Abstract
The most common form of senile dementia is Alzheimer’s disease (AD), which is characterized by the extracellular deposition of amyloid β-peptide (Aβ) plaques and the intracellular formation of neurofibrillary tangles (NFTs) in the cerebral cortex. Tau abnormalities are commonly observed in many neurodegenerative diseases including AD, Parkinson’s disease, and Pick’s disease. Interestingly, tau-mediated formation of NFTs in AD brains shows better correlation with cognitive impairment than Aβ plaque accumulation; pathological tau alone is sufficient to elicit frontotemporal dementia, but it does not cause AD. A growing amount of evidence suggests that soluble Aβ oligomers in concert with hyperphosphorylated tau (pTau) serve as the major pathogenic drivers of neurodegeneration in AD. Increased Aβ oligomers trigger neuronal dysfunction and network alternations in learning and memory circuitry prior to clinical onset of AD, leading to cognitive decline. Furthermore, accumulated damage to mitochondria in the course of aging, which is the best-known nongenetic risk factor for AD, may collaborate with soluble Aβ and pTau to induce synapse loss and cognitive impairment in AD. In this review, I summarize and discuss the current knowledge of the molecular and cellular biology of AD and also the mechanisms that underlie Aβ-mediated neurodegeneration.
Keywords: Alzheimer’s disease, amyloid β-peptide, APP, neurodegeneration, tau
INTRODUCTION
In 1906, Alois Alzheimer identified neuritic plaques and neurofibrillary tangles (NFTs) as prominent neuropathologic features in the brains of certain patients, and the pattern was eventually called Alzheimer’s disease (AD; Goedert and Spillantini, 2006). In the 1980s, amyloid β-peptide (Aβ) and hyperphosphorylated tau (pTau) were recovered as major components of, respectively, neuritic plaques and NFTs of AD (Grundke-Iqbal et al., 1986; Ihara et al., 1986; Kang et al., 1987; Kosik et al., 1986; Masters et al., 1985). The amyloid hypothesis proposes that deposition of Aβ plaques in the brain is the primary cause of AD (Hardy and Higgins, 1992; Hardy and Selkoe, 2002). However, histologically determined numbers of insoluble Aβ plaques in the brain do not correlate with dementia severity (Hardy and Selkoe, 2002; De Strooper and Karran, 2016). Rather, the tau pathology observed in AD brains correlates better with the degree of cognitive decline (Hardy and Selkoe, 2002; Spires-Jones and Hyman, 2014). Multiple studies strongly suggest that abnormal elevation in pTau is necessary but not sufficient to cause AD (Huang and Mucke, 2012; Hutton et al., 1998; Nelson et al., 2012), so what does cause it? This review provides the current understanding of the proteolytic processing of β-amyloid precursor protein (APP), the functional roles of APP-derived fragments, and AD-causing mutations, and it discusses the molecular and cellular mechanisms that underlie the pathogenesis of neurodegenerative AD.
PROTEOLYTIC PROCESSING OF β-AMYLOID PRECURSOR PROTEIN
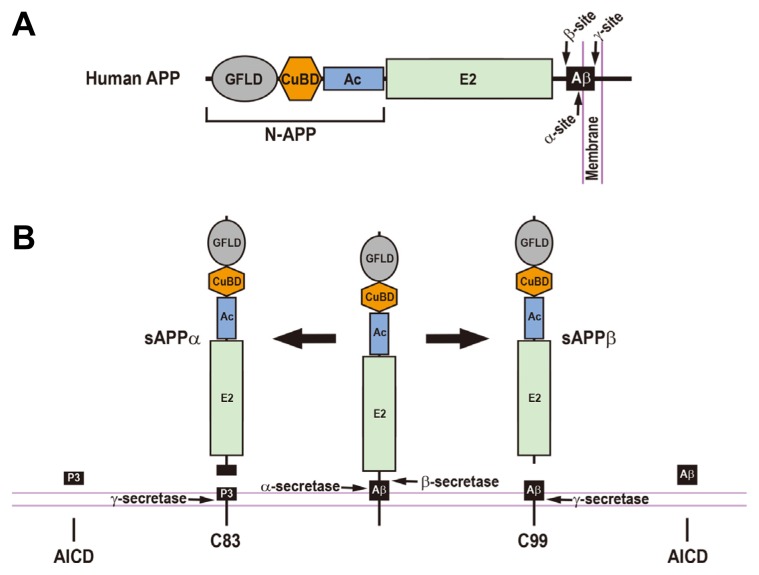
Aβs, which largely consist of peptides that are 40 and 42 amino acids long (Aβ40 and Aβ42), are produced by sequential APP proteolytic processes by β-secretase (BACE) and γ-secretase (Figs. 1A and 1B; Esler and Wolfe, 2001; Goedert, 2015; Reinhard et al., 2005); Aβ42 is more aggregation-prone and more neurotoxic than Aβ40 (Jarrett et al., 1993; Klein et al., 1999). In the amyloidogenic pathway, human APP is first cleaved by β-secretase, generating a large soluble ectodomain (sAPPβ) and the C-terminal C99 peptide (Fig. 1B), and proteolytic cleavages of the C99 peptide by γ-secretase release Aβs and the APP intracellular domain (AICD; Figs. 1B and 2). Alternatively, in the non-amyloidogenic pathway, human