Abstract
The evaluation of venous drainage patterns prior to surgery for skull base meningioma is important owing to their deep location and the vulnerability of surrounding vascular structures. In recent years, the microsurgical skull base approach has matured as a surgical technique, making it an important option for reducing complications related to skull base meningioma surgery. In addition, knowledge of the venous anatomy can prevent venous drainage route disturbance and potentially life-threatening complications. Hence, this topic review aimed to provide an overview of normal venous anatomy as it relates to the microsurgical skull base approach, discuss known changes in venous drainage routes that are associated with the progression of skull base meningioma and the selection of an appropriate operative approach with the highest likelihood of preserving venous drainage structures.
Keywords: intracranial venous circulation, skull base meningioma, skull base approach, transpetrosal approach
Introduction
The basic principal of a skull base surgical approach is to avoid brain compression by extending the bone resection. However, bridging veins and sinuses limit the approach window and can sometimes lead to unpredictable venous complications that disrupt venous circulation. Anatomical knowledge of the venous circulation is vital for neurosurgeons; however, the intracranial venous route is highly complicated.
Damage to the venous circulation is not usually a problem in surgery for anterior cranial fossa meningioma, except in cases of anterior clinoidal meningioma associated with the drainage route of the superficial middle cerebral vein (SMCV). However, for middle and posterior cranial fossa meningioma, the presence of many bridging veins and sinuses requires that neurosurgeons have the knowledge and capability of preserving these venous drainage routes during surgery. Thus, this review is focused on the anatomy of the venous circulation in the middle and posterior cranial fossa.
Overview of the intracranial venous circulation
The intracranial venous circulation system is divided into the supratentorial and infratentorial venous systems. The supratentorial venous system is formed by the superficial and deep cerebral venous systems, connected by the medullary vein. The medullary vein consists of the superficial medullary vein, deep medullary vein, and transmedullary vein. The superficial and deep medullary veins collect venous flow from the pial and subependymal veins. The transmedullary vein connects the superficial and deep medullary veins, and sometimes serves as a collateral route when tumor progression disturbs the venous circulation to the superior sagittal sinus. The superficial cerebral veins collect into four groups of bridging veins: (1) the superior sagittal group that drains into the superior sagittal sinus, (2) the sphenoidal group that drains into the sphenoparietal or cavernous sinus (CS), (3) the tentorial group that converges on the sinuses in the tentorium, and (4) the falcine group that drains into the inferior sagittal or straight sinus.1) The superficial cerebral veins include three major anastomotic veins: the vein of Trolard, which is the largest anastomotic vein connecting to the superior sagittal sinus; the vein of Labbé, which is the largest anastomotic vein connecting to the transverse sinus; and the SMCV. The deep cerebral veins collect into channels that course through the walls of the ventricles and basal cisterns and converge on the internal cerebral, basal, and great veins.
Delion et al.2) similarly classified the infratentorial venous system in terms of superficial and deep veins. Within this system, the superficial veins travel along the surface of the cerebellum and drain the cerebellar cortex. In contrast, the deep veins traverse the fissures between the cerebellum and brainstem. These veins are divided into three groups: (1) the superior group that drains into the great vein, (2) the posterior group that drains into the transtentorial sinus, and (3) the lateral group that drains into the superior petrosal sinus (SPS).
The most unique feature of the intracranial venous circulation is that the intracranial vein does not have a venous valve; as a result, venous blood flow can drain in both directions. Moreover, the cortical veins are linked by numerous anastomoses, allowing the development of a collateral circulation and potentially explaining the good prognoses of some cerebral venous thromboses. These features complicate our understanding of the intracranial venous circulation. Normal venous drainage patterns have been reported in previous studies.3–5) In particular, angiography, 3-dimensional computed tomographic venography (3D-CTV) and magnetic resonance imaging have proven useful for evaluating the preoperative venous circulation.5–12) 3D-CTV is useful for understanding the anatomical relationship between venous structure and bone structure; however, neurosurgeons should pay careful attention to the middle cranial fossa vein because the determination of whether this vein runs along the surface of basal plane of the temporal lobe or along the interdural space of the middle cranial fossa is sometimes difficult. In the next chapter, we provide more detailed discussions of each venous circulation system relevant to skull base approaches.
