings that melatonin enhances autophagic activity via the SIRT1 signaling pathway to suppress oxidative stress-induced senescence (Nopparat et al., 2017).
Another stressful condition where autophagy may play a homeostatic role to suppress cellular senescence is mitochondrial dysfunction. Along with cellular senescence, mitochondrial dysfunction is a hallmark of aging and it is accompanied with increased levels of mitochondrial superoxide generation (Lopez-Otin et al., 2013). Uncoupling mitochondria reduces mitochondrial superoxide generation, telomere dysfunction, and cellular senescence, indicating a major role of mitochondria ROS during cellular senescence (Passos et al., 2007). Interestingly, dysfunctional mitochondria cause a distinct secretory phenotype from the SASP that lacks the IL-1 dependent inflammatory arm (miDAS, mitochondrial dysfunction-associated senescence) (Wiley et al., 2016). miDAS cells have lower NAD+/NADH ratios, which cause both the growth arrest and prevent the IL1-associated SASP through AMPK-mediated p53 activation. Removing mitochondria from senescent cells through parkin-mediated mitophagy, a selective type of autophagy for dysfunctional mitochondria, reduces several characteristics of cellular senescence mentioned above, while preserving ATP production via enhanced glycolysis (Ito et al., 2015; Korolchuk et al., 2017). Mechanistically, ATM, Akt, and mTORC1 phosphorylation cascade relay a signal from DNA damage response toward PGC-1β dependent mitochondrial biogenesis, which in turn stabilize cellular senescence via a positive feedback loop involving ROS and the DDR.
The homeostatic function of autophagy in suppressing cellular senescence was highlighted again from a recent non-biased high-throughput screening to identify small molecules that alleviate cellular senescence (Kang et al., 2017). The Park’s group identified KU-60019, the ataxia telangiectasia mutated (ATM) kinase inhibitor, effectively alleviates replicative senescence. ATM phosphorylates the vacuolar ATPase V1 subunit ATP6V1G1, which decreases the dimerization of ATP6V1G1 with another vacuolar ATPase V1 subunit ATP6V1E1 that impairs the activity of autophagy-lysosome pathway. Suppressing ATM activity restores the dimerization and the steady-state pH of the lysosome. Subsequently, enhanced function of autophagy-lysosome pathway recovers mitochondrial function and induces reprograming from glycolysis to oxidative phosphorylation, which in turn alleviates senescence phenotypes.
Together, these data support an anti-senescence role of autophagy that mainly acts in the homeostatic pathway and relieves the burden of senescence-inducing stressors.
DUAL ROLES OF AUTOPHAGY IN THE REGULATION OF CELLULAR SENESCENCE
Whereas above studies that have examined an inverse relationship between autophagy and cellular senescence may seem to describe a passive role of autophagy, it is possible that autophagy directly suppresses some of senescence pathways as seen in the case of the positive relationship where TASCC facilitates the massive synthesis of the SASP factors (Narita et al., 2011). Indeed, we recently identified such a direct role of autophagy in cellular senescence (Kang and Elledge, 2016; Kang et al., 2015). During a search of the specific regulator for the SASP, we found that the GATA4-NF-κB pathway mainly regulates the SASP. GATA4 protein, but not mRNA, increased during cellular senescence, mainly due to the increased protein stability. Surprisingly, this regulation is mediated by selective autophagy, not the ubiquitin proteasome system: under normal conditions, GATA4 binds to the specialized autophagic receptor called SQSTM1/p62 for degradation by selective autophagy. Upon senescence inducing stimuli, however, selective autophagy of GATA4 is disabled and GATA4 becomes stabilized to activate the SASP program and cellular senescence. Interestingly, but consistent with studies from the Narita’s group, GATA4 requires a ‘general’ autophagy activity, very likely the TASCC, to fully induce cellular senescence: a specialized type of selective autophagy actively suppresses cellular senescence through the degradation of the SASP regulator, GATA4, whereas general autophagy supports senescence transition through the TASCC. Thus, our studies provided the first clue to resolve apparently contradictory roles of autophagy in the regulation of cellular senescence.