APP is sequentially cleaved by α-secretase and γ-secretase, creating a soluble ectodomain (sAPPα), P3 peptide, and the AICD (Fig. 1B). P3 peptide corresponds to Aβ17-40 and Aβ17-42 and is non-amyloidogenic (Fig. 2; Selkoe, 1998). Therefore, α- and β-secretases have opposing effects on generating Aβs due to their competitive cleavages of APP. In support of this, increased α-secretase activity through protein kinase C stimulation decreases Aβ generation (Hung et al., 1993; Lin et al., 1999; Skovronsky et al., 2000), whereas BACE1 knockout mice lack brain Aβs (Cai et al., 2001; Luo et al., 2001). γ-Secretase, a multi-protein complex that consists of at least four subunits—presenilin (PS), nicastrin, Aph-1, and Pen-2—functions as a transmembrane aspartyl protease that plays a critical role in not only generating Aβs but also determining Aβ42 to Aβ40 ratios (Bai et al., 2015; De Strooper, 2003; Esler and Wolfe, 2001). Consistent with this notion, PS1/PS2 double-null mutation resulted in the absence of Aβ production and complete inactivation of γ-secretase (Herreman et al., 2000; Zhang et al., 2000). The γ-secretase-mediated processing of APP is one of the best-characterized examples for regulated intra-membrane proteolysis (RIP) that is an evolutionarily conserved mechanism from bacteria to humans (Brown et al., 2000). RIP of APP can be directly modulated by several γ-secretase associated proteins including TMP21, pigeon homologue protein, and proton myo-inositol cotransporter (Chen et al., 2006; He et al., 2010; Teranishi et al., 2015; Wakabayashi et al., 2009; Zhou et al., 2005). Because the known prerequisite for a γ-secretase substrate is the release of its bulky extracellular domain (Esler and Wolfe, 2001; Struhl and Adachi, 1998), another regulation is indirectly mediated by competitive α-secretase and β-secretase functions that are required for shedding the APP extracellular domain. Therefore, RIP of APP by these secretases is responsible for Aβ production, and it also plays a critical role in determining the ratio of Aβ42 to Aβ40. Understanding the regulatory mechanisms and alterations in RIP of APP in AD patients is necessary for identifying novel therapeutic targets for treating AD.
Fig. 1.
The Regulated Proteolytic Processing of Human β-Amyloid Precursor Protein and the Resulting Cleavage Fragments.
(A) Schematic diagram of human β-amyloid precursor protein (APP). The arrows represent the cleavage sites by α-, β-, and γ-secretases. GFLD, growth factor-like domain; CuBD, copper-binding domain; Ac, acidic domain; E2, APP extracellular carbohydrate domain; Aβ, amyloid β-peptide; N-APP, a cleaved N-terminal fragment of APP. (B) Human APP can be processed through either amyloidogenic or non-amyloidogenic pathways. In the amyloidogenic pathway (right), the proteolytic cleavage of the APP by β-secretase produces a large soluble ectodomain of APP (sAPPβ) and a membrane-associated C-terminal fragment (C99). The C99 is subsequently cleaved by γ-secretase, releasing an amyloid β-peptide (Aβ) and an APP intracellular domain (AICD). In a non-amyloidogenic pathway (left), α-secretase-mediated cleavage of the APP generates a soluble ectodomain of APP (sAPPα) and a membrane-tethered C-terminal fragment (C83). The subsequent cleavage of the C83 by γ-secretase gives rise to a P3 peptide and an AICD.
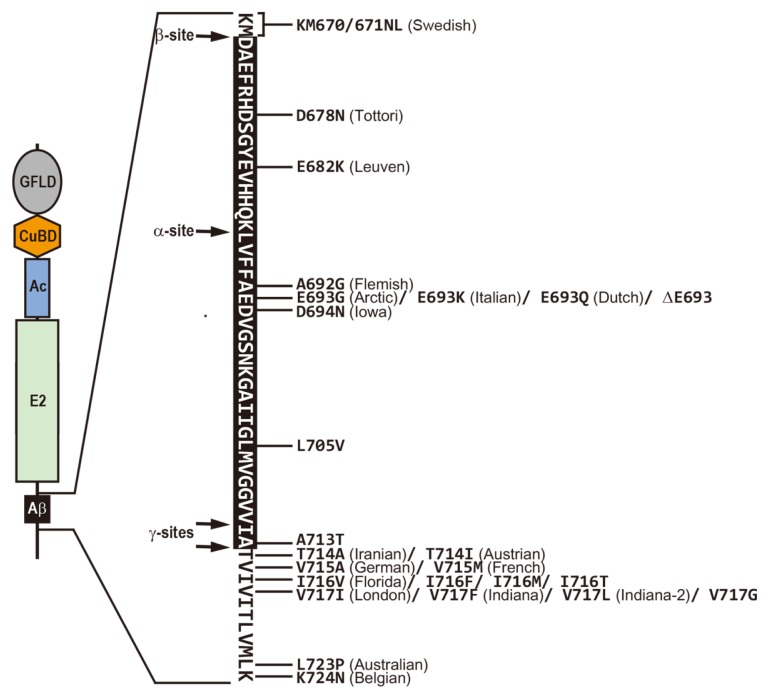
Fig. 2.
Human APP Mutations that Cause Familial Alzheimer’s Disease.
The amino acid sequence of the amyloid β-peptide (black box) and flanking transmembrane regions is presented; the horizontal arrows indicate the cleavage sites by α-, β-, andγ-secretases. Many missense and deletion mutations within the APP were discovered to cause an inherited form of Alzheimer’s disease.
