Abstract
Postmenopausal atrophic vagina (PAV) is the thinning of the walls of the vagina and decreased lugae of the vagina. PAV is caused by decreased estrogen levels in postmenopausal women. However, the harmful effects of hormone replacement therapy (HRT) have resulted in considerable caution in its use. Various estrogen agonist treatment options are available. Vitamin D is influences the regulation of differentiation and proliferation of various cells, especially tissues lining stratified squamous epithelium, such as the vaginal epithelium. In this study, we hypothesized that vitamin D could provide an alternative and a safe treatment option for PAV by promoting the proliferation and differentiation of the vaginal epithelium. Thirty six patients were enrolled in this case-control study. Vitamin D associated proteins in a vitamin D and sex hormone treated vaginal epithelial cell line as well as normal and PAV tissues were measured. To confirm of cell-to-cell junction protein expression, cell line and tissue studies included RT-PCR, immunohistochemistry staining, and immunoblot analyses. The expression of cell-to-cell junction proteins was higher in women with symptoms of atrophic vagina tissue compared to women without the symptoms. Vitamin D stimulated the proliferation of the vaginal epithelium by activating p-RhoA and Erzin through the vitamin D receptor (VDR). The results suggest that vitamin D positively regulates cell-to-cell junction by increasing the VDR/p-RhoA/p-Ezrin pathway. This is the first study to verify the relationship of the expression of RhoA and Ezrin proteins in vaginal tissue of PAV.
Keywords: cholecalciferol, ezrin, rho A, vagina
INTRODUCTION
Postmenopausal atrophic vagina (PAV) is caused by a deficient maturation of vaginal mucosa secondary to a low level of estradiol (E2) or progesterone (P). PAV symptoms include irritation, itchiness and dryness in the vagina, which can lead to the thinning of the walls of vagina, dyspareunia, sexual dysfunction, and urinary incontinence. The North American Menopause Society and International Menopause Society reported that 10–50% of postmenopausal women were experiencing vaginal atrophy symptoms (Wills et al., 2012). An online survey in 2008 estimated that 45% (2,290 respondents) of postmenopausal women in the United States over 45 years of age felt or had formerly experienced vaginal discomfort (Kingsberg and Krychman, 2013). Postmenopausal women with atrophic vagina need to be provided non-hormonal or hormonal treatments to relieve the symptom and to increase their quality of life.
Menopausal symptoms in women are generally recommended for hormone replacement therapy (HRT). This updated statement further distinguishes the emerging differences in the therapeutic benefit-risk ratio between estrogen therapy (ET) and combined estrogen-progestogen therapy (EPT) at various ages and times since the onset of menopause (Suckling et al., 2006b). Ten to forty percent of postmenopausal women with symptomatic vaginal atrophy require estrogen or progesterone replacement therapy, which has proven efficacy (Yildirim et al., 2004b). However, tolerability and safety concerns with the reversal of low estrogen level in the body or substitution by other hormones have been raised, including the risks of various cancers and inflammation (Muhammad et al., 2013a). Estrogen-based HRT is effective in treating symptoms of vaginal atrophy in postmenopausal women (Suckling et al., 2006a). However, the harmful effects of HRT have also resulted in considerable caution in the manner in which it is currently use (Muhammad et al., 2013b). Menopause is the stage of a woman’s reproductive life that is accompanied by estrogen deficiency. This deficiency has many negative effects on the vaginal mucosa, such as dryness, irritation, and vaginal atrophy (Minkin et al., 2013; Reiter, 2013). Although vaginal estrogens are an effective treatment for vulvovaginal atrophy, there are concerns in some women with a history of breast or endometrial hyperplasia (Unfer et al., 2004). Another report claimed that systemic estrogen can increase the risks of thrombosis and breast cancer (Notelovitz et al., 2002). So, nonhormonal alternative treatment is necessary to alleviate the adverse effects of exogenous estrogen.
A non-estrogenic medications for PAV treatment is ospemifene, a tissue-specific estrogen receptor (ER) agonist/antagonist (Constantine et al., 2014). This treatment has a long-term (up to 1 year) safety profile without adverse effects (Portman et al., 2014). However, the drug has been linked with uterine cancer, blood clot formation, cataracts, and pulmonary embolism (Muhammad, Maznah et al., 2013a).
Vitamin D has an antitumor effect (Deeb et al., 2007). Many epidemiological studies that have sought to determine associations between vitamin D status and the risk and mortality rates of a number of cancers (Giovannucci et al., 2006; Schwartz and Skinner, 2007). Vitamin D participates in the regulation of differentiation and proliferation of various cells (Woo et al., 2015), especially tissues lining the stratified squamous epithelium, including the vaginal epithelium (Yildirim et al., 2004b). In a previous empirical study, it was reported that vitamin D not only improves vaginal symptoms, but also increases vaginal maturation value. However, improvement of effects of vaginal symptoms showed differences depending on usage of vitamin D (Yildirim et al., 2004c). In an animal model where menopause was induced, vitamin D increased the appearance of protein cornifin beta and induced the increase of vaginal epithelium (Abban et al., 2008). In addition, the researchers reported in the previous study that the appearance of vitamin D receptor, estrogen receptor, and ezrin in premenopausal vaginal epithelium, but there has been no direct molecular study on vitamin D and vagina (Kim et al., 2014). Vitamin D regulates Ezrin protein; one of the components of Moesin/Ezrin/Radixin/Merlin (MERM) proteins (Abban et al., 2008). Ezrin regulates actin-binding proteins responsible for plasma membrane interaction and cell-to-cell junction. Ezrin is extensively expressed in the vaginal wall, where it helps govern the strength and the flexibility of the tubular structure by modulating vaginal cell-to-cell junctions. The strong vaginal wall with the substantial cell-to-cell junctions regulates the vaginal microbial environment, including pH and flexibility (Fadiel et al., 2008).