Sphenoidal Group
SMCV
SMCV variation is based on the relative development of anastomoses. Drainage of the SMCV was classified by Hacker to include the sphenoparietal sinus, sphenobasal vein, sphenopetrosal vein, or the cortical veins in the absence of a definitive SMCV.13) Suzuki et al. reclassified SMCV drainage into seven patterns using 3D-CTV (Fig. 1);5) these reclassified patterns are useful for understanding venous drainage in the context of surgery, as the preservation of these veins is considered necessary to avoid injury.14)
Fig. 1.
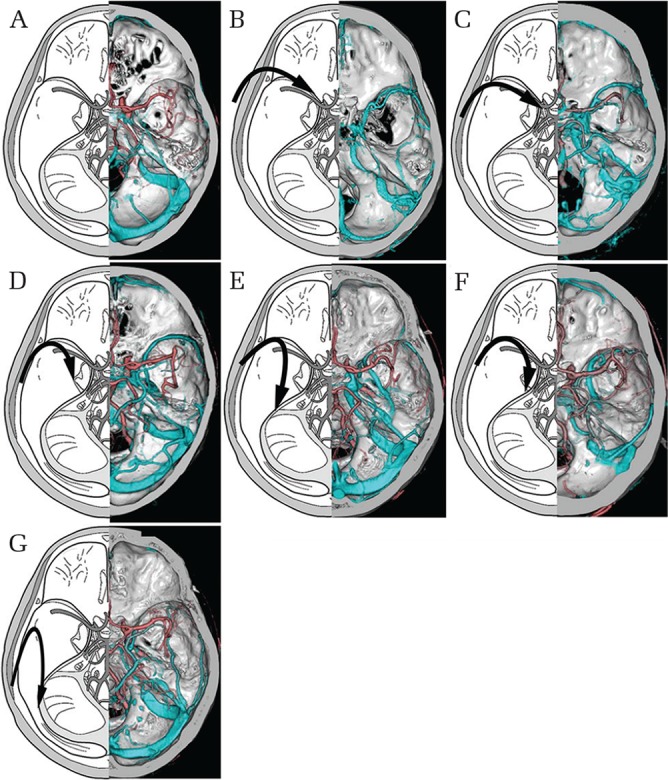
Schematic depictions of superficial middle cerebral vein (SMCV) drainage patterns. (A) Undeveloped type: the SMCV is absent. (B) Sphenoparietal sinus type: the SMCV enters the CS through the sphenoparietal sinus. (C) Cavernous capture type: the SMCV enters the anterior end of the CS directly. (D) Emissary type: the SMCV courses along the lesser wing and turns inferiorly to reach the pterygoid plexus through the middle cranial fossa basal foramen. (E) Basal type: the SMCV runs along the Sylvian fissure, turns downward posteriorly along the middle cranial fossa, and runs along its floor lateral to the foramen ovale to join the transverse sinus. (F) Superior petrosal type: the SMCV runs along the lesser wing, turns downward posteriorly without connecting with the sphenoparietal sinus or CS, runs along the middle of the cranial floor medial to the foramen ovale and lateral to the CS, and joins the SPS. (G) Squamosal type: the SMCV turns directly backward along the inner aspect of the temporal squama without turning medially to connect with the sinus and runs posteriorly to connect with the transverse sinus or lateral tentorial sinus. (H) Combined type: the SMCV is composed of a combination of any of the above types.
The SMCV is connected to the superior sagittal sinus by the great anastomotic vein of Trolard and to the transverse sinus by the posterior anastomotic vein of Labbé.15) In 53% of cases, the deep middle cerebral vein and SMCV are connected,16) and this connection serves as an important collateral route when the proximal position of the SMCV is occluded by a tumor. The drainage pattern of the SMCV influences the selection of a microsurgical skull base approach with middle cranial fossa dura incision.