PROTEOLYTIC FRAGMENTS OF β-AMYLOID PRECURSOR PROTEIN
Emerging evidence suggests that soluble Aβ oligomers are the major neurotoxic species and are responsible for the progressive neurodegeneration in AD (Lacor et al., 2007; Li et al., 2009; Lue et al., 1999; McLean et al., 1999; Shankar et al., 2008). The soluble Aβ oligomers were shown to disrupt cognitive function when injected intracerebroventricularly in rats (Cleary et al., 2005; Shankar et al., 2008). Consistent with this, impaired learning and memory were observed in transgenic mice that overexpress full-length human APP751 with the familial Swedish mutation and the Aβ dimer-stabilizing mutation (APS679C), leading to the generation of highly soluble Aβ dimers (Müller-Schiffmann et al., 2015). The soluble Aβ oligomers rapidly downregulate membrane expression of postsynaptic spine proteins including N-methyl-D-aspartate (NMDA) type glutamate and EphB2 receptors, leading to long-term potentiation (LTP) inhibition as well as enhancing long-term depression (LTD; Lacor et al., 2007; Shankar et al., 2008). Furthermore, the physiological functions of soluble Aβ40 and Aβ42 have been proposed to be involved in the control of cholesterol de novo synthesis and sphingomyelin levels, respectively (Grimm et al., 2005). Interestingly, proteolytic fragments of APP other than Aβ species were reported to have neuroprotective or neurotoxic activities (Chasseigneaux and Allinquant, 2012). Both sAPPα and sAPPβ were shown to mediate neuroprotective function, but the neuroprotective effect of sAPPα was approximately 100 times more potent than sAPPβ (Furukawa et al., 1996). The neuroprotective function of sAPPα is further supported by the finding that sAPPα knock-in mice showed almost complete restoration of the anatomical, behavioral, and electrophysiological abnormalities observed in APP knock-out mice (Ring et al., 2007). In contrast, neurotoxic sAPPβ activity was also reported in mammals. Neurotrophic factor depletion increases sAPPβ production and further induces proteolytic cleavage of sAPPβ, releasing a cleaved N-terminal fragment, N-APP, which triggers axon degeneration and neuronal cell death through binding to death receptor 6 (Fig. 1A; Nikolaev et al., 2009). The AICD, which is produced from both amyloidogenic and non-amyloidogenic pathways (Fig. 1B), has been implicated in the pathological process of AD (Nhan et al., 2016). AICD overexpression in transgenic mice was shown to induce AD-like pathological features including GSK-3β upregulation, tau hyperphosphorylation and its age-dependent aggregation, and hippocampal neurodegeneration (Ghosal et al., 2009; Ryan and Pimplikar, 2005). However, these AD-like phenotypes were not reproduced in independently generated transgenic mice that overexpressed the same AICD (Giliberto et al., 2010), and the cause of this discrepancy remains unclear. In contrast, impaired calcium signaling observed in APP−/− null-mutant cells can be restored by reintroducing an intact AICD, suggesting its functional role in calcium homeostasis (Hamid et al., 2007; Leissring et al., 2002). In addition, AICD overexpression in cultured cells resulted in cell death (Müller et al., 2008), and this apoptotic potential of AICD appears to be conserved between humans and Drosophila (Gunawardena and Goldstein, 2001; Hong et al., 2017; Wang et al., 2014). In summary, soluble Aβ oligomers are now considered to be the primary initiators of neurodegenerative AD processes. In addition, the neurotoxic activity of N-APP and AICD raises the possibility that soluble Aβ oligomers can collaborate with N-APP and AICD to accelerate neurodegeneration in AD patients (Fig. 3). Another possibility is that soluble Aβ oligomers antagonize the neuroprotective functions of sAPPα, sAPPβ, and AICD. One intriguing question is which receptors and signaling components directly respond to soluble Aβ oligomers and result in neuro-degeneration. Therefore, it will be important to identify the crucial genes that link soluble Aβ oligomers to synaptic impairment that correlates with dementia severity in AD.
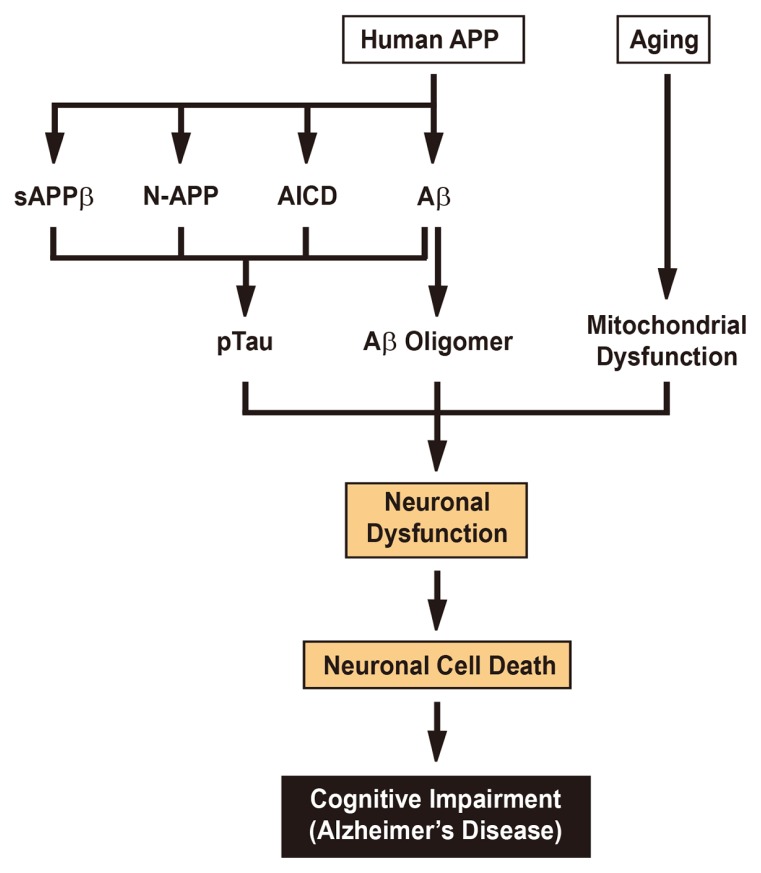
Fig. 3.