Vitamin D receptor (VDR) is up-regulated by vitamin D. VDR is a nuclear receptor that is an upstream protein associated with Ezrin and the Ras homolog gene family (RhoA) (Mora et al., 2008; Zhang et al., 2013). VDR is mediated by calcium influx that activates RhoA. (Ordóñez-Morán et al., 2008) Ezrin is activated and influenced by the VDR, and is also involved in the formation of actin cytoskeleton and complex in human endothelial cells (Choi, 2012). RhoA is stimulated by calcium, and enables various cellular functions including regulation of cell motility, epithelial layer adhesion, cytokinesis, and cell polarity. The activated RhoA signals affect cellular structures, such as cell junction proteins (Ezrin), actin network, intermediate filaments, membrane vesicles, and integrin adhesion complexes (Kher and Worthylake, 2012). In addition, RhoA functions with downstream Ezrin proteins that stimulate epithelial cell proliferation, differentiation, and gene expression (Farkas et al., 2012).
In this study, we hypothesized that vitamin D is involved in vaginal epithelial proliferation and differentiation. We compared the effect of vitamin D on vaginal epithelial cell proliferation with female hormones. We measured the amount of vitamin D associated proteins in vitamin D and sex hormone treated vaginal epithelial cells as well as normal and post-menopausal atrophic vaginal tissues. We also examined the underlying mechanisms of vitamin D in improving atrophic vaginal structure in the human vagina.
MATERIALS AND METHODS
Tissue preparation
Thirty six patients were enrolled for this case control study-19 premenopausal women (PRE), 13 women with the PAV and 4 postmenopausal women without symptoms of atrophic vagina (PON), who either had hysterectomy, anterior repair, posterior repair or LeFort repair in a tertiary university hospital from July 2012 to January 2014. 13 PAV received vaginal cream or tablets for more than 2 months. The atrophic vagina tissue used in this study was acquired from a patient who received surgery for incomplete uterovaginal prolapse, and from patients who had atrophic vaginitis after menopause and received estrogen supplements. Exclusion criteria were detection of cancer in tissue examination, use of medicines for internal medical diseases at the time of specimen collection, and any consistent use vitamin D within the preceding three months. This study was approved by the relevant local Institutional Review Board. Fully informed consent was obtained from each patient before treatment. Patient information, including age, body weight, height, coexisting diseases, and drug prescriptions, were obtained from the electronic medical records. Vaginal tissues (0.5 × 0.5 cm) of menopausal women who underwent hysterectomy, anterior repair, posterior repair, or LeFort procedure were acquired for this study. Half the tissues were fixed using 4% paraformaldehyde and half were stored at −70°C. This study is registered at Clinical Research Information Service (CRIS, http://cris.nih.go.kr, KCT0001559).
Relative quantitative reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was purified from tissues using RNeasy mini kits following the manufacturer’s instructions (QIAGEN, USA). Concentration and purify of total RNA were determined using a NanoDrop 2000 apparatus (NanoDrop Technologies, USA). cDNA was synthesized according to the manufacturers’ instructions with the SensiFast™ cDNA Synthesis Kit (Bioline, Taunton, MA, USA). Synthesized cDNA was used for RT-PCR with EconoTaq PLUS Green 2X premix (Lucigen, USA). The sense and antisense primers used were: human RhoA: agc ttg tgg taa gac atg ctt g and gtg tcc cat aaa gcc aac tct ac; human Ezrin: tga ggc aga gaa gaa cga g and caa gta tgg cac aga tgg aag; for human glyceraldehyde-3-phosphate dehydrogenase (GAPDH): ctg agc tga acg gga agc tc and aaa ggt gga gtg ggt gt.
Immunohistochemical analyses
All vaginal tissues were fixed in 4% formaldehyde solution (PFA) and embedded in paraffin. The blocks were cut into 4 μm thick sections. Each section was dewaxed and rehydrated in xylene and a graded ethanol series. Slides were heated at 90–100°C in a microwave oven for 10 min with 0.1 M trisodium citrate buffer (pH 6.0) for antigen retrieval. After 1 h of cooling at room temperature, the slides were washed in Tris-buffered saline containing 0.1% (v/v) Tween 20 (TBS-T) three times for 5 min each wash. Peroxidase activity was blocked by exposure of the sections to 0.3% hydrogen peroxide for 30 min. The slides were washed three times with TBS-T prior to incubation with blocking buffer at room temperature for 1 h in a humidified chamber. The primary antibodies (anti-RhoA [total RhoA], anti-p-RhoA, anti-Ezrin [total Ezrin] or anti-p-Ezrin) were applied in a moist chamber overnight at 4°C. Binding of antibodies were identified immunfluorescence using an EXPOSE Mouse and Rabbit Specific Immunohistochemistry kit (Abcam, USA) containing secondary antibody bound to horseradish peroxidase and 3, 3′-diaminobenzidine as the peroxidase colorimetric substrate. The procedure was done as described by the manufacturer. Similar tissue sections were immune-stained with non-specific IgG as the negative control. Tissues with brown-colored staining were considered as positive. Background staining was not counted in any case.