UNRAVELING THE DEEPLY INTERTWINED RELATIONSHIP BETWEEN AUTOPHAGY AND CELLULAR SENESCENCE WITH A TOOLKIT OF DIFFERENTIAL DIAGNOSIS
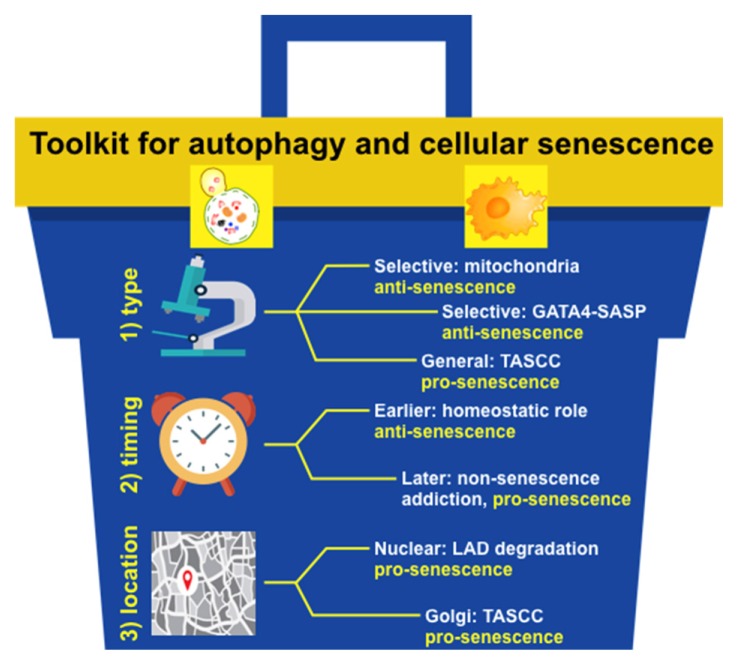
Currently, it is clear that autophagy acts as either a pro-senescence or an anti-senescence mechanism (Gewirtz, 2013). But by what mechanisms? Not so much. Based on our discussion presented above, we would like to provide a toolkit of differential diagnosis to resolve the conundrum how autophagy has such a dual role: a type of autophagy involved, when autophagy acts, and where autophagy acts.
A type of autophagy involved: general autophagy seems to act as an anti-senescence mechanism by maintaining homeostasis under either normal or stress-induced conditions. However, its timing of activation might be a differential factor that we will discuss below in ‘2) When autophagy acts’. When autophagy degrades only a certain type of substrates, we collectively call it ‘selective autophagy’ (Jin et al., 2013; Johansen and Lamark, 2011; Shaid et al., 2013). However, in fact, it is all different types of autophagy, as each has its unique substrate, very similarly to an E3 ubiquitin ligase in the ubiquitin proteasome system. So, when considered for the role of selective autophagy in the regulation of cellular senescence, one should consider more its substrates and autophagic receptors that allow for autophagy to have a specificity. For example, p62-dependent selective autophagy of GATA4 acts as an anti-senescence mechanism (Kang et al., 2015), yet LC3B-lamin B1-dependent selective autophagy of nuclear lamina acts as a pro-senescence mechanism (Dou et al., 2015). It will be of a particular interest to find additional autophagy receptor-substrate pairs that act in the senescence regulatory network.
When autophagy acts: under normal conditions, general autophagy acts as an anti-senescence mechanism as mentioned above. Earlier action of general autophagy under stress-induced conditions also represents a homeostatic response, so it is mostly anti-senescent. Once cells over a certain time point during cellular senescence, however, general autophagy may become pro-senescent, as it may sustain a viability of senescent cells by decreasing the burden of stresses that senescent cells must cope with: non-senescence addiction. For example, senescent cells do not divide, so they cannot dilute toxic substances as in the case of fully differentiated cells. Senescent cells also secrete many factors, which elicit ER stress and an unfolded protein response (Coppe et al., 2010). All of them can disrupt proteostasis in the cell, which in turn eventually cause cell death unless general autophagy delays or dampens it. It will be critical to determine when will be a clear cut to differentiate the role of autophagy in controlling cellular senescence. This will be very likely context dependent, influenced by a type of senescence-inducing stressors.
Where autophagy acts: this might be a tool that is mostly unappreciated. The TASCC that provides amino acids for activating a local protein synthesis of the SASP factors is the best example for this (Narita et al., 2011). Nuclear autophagy may present this type of tools, disrupting nuclear membrane integrity and thus causing abnormal changes in gene expression (Dou et al., 2015). It will be particularly interesting to search this type of regulation in highly polarized cells such as epithelial cell types, since they have more distinct spaces inside the cell than non-polarized cells.