The Main Drivers of Neurodegeneration in Alzheimer’s Disease.
Neuronal dysfunction and cell death are responsible for the development of Alzheimer’s disease. Accumulating evidence suggests that abnormally elevated tau hyperphosphorylation, pathogenic Aβ oligomers, and mitochondrial dysfunction cooperate to drive the neuronal dysfunction and cell death that underlie cognitive impairment. Although the amyloid hypothesis supports that neurotoxic Aβ primarily induces tau pathology, other proteolytic fragments of the human APP including sAPPβ, N-APP, and AICD, also appear to contribute to tau alterations. In addition, aging, which is tightly associated with mitochondrial dysfunction, can serve as the most significant nongenetic risk factor for Alzheimer’s disease.
GENETIC BASIS OF ALZHEIMER’S DISEASE
The two major types of AD are familial and sporadic (Tanzi, 2012). Early-onset familial Alzheimer’s disease (EO-FAD), which accounts for less than 5% of AD, is a fully penetrant autosomal-dominant disorder resulting from rare mutations in APP, PS1 and PS2 (Tanzi, 2012). In contrast, late onset sporadic Alzheimer’s disease (LO-SAD), which represents most cases of AD, is likely influenced by the combined action of multiple genetic susceptibilities and environmental risk factors (Lambert and Amouyel, 2011). One major genetic risk factor for LO-SAD is the ɛ4 allele of the apolipoprotein E gene (APOE4), which plays a crucial role in regulating cholesterol metabolism (Corder et al., 1993; Strittmatter et al., 1993). The contribution of APOE4 to AD pathogenesis is associated with APOE4-mediated modulation of Aβ aggregation and clearance (Bu, 2009). Many additional genes have been identified as genetic risk factors for LO-SAD (Bu, 2009; Lambert et al., 2013; Small and Petsko, 2015; Tanzi, 2012). Although there are differences in the genetic pathological causes between EO-FAD and LO-SAD, they show remarkable similarities in their pathophysiology and clinical symptoms (Bateman et al., 2011). First, similar patterns of senile Aβ plaques, NFTs, and cerebral amyloid angiopathy are observed in both forms of AD. Second, structural and functional neuroimaging studies indicate that hippocampal and medial-temporal lobe atrophies are commonly found in both types of AD. Third, both AD forms share temporo-parietal hypometabolism and episodic memory and judgment impairment, which are responsible for cognitive deficits. In addition, myoclonus and seizures are also frequently observed. Finally, changes in biochemical markers including cerebrospinal fluid Aβ42, tau, and pTau, during the neurodegeneration process are also very similar in both AD forms. These similarities in neuropathology strongly suggest the presence of common pathogenic biochemical processes underlying both EO-FAD and LO-SAD. Therefore, knowledge and disease-modifying drugs for AD obtained from studies on EO-FAD could be useful in providing preventive treatments for LO-SAD.
To date, a total of 25 pathogenic mutations (except for duplications) in APP associated with EO-FAD have been defined (http://www.molgen.ua.ac.be/ADMutations/) (Fig. 2). Most missense mutations in APP are clustered near the proteolytic cleavage sites that are responsible for Aβ generation and clearance (Fig. 2), and they commonly increase the ratio of Aβ42 to Aβ40 (Tanzi, 2012). More than 200 mutations in PS1 and 16 in PS2 have been reported to cause EO-FAD (http://www.molgen.ua.ac.be/ADMutations/) (Tanzi, 2012). Pathogenic missense mutations in PS1 and PS2 shift the γ-secretase site from γ40 to γ42, resulting in an increase in the Aβ42 to Aβ40 ratio (De Strooper, 2007). Taken together, most missense mutations in APP, PS1, and PS2 associated with EO-FAD appear to converge at a higher Aβ42 to Aβ40 ratio, leading to elevated levels of neurotoxic Aβ oligomers, Aβ plaque deposition, and the development of AD.
Although it is widely accepted that the elevated levels of aggregation-prone Aβ including Aβ42 and the longer forms are the primary pathogenic drivers, cognitive impairment in people with AD correlates better with tau pathology than Aβ plaque deposition (Hardy and Selkoe, 2002; Spires-Jones and Hyman, 2014). Tau is a microtubule-associated protein that regulates the stability of microtubules (MTs) located in axons, thereby contributing to axonal transport and outgrowth (Ballatore et al., 2007). In taupathies including AD, fronto-temporal dementia (FTD), and Parkinsonism, tau is hyperphosphorylated, leading to its disengagement from the MTs and the formation of NFTs (Ballatore et al., 2007). However, mutations in the human tau, MAPT, which cause MAPT hyperphosphorylation and aggregation with lack of Aβ deposits, lead to FTD but not to AD (Hutton et al., 1998). In addition, neocortical NFTs, which are tightly associated with severe cognitive impairment, are consistently found in brains with AD, suggesting that Aβ pathology acts upstream of tau pathology (Nelson et al., 2012; Huang and Mucke, 2012; Fig. 3). Furthermore, reduction in endogenous tau levels prevents behavioral abnormalities and premature mortality observed in the AD mouse model that overexpresses human APP with a familial AD mutation, with no alternation in Aβ plaque deposition (Roberson et al., 2007). These observations demonstrate that Aβ- and tau-induced neurotoxicity collaborates to cause progressive neurodegeneration and cognitive impairment in AD (Fig. 3).