Cell culture
VK2/E6E7 vaginal cell line (American Type Culture Collection, USA) were grown in keratinocyte serum-free medium (KSFM; GIBCO Life Technologies, USA) supplemented with 50 ug/ml bovine pituitary extract, 0.1 ng/mL epidermal growth factor, 1% penicillin/streptomycin, 1% fungi zone, and 0.4 mM CaCl2, at 37°C and 5% CO2. Cells were split to 1:3 at 60% confluence and stored in liquid nitrogen.
Cell viability
The effect of Vitamin D (1α,25-dihydroxyvitamin D3; Sigma-Aldrich Corp., USA) and estrogen (E2; Sigma-Aldrich Corp.) on VK2/E6E7 were measured in a viability assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich Corp.) assay. VK2/E6E7 cells per well were seeded at a density of 5 × 103 cells/100 μL in wells of a 96-well plate and incubated at 37°C for 24 h. After 24 h, the cells were maintained on starvation medium for 24 h before exposure to three different concentrations of Vitamin D ranging from 10−10 M to 10−7 M and E2 ranging from 10−9 M to 10−7 M for 48 h. MTT solution 40 μl (5 mg/ml in hosphate buffer saline, PBS) was supplied to each well and incubated at 37°C for 4 h. After incubation, the culture medium was removed, 100 μl of dimethyl sulfoxide (DMSO) was added to each well, and the plate was agitated at room temperature for 30 min to dissolve the precipitate. The absorbance was measured at wavelength of 565 nm using a microplate reader.
Small interfering (si)RNA transfection assay
VK2/E6E7 monolayers were suspended and treated with 0.05% trypsin (GenDEPOT, USA). Cells (3.5 × 105) were dispensed in wells of a 6-well plate and grown for 24 h to about 50% confluence in KSFM. The cells were treated with Lipofectamine 2000 (Invitrogen, USA) in opti-MEM medium (Gibco, USA) for 4 h. VDR siRNA templates were as follows: 5′-GCA UGU AGG AGA AUC CAA GCG AGG T-3′, 5′-GGC AGC GAA AAG AUG UAA ACA GUG C-3′, and 5′-ACA CUU GUA AGU UGC UAA ACG AGT C-3′. The VDR treatment group was transfected by 25 nM VDR siRNA using the instructions from Lipofectamine 2000 reagent. The cells were harvested 24 h later for verification of VDR mRNA and protein expression. Experiments were independently performed three times.
Real-time-PCR analysis
Total RNA was extracted from transfected VK2/E6E7 cells using Trizol Reagent, and RNA purity was determined by a ratio of 1.8–2.0 of absorbance. The RNA was then considered suitable for cDNA synthesis using the SensiFast™ cDNA Synthesis Kit and SensiFast™ SYBR Hi-Rox Kit (Bioline, UK) and Step-One Plus Real-time PCR system (Applied Biosystems, USA) were used for quantitative real-time PCR. The sense and antisense primers used for human VDR were 5′-cac tat tca cct gcc cct tc-3′, 5′-ctt cct ctg cac ttc ctc atc-3′.
Western blotting
The VK2/E6E7 was plated and grown to 70–80% confluence in 60 mm-diameter petri dishes. After 24 h, VK2/E6E7 was maintained in the aforementioned starvation medium for another 24 h before exposure to the established optimal concentrations of Vitamin D (10−8 M) and E2 (10−9 M). For immunodetection of each protein, VK2/E6E7 and tissues were harvested and lysed in lysis buffer consisting of 15 mM sodium chloride (NaCl), 1.0% Triton X-100, 0.5% desoxycholic acid sodium salt, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris (pH 8.0), Phosphatase Inhibitor Cocktail 2, 3 (Sigma-Aldrich) and 1 mM EDTA Protease Inhibitor Cocktail (Roche, Germany). After debris were removed at 12,659 × g, 4°C, the liquids were assembled, and protein concentration was determined by the DC protein assay (Bio-Rad Laboratories, USA). The protein lysate was separated by 8, 10 or 15% SDS-PAGE, and the resolved proteins were transferred electrophoretically onto a polyvinylidene fluoride membrane. Blots were blocked with skimmed milk 5% (w/v) in TBS-T) for 1 h at room temperature. After washing three times with TBS-T, each blot was incubated at 4°C overnight with antibody to anti-RhoA (total RhoA; 1:100, Santa Cruz Biotechnology, USA), anti-p-RhoA (phospho-RhoA; 1:100, Santa Cruz Biotechnology), anti-Ezrin (total Ezrin; 1:1000, Abcam) or anti-Thr567 phospho-Ezrin (p-Ezrin; 1:1000, Abcam) in a separate membrane. The membranes were further incubated with anti-mouse or rabbit IgG-conjugated with horseradish peroxidase for 1 h. Revelation was performed by enhanced chemiluminescence.
Statistical analyses
Data were entered into Excel (Microsoft 2007 Office; Microsoft, USA), and statistical analyses were performed using SPSS Ver. 14.0 (SPSS Inc, USA). Parametric data are presented as the mean (standard error) and non-parametric data are presented as the median (minimum – maximum). Continuous variables were analyzed by Mann-Whitney U test or Student’s t-test. More than three groups were compared using the Kruskal-Wallis test. It is considered to be statistically significant when the p-value was < 0.05. We also evaluated correlations between total RhoA/p-RhoA or total Ezrin/p-Ezrin expression using the Pearson chi-squared test.