CS
The development of the cerebral vein starts with the formation of three meningeal plexuses until the fourth week of embryonic life. Thereafter, the intracranial venous system is formed by venous anastomosis and degeneration. The CS has an important role in the frontal and temporal venous circulations. The anterior part of the CS connects to the superior ophthalmic vein, the anterolateral part connects to the superficial Sylvian vein, the lateral part connects to the sinus of the middle meningeal vein, and the posterior part connects to the superior and inferior petrosal sinus, although these connections are subject to some variability. The bilateral CS is connected by the intercavernous sinus (anterior and posterior) and basilar sinus. The lateral wall of the CS is usually composed of two layers of dura mater.17–19) In one cadaver study, a venous channel was present between these layers in 24% of specimens.20) When present, this channel connects with the SMCV and runs posteriorly through the middle cranial fossa towards the pterygoid plexus, transverse sinus, or SPS.5) Thus, the CS is a likely candidate for a collateral route when the original drainage route is disturbed by meningioma progression.
Tentorial Group
SPS
The SPS connects the CS and the junction of the transverse and sigmoid sinuses. Accordingly, the SPS usually joins the cerebellum and brainstem bridging veins. In some cases, the SMCV empties into the SPS in a structural pattern known as the sphenopetrosal sinus.1) SPS patterns have been classified into four types based on whether the sinus empties into the CS (medial type), transverse sinus-sigmoid sinus junction (lateral type), both (complete type), or neither (absent type). Matsushima et al. reported the frequencies of SPS patterns in subjects as follows: 60% complete type, 37% lateral type, and 3% medial type.4) Adachi et al. reported that the proportion of the hemispheres with complete and medial type patterns was significantly lower and the proportion of the hemispheres with lateral and absent type patterns was significantly higher in petrocrival meningioma (PCM) cases.7) In surgical contexts, the prevention of venous flow from the cerebrum to the SPS via the petrosal vein is more important than patency of the SPS.
Superior petrosal vein
Extensive microsurgical anatomical studies on cadavers21) have allowed classification of the superior petrosal veins into three groups.22) In Type I, the superior petrosal vein empties into the superior petrosal sinus above or lateral to the boundaries of the internal acoustic meatus. In Type II, the superior petrosal vein empties into an area between the lateral limit of the trigeminal nerve at Meckel’s cave and the medial limit of the facial nerve at the internal acoustic meatus. In Type III, the superior petrosal vein empties into the SPS above or medial to the boundaries of Meckel’s cave. Type II is the most common pattern (72% of cases) and Type III is the least common pattern (9% of cases).22) The relationship between the petrosal vein and cerebellopontine angle (CPA) meningioma has been reported using the above classifications based on tumor attachment in both PCM and anterior petrous meningioma; the most common vein type was Type III.23)
In a recent anatomical report by Matsushima et al.,4) four major vein groups were described as tributaries of the superior petrosal vein: (1) the petrosal group, draining the fourth ventricle, lateral medulla, cerebellopontine fissure, and petrosal cerebellar surface; (2) the posterior mesencephalic group, draining the area facing the cerebello-mesencephalic fissure; (3) the anterior mesencephalic group, draining the anterior-lateral portion of the midbrain and pons; and (4) the tentorial group, draining the lateral portion of the cerebellar surface facing the tentorium. The petrosal group is the largest group and the vein of the cerebellopontine fissure is the largest tributary. Collateral anastomosis between the petrosal vein and the Galenic system is common.24) Mizutani et al. reported that the basal vein via the pontotrigeminal vein serves as a collateral route for the superior petrosal vein in PCM.25) The risk of sacrificing the superior petrosal veins is well established and can lead to venous complications;23,26–38) however, there is a very low rate of serious complications related to microvascular decompression (MVD) procedures, in part due to the established practice of coagulating the vein for decompression and/or to achieve better exploration of the nerve.39) Several authors have reported the importance of preserving venous flow via the petrosal vein during the petrosal approach.22,40)