SYNAPTIC DYSFUNCTION AND NEURONAL CELL DEATH IN ALZHEIMER’S DISEASE
Synapse loss in the neocortex and hippocampus, which play a critical role in learning and memory (Kandel et al., 2014), is an early pathological hallmark of AD and correlates strongly with cognitive impairment (Arendt, 2009; DeKosky and Scheff, 1990; Terry et al., 1991). What are the cellular and molecular mechanisms underlying synapse loss? A great deal of evidence supports that soluble Aβ oligomers contribute to synapse dysfunction and loss by acting on multiple synaptic receptors including NMDA-type glutamate and α7-nicotinic acetylcholine (α7-nACh) receptors (Palop and Mucke, 2016; Spires-Jones and Hyman, 2014). NMDA receptors are required for generating both long-term potentiation and long-term depression but in distinct manners (Kandel et al., 2014). LTP is a synaptic plasticity mechanism that contributes to memory encoding in the hippocampus (Kandel et al., 2014); in contrast, LTD in the hippocampus is involved in memory decay (Kandel et al., 2014). NMDA receptor-induced large Ca2+ influx during LTP promotes the incorporation of αamino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA)-type glutamate receptors into synapses, leading to dendritic spine growth, whereas NMDA receptor-mediated reduced Ca2+ influx during LTD decreases the levels of AMPA receptors at the synapses and results in dendritic spine loss (Kandel et al., 2014). Several studies have shown that soluble Aβ oligomers inhibit LTP and induce progressive spine loss via downregulation of NMDA receptors, followed by synaptic removal of AMPA receptors (Chen et al., 2000; Cullen et al., 1997; Hsieh et al., 2006; Shankar et al., 2007; Walsh et al., 2002). In addition, soluble Aβ oligomers interfere with glutamate uptake at the synapses, resulting in enhanced LTD (Li et al., 2009). Consistent with this, NMDA receptor antagonists such as memantine and NitroMemantine can compromise Aβ-induced synaptic dysfunction and cognitive impairment (Figueiredo et al., 2013; Talantova et al., 2013; Zhu et al., 2013). Taken together, Aβ-mediated LTP inhibition, LTD enhancement, and synapse loss appear to underlie cognitive impairment in AD (Fig. 3; Palop and Mucke, 2010).
Soluble Aβ oligomers were shown to activate α7-nACh receptors expressed in Xenopus oocytes (Dineley et al., 2002). Interestingly, α7-nACh receptor blockade reduces the production of Aβ and Aβ-induced synapse loss, indicating the presence of positive feedback between α7-nACh receptors and Aβ (Wei et al., 2010). Furthermore, substantial loss of cholinergic neurons in the nucleus basalis of Meynert was observed in AD brains, supporting the cholinergic hypothesis (Bartus et al., 1982; Whitehouse et al., 1981). Indeed, cholinesterase inhibitors including donepezil, galantamine, and rivastigmine, which inhibit acetylcholine degradation, are used to treat patients with early-stage AD (Canter et al., 2016; Zhu et al., 2013).
Progressive and substantial neuronal cell death in vulnerable brain regions including the hippocampus, nucleus basalis, and entorhinal cortex is an another pathological hallmark of AD (Gómez-Isla et al., 1996; Simić et al., 1997; Whitehouse et al., 1981). Consistent with this, significant neuronal loss was observed in many different transgenic mouse models that commonly contain EO-FAD mutations in human full-length APP (Wirths and Bayer, 2010). In addition, overexpression of N-terminally truncated AβpE3-42 peptide, which lacks two N-terminal amino acids and carries a pyroglutamate at position 3, but not full-length Aβ42 in transgenic mice induces robust neuronal loss (McGowan et al., 2005; Wirths et al., 2009). Because tau abnormalities cause neuronal cell death (Gendron and Petrucelli, 2009), these findings suggest that neurotoxic Aβ and tau collaborate to mediate neuronal loss (Fig. 3). Neuronal cell death observed in AD is also associated with mitochondrial dysfunction (Knott et al., 2008; Reddy and Beal, 2008). Dynamin-related protein 1 was reported to mediate Aβ-related mitochondrial dysfunction and subsequent neuronal loss (Cho et al., 2009). In addition, a growing body of evidence suggests that mitochondrial dysfunction plays a critical role in the pathogenesis of AD (Reddy and Reddy, 2011; Swerdlow et al., 2010). Mitochondrial dysfunction in the brains of AD patients correlates with a wide range of mitochondrial abnormalities including mitochondrial DNA defects, decreased levels of mitochondrial enzyme activities, impaired mitochondrial trafficking, and increased oxidative stress (Reddy and Reddy, 2011; Swerdlow et al., 2010). Because mitochondria accumulate reactive oxygen species-induced damage with age, it seems that the most prominent nongenetic risk factor for AD, aging, in concert with soluble Aβ oligomers and pathological tau, contributes to AD pathogenesis through mitochondrial dysfunction (Fig. 3; Canter et al., 2016; Tu et al., 2014).