RESULTS
Expression of p-RhoA and p-Ezrin in vaginal tissues
Clinical characteristics of patients who provided vaginal tissue samples are summarized in Table 1. There were no differences in weight and body mass index between the premenopausal (PRE), PAV, and normal postmenopausal (PON) groups, although the height of premenopausal women was higher than the other groups. However, the difference was minimal and had no clinical significance. The mean age in PRE, PAV, and PON group was 44.0, 69.6 and 72.0 years, respectively. There was no significant difference in the age between postmenopausal groups (p = 0.765; data not shown).
Table 1.
Clinical characteristics of the study subjects
| Age (years)* | Height (m)† | Weight (kg)‡ | BMI (kg/m2)§ | ||
|---|---|---|---|---|---|
|
|
|||||
| Mean | SE | Median | Median | Median | |
| PRE (n = 19) | 44.0 | 1.034 | 1.59 (1.50–1.65) | 60.90 (51.30–71.80) | 23.48 (20.04–28.58) |
| PAV (n = 13) | 69.6 | 2.217 | 1.50 (1.44–1.56) | 59.74 (49.70–67.30) | 25.61 (22.68–30.66) |
| PON (n=4) | 72.0 | 2.469 | 1.54 (1.41–1.55) | 55.90 (46.70–66.80) | 24.15 (22.46–27.77) |
P value by Kruskal-Wallis test:
0.000,
0.000,
0.437,
0.060
Body mass index = weight (kg)/height (m)2
SE: Standard Error, Median: Median (min–mix)
PRE; premenopausal women, PAV; postmenopausal women with atrophic vagina, PON; postmenopausal women without atrophic vagina
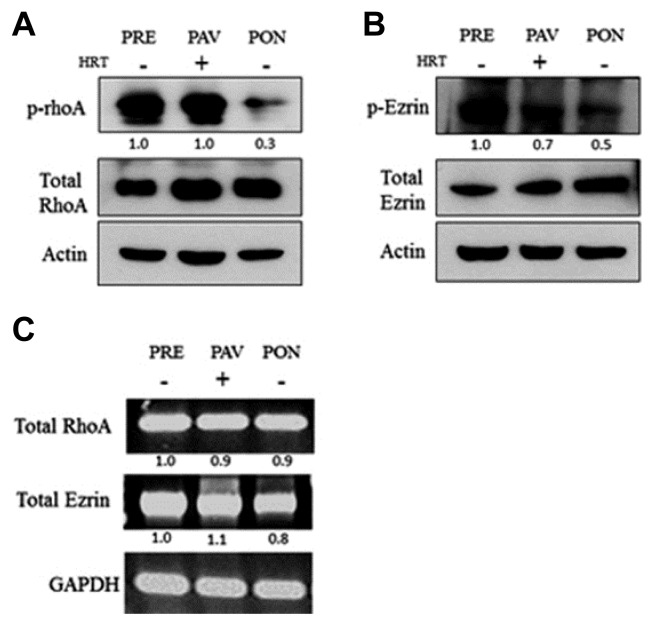
We evaluated whether activating RhoA and Ezrin was associated with PRE. To measure the expression of p-RhoA and p-Ezrin in the patients’ vaginal tissue samples, we performed Western blot, RT-PCR (Fig. 1), and immunohistochemistry (Fig. 2). The expression of p-RhoA and p-Ezrin was higher in PRE and PAV vaginal tissue compared to PON vaginal tissue. Interestingly, expression in PAV samples increased to levels similar to PRE upon estrogen hormone treatment. This suggests that the activation of RhoA and Ezrin may have regulated vaginal atrophy at the upstream initiation steps (Fig. 1).
Fig. 1.
Activation of RhoA and Ezrin in the vaginal tissue.
(A) Protein expression levels of p-RhoA, RhoA, and Actin in PRE, PAV, and PON were assessed by Western blotting. Shown is the average of three independent experiments, each performed in triplicate. Total RhoA and Actin was represented as the amount of the loaded protein. (B) Expression levels of p-Ezrin, Ezrin, and Actin in PRE, PAV, and PON were assessed by Western blotting. Data represent the average of three independent experiments, each performed in triplicate. Total Ezrin and Actin was represented as the amount of the loaded protein. (C) Expression levels of mRNA of total RhoA and Ezrin in PRE, PAV, and PON were assessed by RT-PCR.
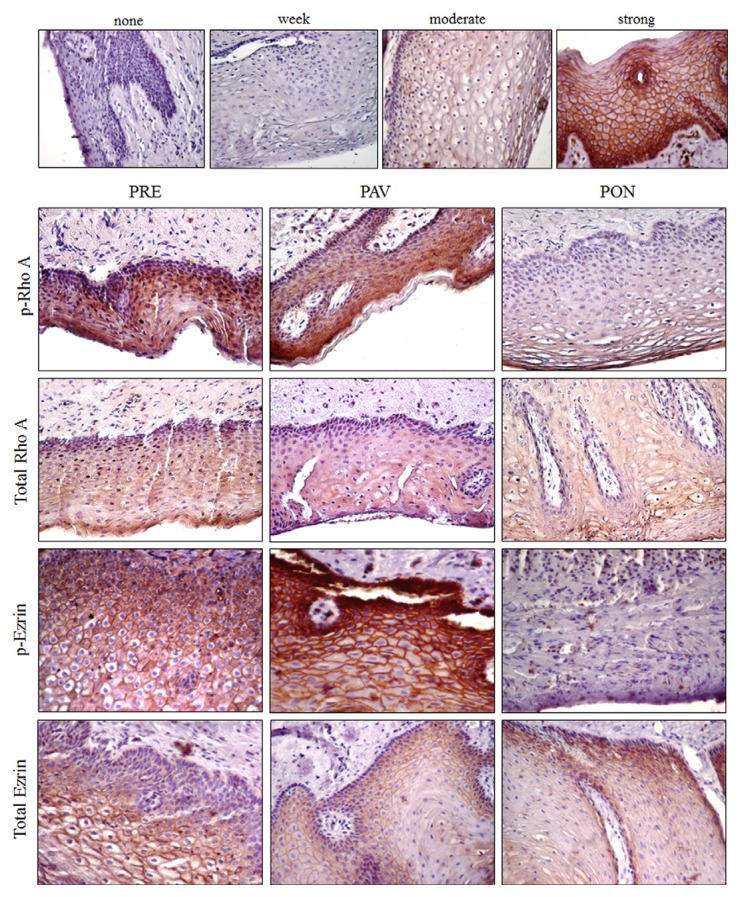
Fig. 2.