Falcine Group
Deep cerebral veins
The deep cerebral veins traverse the walls of the ventricles and basal cisterns and converge on the internal cerebral, basal, and great veins.1) Anatomical variation of the straight sinus has been noted in association with the infratentorial supracerebellar approach for the surgical treatment of pineal lesions.41) The basal vein of Rosenthal (BVR) is sometimes associated with the skull base approach. This vein exits the anteromedial part of the temporal lobe and collects venous flow from the insular cortex, medial side of the frontal lobe, and brain stem, and drains into the vein of Galen. The BVR is classified into three segments: (1) the anterior segment, which collects venous flow from the anterior cerebral vein, deep middle cerebral vein, inferior striate vein, posterior front-orbital vein, and olfactory vein; (2) the middle segment, which collects venous flow from the peduncular vein, inferior ventricular vein, and the vein from the medial side of the temporal lobe; (3) the posterior segment, which collects venous flow from the veins of the thalamus and cerebral peduncle and the lateral mesencephalic vein.1) Five drainage routes of have been classified for the BVR: (1) the great vein of Galen, (2) the cavernous sinus or sphenoparietal sinus, (3) the SPS via the lateral mesencephalic vein, (4) the SPS via the peduncular vein, and (5) the transverse sinus or straight sinus via the tentorium.42) Neurosurgeons should carefully note BVR drainage pattern variations in order to reduce the likelihood of venous complications.43) In particular, tentorium incisions in the anterior transpetrosal approach should be performed with extreme care to preserve the BVR.44)
Changes in the venous circulation in skull base meningioma
In meningioma, the surrounding venous structures are obstructed by gradual tumor progression, and congestive venous flow is drained through a collateral venous route.45) Considering the relationship between skull base meningioma and the venous circulation, three major intracranial venous circulation routes can be classified as follows: (1) superficial venous flow to the internal jugular vein via the superior sagittal sinus, transverse sinus, and sigmoid sinus; (2) an internal jugular venous route through the CS to the inferior petrosal sinus; and (3) a route via the pterygoid plexus through the SMCV and CS. Adachi et al.7) studied changes in venous drainage routes in PCM and proposed three hypotheses based on the study findings. First, the progression of PCM impairs the drainage route involving the medial part of the SPS and/or the drainage route from the SMCV to the CS. Second, impaired venous drainage causes a change in route; specifically, impaired venous flow drains through the emissary foramen and/or greater anastomosis of the SMCV. Third, if the emissary foramen route functions in a compensatory capacity when the original SMCV drainage to the CS is impaired, greater anastomosis of the SMCV is not necessary. In contrast, if SMCV drainage to the emissary foramen does not compensate for impairment of the original SMCV drainage to the CS, greater anastomosis of the SMCV is necessary (Fig. 2). Moreover, the study findings showed that SMCV drainage to the pterygoid plexus via the emissary foramen was the important drainage route in PCM.
Fig. 2.
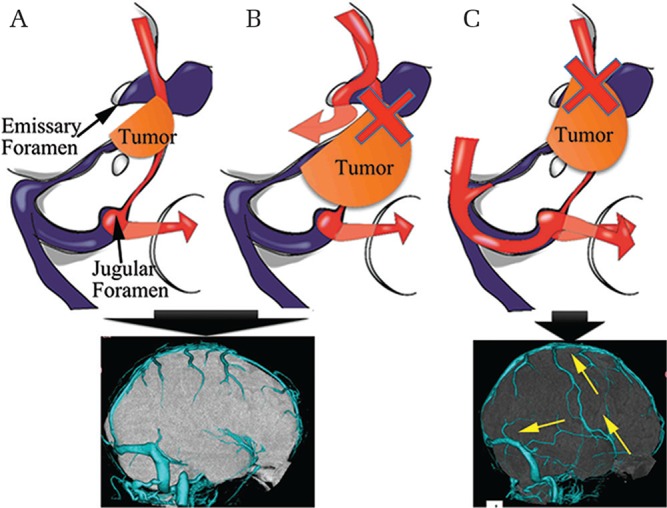
Schematic depictions of the relationship between greater anastomosis and superficial middle cerebral vein (SMCV) drainage pattern. When the SMCV drains into the cavernous sinus (CS) (A) or pterygoid plexus (B), venous drainage occurs via the jugular foramen and emissary foramen. In these cases, greater anastomosis of the SMCV is not required to prevent congestive venous flow. When SMCV drainage to the emissary foramen does not compensate for the impairment of original SMCV drainage to the CS (C), congestive venous flow drains through the jugular foramen via the connection between the vein of Trolard and the vein of Labbè.