CONCLUSION
Tau pathology better correlates with cognitive decline in AD than does substantial Aβ plaque deposition, and the amyloid hypothesis suggests that the tau-mediated formation of NFTs observed in AD results from abnormally elevated Aβ. Recent evidence supports that soluble Aβ oligomers are the primary pathogenic drivers of neurodegeneration in AD and further proposes that soluble Aβ oligomers cooperate with pathological tau to progressively degenerate the learning and memory circuitry required for cognitive functions. In addition to neurotoxic Aβ and tau, the other critical risk factor for clinical onset of AD is aging; age-related mitochondrial damage appears to play an important role in Aβ/tau-induced neurodegeneration in AD. Even though great advances have been made in knowledge of the genetic and cellular mechanisms involved in the pathogenesis of AD, unfortunately, there is still no effective treatment that prevents, delays, and cures it. Therefore, better understanding of the pathological functions of neurotoxic Aβ oligomers and precise mechanisms of Aβ-mediated signaling cascades, in addition to defining the molecular link between Aβ/tau and aging, will help to guide therapeutic drug development for AD treatment.
ACKNOWLEDGMENTS
This study was supported by NRF-2013R1A1A4A01011329 (S.J.).
REFERENCES
- Arendt T. Synaptic degeneration in Alzheimer’s disease. Acta Neuropathol. 2009;118:167–179. doi: 10.1007/s00401-009-0536-x. [DOI] [PubMed] [Google Scholar]
- Bai X.C., Yan C., Yang G., Lu P., Ma D., Sun L., Zhou R., Scheres S.H., Shi Y. An atomic structure of human γ-secretase. Nature. 2015;525:212–217. doi: 10.1038/nature14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballatore C., Lee V.M., Trojanowski J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Bartus R.T., Dean R.L., 3rd, Beer B., Lippa A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bateman R.J., Aisen P.S., De Strooper B., Fox N.C., Lemere C.A., Ringman J.M., Salloway S., Sperling R.A., Windisch M., Xiong C. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M.S., Ye J., Rawson R.B., Goldstein J.L. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. doi: 10.1016/s0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Cai H., Wang Y., McCarthy D., Wen H., Borchelt D.R., Price D.L., Wong P.C. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer’s disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- Chasseigneaux S., Allinquant B. Functions of Aβ, sAPPα and sAPPβ : similarities and differences. J Neurochem. 2012;120:99–108. doi: 10.1111/j.1471-4159.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- Chen Q.S., Kagan B.L., Hirakura Y., Xie C.W. Impairment of hippocampal long-term potentiation by Alzheimer amyloid β-peptides. J Neurosci Res. 2000;60:65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Chen F., Hasegawa H., Schmitt-Ulms G., Kawarai T., Bohm C., Katayama T., Gu Y., Sanjo N., Glista M., Rogaeva E., et al. TMP21 is a presenilin complex component that modulates γ-secretase but not ε-secretase activity. Nature. 2006;440:1208–1212. doi: 10.1038/nature04667. [DOI] [PubMed] [Google Scholar]
- Cho D.H., Nakamura T., Fang J., Cieplak P., Godzik A., Gu Z., Lipton S.A. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary J.P., Walsh D.M., Hofmeister J.J., Shankar G.M., Kuskowski M.A., Selkoe D.J., Ashe K.H. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Corder E.H., Saunders A.M., Strittmatter W.J., Schmechel D.E., Gaskell P.C., Small G.W., Roses A.D., Haines J.L., Pericak-Vance M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cullen W.K., Suh Y.H., Anwyl R., Rowan M.J. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997;8:3213–3217. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- DeKosky S.T., Scheff S.W. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active γ-Secretase complex. Neuron. 2003;38:9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- De Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8:141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B., Karran E. The Cellular phase of Alzheimer’s disease. Cell. 2016;164:603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- Dineley K.T., Bell K.A., Bui D., Sweatt J.D. β-Amyloid peptide activates α7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- Esler W.P., Wolfe M.S. A portrait of Alzheimer secretases--new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- Figueiredo C.P., Clarke J.R., Ledo J.H., Ribeiro F.C., Costa C.V., Melo H.M., Mota-Sales A.P., Saraiva L.M., Klein W.L., Sebollela A., et al. Memantine rescues transient cognitive impairment caused by high-molecular-weight aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci. 2013;33:9626–9634. doi: 10.1523/JNEUROSCI.0482-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Sopher B.L., Rydel R.E., Begley J.G., Pham D.G., Martin G.M., Fox M., Mattson M.P. Increased activity-regulating and neuroprotective efficacy of α-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- Gendron T.F., Petrucelli L. The role of tau in neurodegeneration. Mol Neurodegener. 2009;4:13. doi: 10.1186/1750-1326-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal K., Vogt D.L., Liang M., Shen Y., Lamb B.T., Pimplikar S.W. Alzheimer’s disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci USA. 2009;106:18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giliberto L., d’Abramo C., Acker C.M., Davies P., D’Adamio L. Transgenic expression of the amyloid-β precursor protein-intracellular domain does not induce Alzheimer’s Disease-like traits in vivo. PLoS One. 2010;5:e11609. doi: 10.1371/journal.pone.0011609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349:1255555. doi: 10.1126/science.1255555. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M.G. A century of Alzheimer’s disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T., Price J.L., McKeel D.W., Jr, Morris J.C., Growdon J.H., Hyman B.T. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M.O., Grimm H.S., Pätzold A.J., Zinser E.G., Halonen R., Duering M., Tschäpe J.A., De Strooper B., Müller U., Shen J., et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-β and presenilin. Nat Cell Biol. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena S., Goldstein L.S. Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron. 2001;32:389–401. doi: 10.1016/s0896-6273(01)00496-2. [DOI] [PubMed] [Google Scholar]