CONCLUSION
The toolkit proposed here is just a prototype to unravel a complex relationship between autophagy and cellular senescence. A deeper understanding of these two critical stress responses will be required to upgrade it to the full version. For this, we need to have a full picture of selective autophagy during cellular senescence and examine very detailed series of time course experiments when and where different types of autophagy act. Since autophagy and cellular senescence play critical roles in many age-related human diseases including cancer, neurodegenerative diseases, and aging itself (He and Sharpless, 2017; Kroemer, 2015; Lapierre et al., 2015; Nah et al., 2015), our efforts here will help fight such devastating diseases and eventually enhance human health.
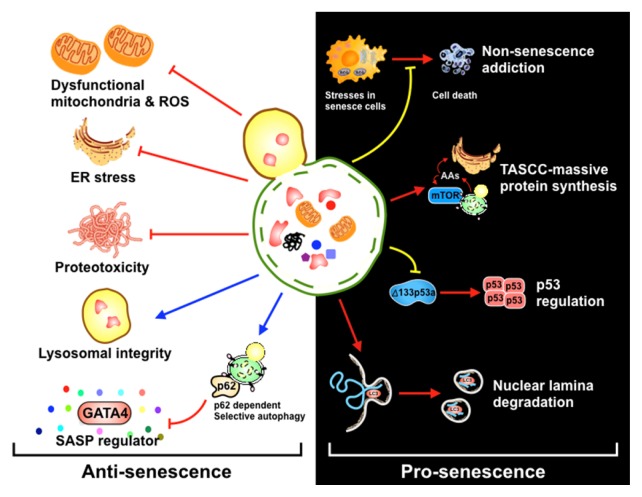
Fig. 1.
Dual roles of autophagy in the regulation of cellular senescence.
(Left) Under normal conditions, autophagy alleviates stressors that can cause cellular senescence such as dysfunctional mitochondria, ROS, and ER stress. Autophagy also maintains the lysosomal integrity, thus acting an anti-senescence mechanism in a passive manner. p62-dependent autophagy, meanwhile, specifically degrades GATA4, a main regulator of the SASP, thereby actively suppressing cellular senescence. (Right) Autophagy function as ‘non-senescence addiction’ that can manage several senescence-associated stresses and thus maintain the viability of senescent cells. In addition, autophagy targets Δ133p53α or lamin B to cause cellular senescence. Lastly, autophagy may provide amino acids to support the massive synthesis of some SASP factors through the TASCC.
Fig. 2.
A prototype toolbox for unraveling a complex relationship between autophagy and cellular senescence.
Three tools are provided: 1) a type of autophagy involved (type), 2) when autophagy acts (timing), and 3) where autophagy acts (location).
ACKNOWLEDGMENTS
This work was supported by grants from the POSCO Science Fellowship of POSCO TJ Park Foundation, the National Research Foundation of Korea (2017R1D1A1B03034795), the SNU invitation program for distinguished scholar, the Research Resettlement Fund for the new faculty of Seoul National University.