Expression of p-RhoA and p-Ezrin in the vaginal epithelium.
Immunohistochemisty demonstrate the expression, and the total amount of p-RhoA and p-Ezrin in each indicated vaginal tissue sample. The expression is recorded as none, weak, moderate, or strong.
Immunohistochemistry was done to evaluate the activation of RhoA and Ezrin in the vaginal epithelium of the PRE, PAV, and PON groups. First, the degree of expression of the positive cell in the epithelium was quantified using the H-scoring method (Fig. 2, upper panel): none (< 5% positive cells), weak (5–10% positive cells), moderate (10–30% positive cells), and strong (> 30% positive cells). PRE and PAV moderately increased the level of p-Rho A according to this H-score. In contrast, the PON group weakly expressed p-RhoA and p-Ezrin, which were localized in the vaginal epithelium. The immunohistochemistry results (Fig. 2) showed that the expression of total RhoA and Ezrin was similar in the vaginal epithelium of all indicated samples that showed the same result as Western blot in Fig. 1.
Vitamin D and E2 induce proliferation of vaginal epithelium cell line VK2/E6E7
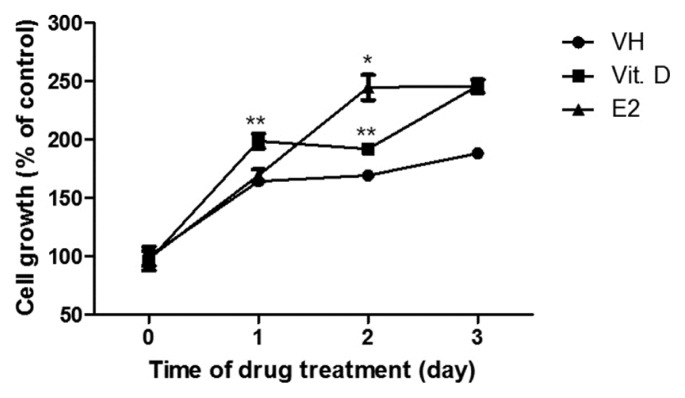
Proliferation of the vaginal epithelium could affect vaginal atrophy. Although recent reports have suggested that vitamin D could regulate cell growth, the role of vitamin D in the vaginal epithelium remains unknown. To evaluate whether vitamin D induces proliferation of the vaginal epithelium, VK2/E6E7 vaginal epithelium cells were treated with vitamin D (10−8 M) and E2, and cell viability was measured 3 days later. The viability of the cells treated with vitamin D continuously for 3 days was significantly increased compared to that of the control group (VH). Cell viability was enhanced 48 h after treatment with E2 (Fig. 3). Therefore, 10−8 M vitamin D was used as an optimal concentration for vaginal epithelium proliferation.
Fig. 3.
Effects of vitamin D and estrogen on vaginal epithelial cell proliferation.
Cells were seeded at 2 × 103 cells/wells in six-well plates and were incubated at 37°C. Cell viability was measured at the indicated times by the MTT assay. Data are shown as the average of 3 independent experiments, each performed in triplicate. The results are expressed as mean ± SEM. Student’s t-test: *p < 0.05 and **p < 0.01
Effects of vitamin D on expression of p-RhoA and p-Ezrin in VK2/E6E7
Vitamin D regulates cell proliferation. But, how vitamin D affects the growth of vaginal epithelium through the VDR is unclear. To ensure a functional connection between vitamin D, p-RhoA, and p-Ezrin in VK2/E6E7, we suppressed the expression of VDR using siRNA in VK2/E6E7 cells. Three different target sites for suppressing VDR were tested for their effectiveness to knockdown vdr using commercially available siRNAs. VDR expression was measured using real-time PCR (Fig. 4). The analysis revealed that siRNA 3 greatly reduced the expression of VDR (approximately 85%) compared to scramble controls in VK2/E6E7. Therefore, we used siRNA 3 to suppress the VDR gene to evaluate the role of vitamin D in VK2/E6E7.
Fig. 4.