- Hamid R., Kilger E., Willem M., Vassallo N., Kostka M., Bornhövd C., Reichert A.S., Kretzschmar H.A., Haass C., Herms J. Amyloid precursor protein intracellular domain modulates cellular calcium homeostasis and ATP content. J Neurochem. 2007;102:1264–1275. doi: 10.1111/j.1471-4159.2007.04627.x. [DOI] [PubMed] [Google Scholar]
- Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- He G., Luo W., Li P., Remmers C., Netzer W.J., Hendrick J., Bettayeb K., Flajolet M., Gorelick F., Wennogle L.P., et al. Gamma-secretase activating protein is a therapeutic target for Alzheimer’s disease. Nature. 2010;467:95–98. doi: 10.1038/nature09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A., Serneels L., Annaert W., Collen D., Schoonjans L., De Strooper B. Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- Hong Y.G., Roh S., Paik D., Jeong S. Development of a reporter system for in vivo monitoring of γ-secretase activity in Drosophila. Mol Cells. 2017;40:73–81. doi: 10.14348/molcells.2017.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H., Boehm J., Sato C., Iwatsubo T., Tomita T., Sisodia S., Malinow R. AMPAR removal underlies Ab-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung A.Y., Haass C., Nitsch R.M., Qiu W.Q., Citron M., Wurtman R.J., Growdon J.H., Selkoe D.J. Activation of protein kinase C inhibits cellular production of the amyloid β-protein. J Biol Chem. 1993;268:22959–22962. [PubMed] [Google Scholar]
- Hutton M., Lendon C.L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP–17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- IIhara Y., Nukina N., Miura R., Ogawara M. Phosphorylated tau protein is integrated into paired helical filaments in Alzheimer’s disease. J Biochem. 1986;99:1807–1810. doi: 10.1093/oxfordjournals.jbchem.a135662. [DOI] [PubMed] [Google Scholar]
- Jarrett J.T., Berger E.P., Lansbury P.T., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. doi: 10.1021/bi00069a001. [DOI] [PubMed] [Google Scholar]
- Kandel E.R., Dudai Y., Mayford M.R. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H.G., Unterbeck A., Salbaum J.M., Masters C.L., Grzeschik K.H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Klein A.M., Kowall N.W., Ferrante R.J. Neurotoxicity and oxidative damage of beta amyloid 1-42 versus beta amyloid 1-40 in the mouse cerebral cortex. Ann N Y Acad Sci. 1999;893:314–320. doi: 10.1111/j.1749-6632.1999.tb07845.x. [DOI] [PubMed] [Google Scholar]
- Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik K.S., Joachim C.L., Selkoe D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacor P.N., Buniel M.C., Furlow P.W., Clemente A.S., Velasco P.T., Wood M., Viola K.L., Klein W.L. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert J.C., Amouyel P. Genetics of Alzheimer’s disease: new evidences for an old hypothesis? Curr. Opin Genet Dev. 2011;21:295–301. doi: 10.1016/j.gde.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Lambert J.C., Ibrahim-Verbaas C.A., Harold D., Naj A.C., Sims R., Bellenguez C., DeStafano A.L., Bis J.C., Beecham G.W., Grenier-Boley B., et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leissring M.A., Murphy M.P., Mead T.R., Akbari Y., Sugarman M.C., Jannatipour M., Anliker B., Müller U., Saftig P., De Strooper B., et al. A physiologic signaling role for the γ-secretase-derived intracellular fragment of APP. Proc Natl Acad Sci USA. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Hong S., Shepardson N.E., Walsh D.M., Shankar G.M., Selkoe D. Soluble oligomers of amyloid b protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Georgievska B., Mattsson A., Isacson O. Cognitive changes and modified processing of amyloid precursor protein in the cortical and hippocampal system after cholinergic synapse loss and muscarinic receptor activation. Proc Natl Acad Sci USA. 1999;96:12108–12113. doi: 10.1073/pnas.96.21.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue L.F., Kuo Y.M., Roher A.E., Brachova L., Shen Y., Sue L., Beach T., Kurth J.H., Rydel R.E., Rogers J. Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Bolon B., Kahn S., Bennett B.D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- Masters C.L., Simms G., Weinman N.A., Multhaup G., McDonald B.L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci USA. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan E., Pickford F., Kim J., Onstead L., Eriksen J., Yu C., Skipper L., Murphy M.P., Beard J., Das P., et al. Aβ42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C.A., Cherny R.A., Fraser F.W., Fuller S.J., Smith M.J., Beyreuther K., Bush A.I., Masters C.L. Soluble pool of Aβ amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Müller T., Meyer H.E., Egensperger R., Marcus K. The amyloid precursor protein intracellular domain (AICD) as modulator of gene expression, apoptosis, and cytoskeletal dynamics-relevance for Alzheimer’s disease. Prog Neurobiol. 2008;85:393–406. doi: 10.1016/j.pneurobio.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Müller-Schiffmann A., Herring A., Abdel-Hafiz L., Chepkova A.N., Schäble S., Wedel D., Horn A.H., Sticht H., de Souza Silva M.A., Gottmann K., et al. Amyloid-β dimers in the absence of plaque pathology impair learning and synaptic plasticity. Brain. 2015;139:509–525. doi: 10.1093/brain/awv355. [DOI] [PubMed] [Google Scholar]