REFERENCES
- Amaravadi R.K., Yu D., Lum J.J., Bui T., Christophorou M.A., Evan G.I., Thomas-Tikhonenko A., Thompson C.B. Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest. 2007;117:326–336. doi: 10.1172/JCI28833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boya P., Reggiori F., Codogno P. Emerging regulation and functions of autophagy. Nat Cell Biol. 2013;15:713–720. doi: 10.1038/ncb2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capparelli C., Chiavarina B., Whitaker-Menezes D., Pestell T.G., Pestell R.G., Hulit J., Ando S., Howell A., Martinez-Outschoorn U.E., Sotgia F., et al. CDK inhibitors (p16/p19/p21). induce senescence and autophagy in cancer-associated fibroblasts, “fueling” tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle. 2012;11:3599–3610. doi: 10.4161/cc.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs B.G., Durik M., Baker D.J., van Deursen J.M. Cellular senescence in aging and age-related disease: from mechanisms to therapy. Nat Med. 2015;21:1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- Coppe J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Pezze P., Nelson G., Otten E.G., Korolchuk V.I., Kirkwood T.B., von Zglinicki T., Shanley D.P. Dynamic modelling of pathways to cellular senescence reveals strategies for targeted interventions. PLoS Comput Biol. 2014;10:e1003728. doi: 10.1371/journal.pcbi.1003728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z., Xu C., Donahue G., Shimi T., Pan J.A., Zhu J., Ivanov A., Capell B.C., Drake A.M., Shah P.P., et al. Autophagy mediates degradation of nuclear lamina. Nature. 2015;527:105–109. doi: 10.1038/nature15548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z., Ivanov A., Adams P.D., Berger S.L. Mammalian autophagy degrades nuclear constituents in response to tumorigenic stress. Autophagy. 2016;12:1416–1417. doi: 10.1080/15548627.2015.1127465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Kepp O., Vander Heiden M.G., Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Dis. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- Garcia-Prat L., Martinez-Vicente M., Perdiguero E., Ortet L., Rodriguez-Ubreva J., Rebollo E., Ruiz-Bonilla V., Gutarra S., Ballestar E., Serrano A.L., et al. Autophagy maintains stemness by preventing senescence. Nature. 2016;529:37–42. doi: 10.1038/nature16187. [DOI] [PubMed] [Google Scholar]
- Gewirtz D.A. Autophagy and senescence: a partnership in search of definition. Autophagy. 2013;9:808–812. doi: 10.4161/auto.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Tai H., Wang X., Wang Z., Zhou J., Wei X., Ding Y., Gong H., Mo C., Zhang J., et al. AMPK activation protects cells from oxidative stress-induced senescence via autophagic flux restoration and intracellular NAD(+). elevation. Aging Cell. 2016;15:416–427. doi: 10.1111/acel.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa I., Fujita K., Jenkins L.M., Hiyoshi Y., Mondal A.M., Vojtesek B., Lane D.P., Appella E., Harris C.C. Autophagic degradation of the inhibitory p53 isoform Delta133p53alpha as a regulatory mechanism for p53-mediated senescence. Nat Commun. 2014;5:4706. doi: 10.1038/ncomms5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.H., Yang P.M., Chuah Q.Y., Lee Y.J., Hsieh Y.F., Peng C.W., Chiu S.J. Autophagy promotes radiation-induced senescence but inhibits bystander effects in human breast cancer cells. Autophagy. 2014;10:1212–1228. doi: 10.4161/auto.28772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Araya J., Kurita Y., Kobayashi K., Takasaka N., Yoshida M., Hara H., Minagawa S., Wakui H., Fujii S., et al. PARK2-mediated mitophagy is involved in regulation of HBEC senescence in COPD pathogenesis. Autophagy. 2015;11:547–559. doi: 10.1080/15548627.2015.1017190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M., Liu X., Klionsky D.J. SnapShot: selective autophagy. Cell. 2013;152:368–368 e362. doi: 10.1016/j.cell.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen T., Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–296. doi: 10.4161/auto.7.3.14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Avery L. To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4:82–84. doi: 10.4161/auto.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Avery L. Systemic regulation of autophagy in Caenorhabditis elegans. Autophagy. 2009a;5:565–566. doi: 10.4161/auto.5.4.8171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Avery L. Systemic regulation of starvation response in Caenorhabditis elegans. Genes Dev. 2009b;23:12–17. doi: 10.1101/gad.1723409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Elledge S.J. How autophagy both activates and inhibits cellular senescence. Autophagy. 2016;12:898–899. doi: 10.1080/15548627.2015.1121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., You Y.J., Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007;21:2161–2171. doi: 10.1101/gad.1573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.T., Park J.T., Choi K., Kim Y., Choi H.J.C., Jung C.W., Lee Y.S., Park S.C. Chemical screening identifies ATM as a target for alleviating senescence. Nat Chem Biol. 2017;13:616–623. doi: 10.1038/nchembio.2342. [DOI] [PubMed] [Google Scholar]