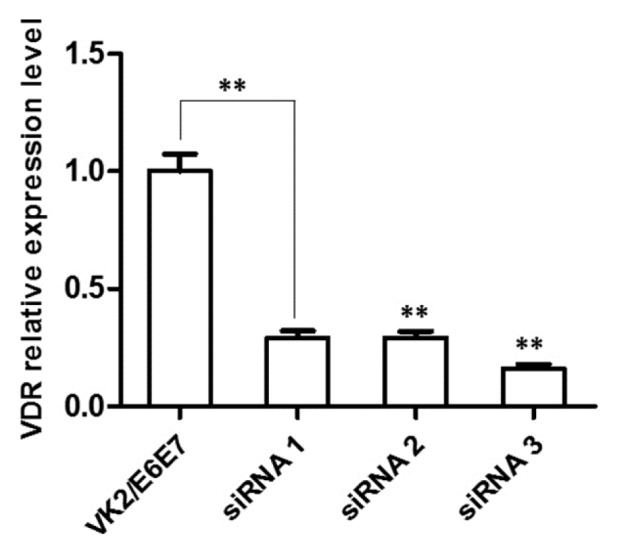
VDR knock-down experiments through siRNA.
Quantification of expression levels of VDR in cells treated with siRNAs that recognize different sites of gene. Data are expressed as the average of three independent experiments, each performed in triplicate. Mean ± SEM. Student’s t-test: *p < 0.05 and **p < 0.01.
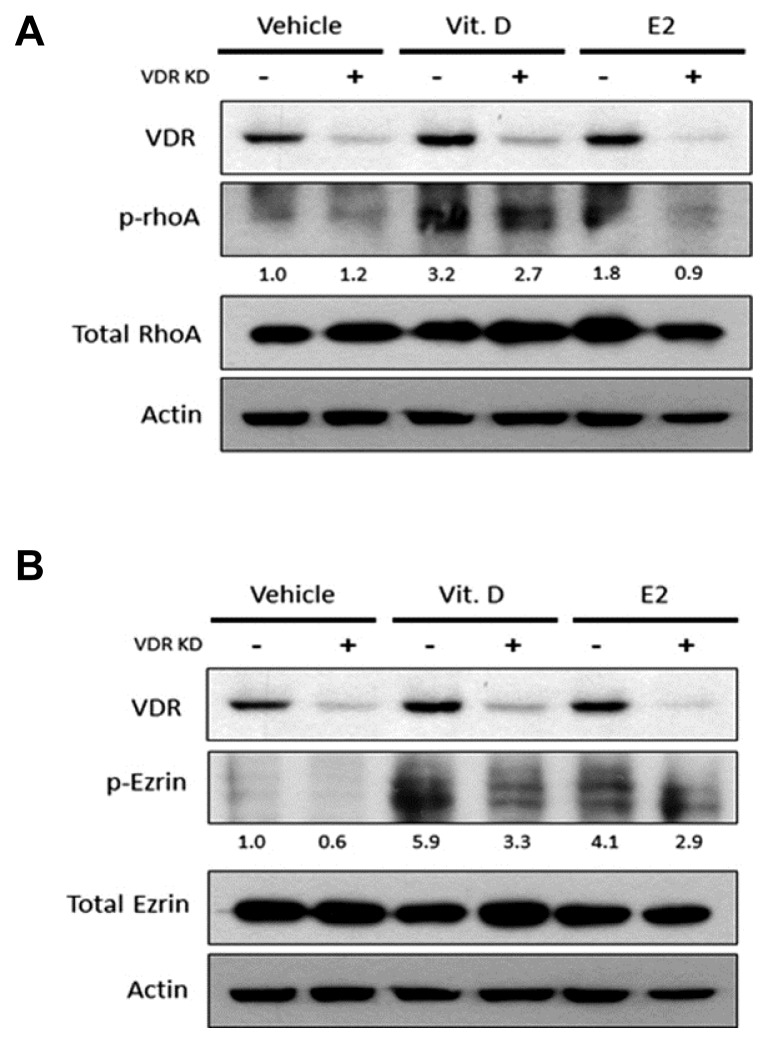
It is unclear if the vitamin D-mediated up-regulation of cell-to-cell junction is directly involved with the increased expressions of p-RhoA and p-Ezrin. To investigate, we treated VK2/E6E7 cells with siRNA3, and performed Western bolt analysis to examine the level of expression of p-RhoA and p-Ezrin. The analysis revealed that the expression of VDR increased in vitamin D treated wild-type (VDR KD -) VK2/E6E7 cells, which enhanced the phosphorylation of RhoA and Ezrin compared to the vehicle control. However, in the VDR knockdown VK2/E6E7, p-RhoA and p-Ezrin were less activated after vitamin D or E2 treatment (Fig. 5). This suggested that vitamin D might positively regulate cell-to-cell junction by increasing activity of the VDR/p-RhoA/p-Ezrin pathway.
Fig. 5.
Activation of RhoA and Ezrin in the vaginal epithelial cell line.
WT and VDR knockdown cells were treated with vitamin D and estrogen. Protein levels of VDR and (A) phosphorylation status of RhoA, (B) phosphorylation status of Ezrin were visualized by Western blotting.
DISCUSSION
The results demonstrate three things. First, phosphorylation of RhoA and Ezrin decreased in PON vaginal tissue sample compared to that found in PRE sample. Second, hormone treatment activated p-RhoA and p-Ezrin in PAV sample to levels similar to PRE. Third, vitamin D mediated the activation of RhoA and Ezrin through the VDR. The collective results implicate vitamin D as a promising therapeutic candidate for treatment of vaginal atrophy.