- Nelson P.T., Alafuzoff I., Bigio E.H., Bouras C., Braak H., Cairns N.J., Castellani R.J., Crain B.J., Davies P., Del Tredici K., et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhan H.S., Chiang K., Koo E.H. The multifaceted nature of amyloid precursor protein and its proteolytic fragments: friends and foes. Acta Neuropathol. 2016;129:1–19. doi: 10.1007/s00401-014-1347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A., McLaughlin T., O’Leary D.D., Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Palop J.J., Mucke L. Amyloid-β-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nat Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat Rev Neurosci. 2016;17:777–792. doi: 10.1038/nrn.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.H., Beal M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.H., Reddy T.P. Mitochondria as a therapeutic target for aging and neurodegenerative diseases. Curr Alzheimer Res. 2011;8:393–409. doi: 10.2174/156720511795745401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard C., Hébert S.S., De Strooper B. The amyloid-β precursor protein: integrating structure with biological function. EMBO J. 2005;24:3996–4006. doi: 10.1038/sj.emboj.7600860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring S., Weyer S.W., Kilian S.B., Waldron E., Pietrzik C.U., Filippov M.A., Herms J., Buchholz C., Eckman C.B., Korte M., et al. The secreted β-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Ryan K.A., Pimplikar S.W. Activation of GSK-3 and phosphorylation of CRMP2 in transgenic mice expressing APP intracellular domain. J Cell Biol. 2005;171:327–335. doi: 10.1083/jcb.200505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D.J. The cell biology of β-amyloid precursor protein and presenilin in Alzheimer’s disease. Trends Cell Biol. 1998;8:447–453. doi: 10.1016/s0962-8924(98)01363-4. [DOI] [PubMed] [Google Scholar]
- Shankar G.M., Bloodgood B.L., Townsend M., Walsh D.M., Selkoe D.J., Sabatini B.L. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar G.M., Li S., Mehta T.H., Garcia-Munoz A., Shepardson N.E., Smith I., Brett F.M., Farrell M.A., Rowan M.J., Lemere C.A., et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simić G., Kostović I., Winblad B., Bogdanović N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J Comp Neurol. 1997;379:482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Skovronsky D.M., Moore D.B., Milla M.E., Doms R.W., Lee V.M. Protein kinase C-dependent α-secretase competes withβ-secretase for cleavage of amyloid-β precursor protein in the trans-Golgi network. J Biol Chem. 2000;275:2568–2575. doi: 10.1074/jbc.275.4.2568. [DOI] [PubMed] [Google Scholar]
- Small S.A., Petsko G.A. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat Rev Neurosci. 2015;16:126–132. doi: 10.1038/nrn3896. [DOI] [PubMed] [Google Scholar]
- Spires-Jones T.L., Hyman B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron. 2014;82:756–771. doi: 10.1016/j.neuron.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter W.J., Saunders A.M., Schmechel D., Pericak-Vance M., Enghild J., Salvesen G.S., Roses A.D. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow R.H., Burns J.M., Khan S.M. The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimers Dis. 2010;20:265–279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talantova M., Sanz-Blasco S., Zhang X., Xia P., Akhtar M.W., Okamoto S., Dziewczapolski G., Nakamura T., Cao G., Pratt A.E., et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA. 2013;110:E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi R.E. The genetics of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006296. doi: 10.1101/cshperspect.a006296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., Hansen L.A., Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Tu S., Okamoto S., Lipton S.A., Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegener. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T., Craessaerts K., Bammens L., Bentahir M., Borgions F., Herdewijn P., Staes A., Timmerman E., Vandekerckhove J., Rubinstein E., et al. Analysis of the γ-secretase interactome and validation of its association with tetraspanin-enriched microdomains. Nat Cell Biol. 2009;11:1340–1346. doi: 10.1038/ncb1978. [DOI] [PubMed] [Google Scholar]
- Walsh D.M., Klyubin I., Fadeeva J.V., Cullen W.K., Anwyl R., Wolfe M.S., Rowan M.J., Selkoe D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang X., Wang Z., Chen Y., Huang X., Hu Y., Zhang R., Ho M.S., Xue L. FoxO mediates APP-induced AICD-dependent cell death. Cell Death Dis. 2014;5:e1233. doi: 10.1038/cddis.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W., Nguyen L.N., Kessels H.W., Hagiwara H., Sisodia S., Malinow R. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2010;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse P.J., Price D.L., Clark A.W., Coyle J.T., DeLong M.R. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- Wirths O., Breyhan H., Cynis H., Schilling S., Demuth H.U., Bayer T.A. Intraneuronal pyroglutamate-Abeta 3-42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;118:487–496. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirths O., Bayer T.A. Neuron loss in transgenic mouse models of Alzheimer’s disease. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/723782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Nadeau P., Song W., Donoviel D., Yuan M., Bernstein A., Yankner B.A. Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch–1. Nat Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- Zhou S., Zhou H., Walian P.J., Jap B.K. CD147 is a regulatory subunit of the γ-secretase complex in Alzheimer’s disease amyloid β-peptide production. Proc Natl Acad Sci USA. 2005;102:7499–7504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.W., Livote E.E., Scarmeas N., Albert M., Brandt J., Blacker D., Sano M., Stern Y. Long-term associations between cholinesterase inhibitors and memantine use and health outcomes among patients with Alzheimer’s disease. Alzheimers Dementia. 2013;9:733–740. doi: 10.1016/j.jalz.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]