- Kang H.T., Lee K.B., Kim S.Y., Choi H.R., Park S.C. Autophagy impairment induces premature senescence in primary human fibroblasts. PloS One. 2011;6:e23367. doi: 10.1371/journal.pone.0023367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Xu Q., Martin T.D., Li M.Z., Demaria M., Aron L., Lu T., Yankner B.A., Campisi J., Elledge S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science. 2015;349:aaa5612. doi: 10.1126/science.aaa5612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D.J., Codogno P. The mechanism and physiological function of macroautophagy. J Innate Immun. 2013;5:427–433. doi: 10.1159/000351979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk V.I., Miwa S., Carroll B., von Zglinicki T. Mitochondria in cell senescence: is mitophagy the weakest link? EBioMedicine. 2017;21:7–13. doi: 10.1016/j.ebiom.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G. Autophagy: a druggable process that is deregulated in aging and human disease. J Clin Invest. 2015;125:1–4. doi: 10.1172/JCI78652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G., Marino G., Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T., Michaloglou C., Mooi W.J., Peeper D.S. The essence of senescence. Genes Dev. 2010;24:2463–2479. doi: 10.1101/gad.1971610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre L.R., Kumsta C., Sandri M., Ballabio A., Hansen M. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy. 2015;11:867–880. doi: 10.1080/15548627.2015.1034410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., He Z., Simon H.U. Autophagy suppresses melanoma tumorigenesis by inducing senescence. Autophagy. 2014;10:372–373. doi: 10.4161/auto.27163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Solimini N.L., Elledge S.J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–837. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Espin D., Serrano M. Cellular senescence: from physiology to pathology. Nature reviews Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- Nah J., Yuan J., Jung Y.K. Autophagy in neurodegenerative diseases: from mechanism to therapeutic approach. Mol Cells. 2015;38:381–389. doi: 10.14348/molcells.2015.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam H.Y., Han M.W., Chang H.W., Kim S.Y., Kim S.W. Prolonged autophagy by MTOR inhibitor leads radioresistant cancer cells into senescence. Autophagy. 2013;9:1631–1632. doi: 10.4161/auto.25879. [DOI] [PubMed] [Google Scholar]
- Narita M., Young A.R., Arakawa S., Samarajiwa S.A., Nakashima T., Yoshida S., Hong S., Berry L.S., Reichelt S., Ferreira M., et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science. 2011;332:966–970. doi: 10.1126/science.1205407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopparat C., Sinjanakhom P., Govitrapong P. Melatonin reverses H2 O2 -induced senescence in SH-SY5Y cells by enhancing autophagy via sirtuin 1 deacetylation of the RelA/p65 subunit of NF-kappaB. J Pineal Res. 2017;63 doi: 10.1111/jpi.12407. [DOI] [PubMed] [Google Scholar]
- Passos J.F., Saretzki G., Ahmed S., Nelson G., Richter T., Peters H., Wappler I., Birket M.J., Harold G., Schaeuble K., et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007;5:e110. doi: 10.1371/journal.pbio.0050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein D.C., Marino G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- Shaid S., Brandts C.H., Serve H., Dikic I. Ubiquitination and selective autophagy. Cell Death Differ. 2013;20:21–30. doi: 10.1038/cdd.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Gamez A., Demaria M. Therapeutic interventions for aging: the case of cellular senescence. Drug Dis Today. 2017;22:786–795. doi: 10.1016/j.drudis.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Tai H., Wang Z., Gong H., Han X., Zhou J., Wang X., Wei X., Ding Y., Huang N., Qin J., et al. Autophagy impairment with lysosomal and mitochondrial dysfunction is an important characteristic of oxidative stress-induced senescence. Autophagy. 2017;13:99–113. doi: 10.1080/15548627.2016.1247143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J.L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen X., Klionsky D.J. Autophagy is a key factor in maintaining the regenerative capacity of muscle stem cells by promoting quiescence and preventing senescence. Autophagy. 2016;12:617–618. doi: 10.1080/15548627.2016.1158373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C.D., Velarde M.C., Lecot P., Liu S., Sarnoski E.A., Freund A., Shirakawa K., Lim H.W., Davis S.S., Ramanathan A., et al. Mitochondrial Dysfunction induces senescence with a distinct secretory phenotype. Cell Metabol. 2016;23:303–314. doi: 10.1016/j.cmet.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A.R., Narita M., Ferreira M., Kirschner K., Sadaie M., Darot J.F., Tavare S., Arakawa S., Shimizu S., Watt F.M., et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009;23:798–803. doi: 10.1101/gad.519709. [DOI] [PMC free article] [PubMed] [Google Scholar]