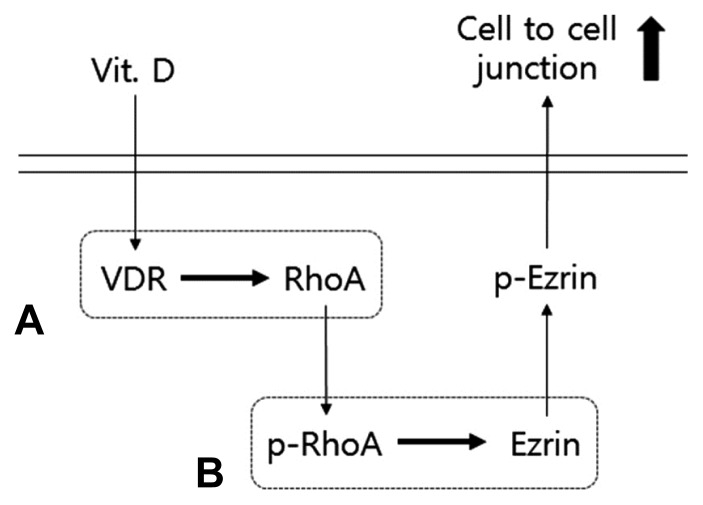
Vitamin D regulates cellular responses through the phosphorylation of RhoA kinases, membrane and cytoplasmic proteins, and nuclear receptors, such as the VDR. VDR-RhoA-Ezrin pathway is a major regulatory pathway that regulates proliferation, differentiation, and survival of the vaginal epithelium. Vitamin D and the VDR form a complex that binds to the vitamin D response element of genes facilitating transcription of genes responsible for calcium transfer in the cytoplasm. In the formation of stress fibers, activated RhoA and Ezrin regulate the actin cytoskeleton (Kosova et al., 2015). Calcium affects cell-to-cell junctions by phosphorylation of RhoA by RhoA kinase (Ordóñez-Morán, Larriba et al., 2008). Presently, RhoA kinase mediated p-Ezrin and Ezrin, which play crucial roles in the cell-to-cell junction in vaginal tissues (Fig. 6).
Fig. 6.
Classical action of Vitamin D on cell to cell junction via VDR-RhoA-Ezrin pathway in the vaginal epithelial cells.
(A) Binding of Vitamin D to the VDR affects RhoA. (B) Through activation and phosphorylation of the small GDP-binding protein RhoA, phosphorylated RhoA increases RhoA kinase activity, which leads to Ezrin phosphorylation. The activated Erin is related with modification of cell-to-cell junction.
Vitamin D affects the activity of VDR in the vagina. VDR has a similar effect to E2 in cell proliferation and differentiation of vaginal epithelium in rats (Yildirim et al., 2004a). RhoA activation is crucial for regulation of adherens junction proteins in differentiated epithelial cells. (Ordóñez-Morán, Larriba et al., 2008) Ezrin is an adherens junction protein that is associated with RhoA activation in osteosarcoma and breast cancer (Chiappetta et al., 2014; Vasco et al., 2015). It was previously reported that estrogen affects a different pathway in the vagina epithelium to VDR-RhoA-Ezrin pathway (Gao et al., 2015). When PON vaginal tissue samples were treated with E2, immunohistochemistry analysis showed the high expression of inactivated RhoA and Ezrin. This supports the view that the mechanism of E2 influence in the vaginal epithelium is different from that of vitamin D.
Our results suggest that vitamin D can be a promising alternative treatment modality for PAV. We demonstrated a superior effect of vitamin D on vaginal epithelization compared to estrogen when tested with a vaginal cell line and human vaginal tissue samples. There are several limitations in our study. The number of tissue samples of premenopausal and postmenopausal women was relatively small. It was not determined whether the expression of RhoA and Ezrin could have been affected by the severity of PAV.
However, to our knowledge, this paper is the first study to verify the relation of the expression of RhoA and Ezrin proteins in vaginal tissue of PAV, and influence of vitamin D and female hormones.
In conclusion, our study suggests that vitamin D influences the proliferation of vagina epithelial layer through VDR-RhoA-Ezrin pathway. Vitamin D may be an alternative therapeutic modality for PAV.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2014R1A1A3049819).
REFERENCES
- Abban G., Yilidirim N.B., Jetten A.M. Regulation of the vitamin D receptor and cornifin beta expression in vaginal epithelium of the rats through vitamin D3. Eur J Histochem. 2008;52:107–114. doi: 10.4081/1200. [DOI] [PubMed] [Google Scholar]
- Chiappetta C., Leopizzi M., Censi F., Puggioni C., Petrozza V., Rocca C.D., Di Cristofano C. Correlation of the Rac1/RhoA pathway with ezrin expression in osteosarcoma. Appl Immunohistochem Mol Morphol. 2014;22:162–170. doi: 10.1097/PDM.0000000000000033. [DOI] [PubMed] [Google Scholar]
- Choi S.D. Ezrin is an essential marker for metastasis of gynecologic cancer. J Korean Society of Menopause. 2012;18:81–93. [Google Scholar]
- Constantine G., Graham S., Koltun W.D., Kingsberg S.A. Assessment of ospemifene or lubricants on clinical signs of VVA. J Sex Med. 2014;11:1033–1041. doi: 10.1111/jsm.12428. [DOI] [PubMed] [Google Scholar]
- Deeb K.K., Trump D.L., Johnson C.S. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- Fadiel A., Lee H., Demir N., Richman S., Iwasaki A., Connell K., Naftolin F. Ezrin is a key element in the human vagina. Maturitas. 2008;60:31–41. doi: 10.1016/j.maturitas.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Farkas A.E., Capaldo C.T., Nusrat A. Regulation of epithelial proliferation by tight junction proteins. Ann N Y Acad Sci. 2012;1258:115–124. doi: 10.1111/j.1749-6632.2012.06556.x. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Liu Y., Rimm E.B., Hollis B.W., Fuchs C.S., Stampfer M.J., Willett W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Institute. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- Kher S.S., Worthylake R.A. Nuanced junctional RhoA activity. Nat Cell Biol. 2012;14:784–786. doi: 10.1038/ncb2553. [DOI] [PubMed] [Google Scholar]
- Kim T.H., Lee H.H., Park J. Immunohistochemical detection of the 1, 25-dihydroxy vitamin D receptor in the human vagina. Iran J Reprod Med. 2014;12:805–810. [PMC free article] [PubMed] [Google Scholar]
- Kingsberg S.A., Krychman M.L. Resistance and barriers to local estrogen therapy in women with atrophic vaginitis. J Sex Med. 2013;10:1567–1574. doi: 10.1111/jsm.12120. [DOI] [PubMed] [Google Scholar]
- Kosova G., Stephenson M.D., Lynch V.J., Ober C. Evolutionary forward genomics reveals novel insights into the genes and pathways dysregulated in recurrent early pregnancy loss. Hum Reprod. 2015;30:519–529. doi: 10.1093/humrep/deu355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkin M.J., Maamari R., Reiter S. Improved compliance and patient satisfaction with estradiol vaginal tablets in postmenopausal women previously treated with another local estrogen therapy. Int J Womens Health. 2013;5:133–139. doi: 10.2147/IJWH.S41897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora J.R., Iwata M., Von Andrian U.H. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S.I., Maznah I., Mahmud R.B., Saeed M.I., Imam M.U., Ishaka A. Estrogen receptor modulatory effects of germinated brown rice bioactives in the uterus of rats through the regulation of estrogen-induced genes. Drug Des Devel Ther. 2013a;7:1409–1420. doi: 10.2147/DDDT.S50861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notelovitz M., Funk S., Nanavati N., Mazzeo M. Estradiol absorption from vaginal tablets in postmenopausal women. Obstet Gynecol. 2002;99:556–562. doi: 10.1016/s0029-7844(01)01385-0. [DOI] [PubMed] [Google Scholar]
- Ordóñez-Morán P., Larriba M.J., Pálmer H.G., Valero R.A., Barbáchano A., Duñach M., de Herreros A.G., Villalobos C., Berciano M.T., Lafarga M. RhoA-ROCK and p38MAPK-MSK1 mediate vitamin D effects on gene expression, phenotype, and Wnt pathway in colon cancer cells. J Cell Biol. 2008;183:697–710. doi: 10.1083/jcb.200803020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman D., Palacios S., Nappi R., Mueck A. Ospemifene, a non-oestrogen selective oestrogen receptor modulator for the treatment of vaginal dryness associated with postmenopausal vulvar and vaginal atrophy: a randomised, placebo-controlled, phase III trial. Maturitas. 2014;78:91–98. doi: 10.1016/j.maturitas.2014.02.015. [DOI] [PubMed] [Google Scholar]
- Reiter S. Barriers to effective treatment of vaginal atrophy with local estrogen therapy. Int J Gen Med. 2013;6:153–158. doi: 10.2147/IJGM.S43192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G.G., Skinner H.G. Vitamin D status and cancer: new insights. Curr OpinClin Nutr Metab Care. 2007;10:6–11. doi: 10.1097/MCO.0b013e328011aa60. [DOI] [PubMed] [Google Scholar]
- Suckling J., Lethaby A., Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006a;31:Cd001500. doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- Suckling J.A., Kennedy R., Lethaby A., Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006b;18:Cd001500. doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- Unfer V., Casini M.L., Costabile L., Mignosa M., Gerli S., Di Renzo G.C. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril. 2004;82:145–148. doi: 10.1016/j.fertnstert.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Vasco V.L., Leopizzi M., Della Rocca C. Ezrin-related Phosphoinositide pathway modifies RhoA and Rac1 in human osteosarcoma cell lines. J Cell Commun Signal. 2015;9:55–62. doi: 10.1007/s12079-015-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills S., Ravipati A., Venuturumilli P., Kresge C., Folkerd E., Dowsett M., Hayes D.F., Decker D.A. Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Practice. 2012;8:144–148. doi: 10.1200/JOP.2011.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S.M., Lim H.S., Jeong K.Y., Kim S.M., Kim W.J., Jung J.Y. Vitamin D promotes odontogenic differentiation of human dental pulp cells via ERK activation. Mol Cells. 2015;38:604–609. doi: 10.14348/molcells.2015.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim B., Abban G., Erdogan B.S. Immunohistochemical detection of 1,25-dihydroxyvitamin D receptor in rat vaginal epithelium. Fertil Steril. 2004a;82:1602–1608. doi: 10.1016/j.fertnstert.2004.07.949. [DOI] [PubMed] [Google Scholar]
- Yildirim B., Kaleli B., Düzcan E., Topuz O. The effects of postmenopausal Vitamin D treatment on vaginal atrophy. Maturitas. 2004b;49:334–337. doi: 10.1016/j.maturitas.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y.G., Wu S., Sun J. Vitamin D, vitamin D receptor and tissue barriers. Tissue Barriers. 2013;1:e23118. doi: 10.4161/tisb.23118. [DOI] [PMC free article] [PubMed] [Google Scholar]