Operative approaches for skull base meningioma considering the venous circulation
The ideal operative approach allows tumor detachment as early as possible, involves few or no cranial nerves or vascular structures in the approach route, and preserves local venous structures. Endoscopic skull base surgery has particular value for this purpose. However, a microsurgical skull base approach is more suitable for skull base meningioma because endoscopic surgery is unable to resolve the problem of handling the dural tail in meningioma. Meningioma saddled from the middle to posterior cranial fossa and posterior cranial fossa meningioma sometimes lead to a controversial approach route. One such meningioma saddled from the middle to posterior cranial fossa is PCM. A transpetrosal approach is appropriate for PCM;46–57) however, some authors have reported postoperative venous complications28,48,51,58) secondary to the impairment of venous drainage routes and modified the transpetrosal approach to prevent these complications.7,59–61) Shibao et al. reported the idea of an epidural and/or subdural modification to the anterior transpetrosal approach for PCM based on the SMCV drainage pattern.61) In the anterior transpetrosal approach, the dura propria of the trigeminal nerve (V3) of the foramen rotundum is peeled away to expose the apex of petrous bone.62,63) This may cause venous complications after surgery due to the obstruction of venous flow through the emissary route (Fig. 2). Adachi et al. suggested that peeling away the dura propria of V3 was not necessary for the anterior transpetrosal approach, as this procedure does explicitly require exposure of the petrous apex (i.e., the petrous apex was successfully drilled without this step).7) The vein of Labbè also has an important role. When the venous drainage route of the SMCV empties into the CS and/or when the emissary foramen is disturbed, greater anastomosis via the vein of Labbè and vein of Trolard is needed to control venous drainage flow. In this situation, the maneuver in which the temporal lobe is lifted away from the middle cranial fossa causes stretching and stenosis of the vein of Labbè, which increases the risk of venous congestion in cases where the entry of the vein of Labbè is caudal to the transverse and sigmoid sinus junction.7) In petroapex meningioma and PCM, it is sometimes unclear whether a retrosigmoid approach or an anterior transpetrosal approach is more suitable. The anterior transpetrosal approach is often more suitable because it can reach the tumor from the inside of the cranial nerves and handle the feeding artery at a comparatively earlier stage. The positional relationship between the petrosal vein and PCM or petroapex meningioma has been reported, with the most common vein pattern being Type III (the superior petrosal vein emptying into the SPS above or medial to the boundaries of Meckel’s cave).23) From this perspective, although a retrosigmoid approach is more suitable for CPA meningioma, the anterior transpetrosal approach is more useful for both PCM and petroapex meningioma because retraction of the cerebellar hemisphere could cause an increase in intracerebellar pressure and aggravate venous congestion. For the retrosigmoid approach, the rate of petrosal vein sacrifice is 42.1–47% and that of postoperative venous complication is 30–37.5%.23,28) In contrast, for the anterior transpetrosal approach, the rate of petrosal vein sacrifice is 25–35.7% and that of postoperative venous complication is 0%.25,64) According to these results, Mizutani et al. suggested that sacrifice of the petrosal vein during the retrosigmoid approach may cause further venous congestion and increase intracerebellar pressure, leading to venous complications.25)
Unnecessary and prolonged brain compression due to retraction should be avoided as this maneuver can disturb the venous circulation.65–67) For example, the anterior transpetrosal approach requires lifting of the temporal lobe. In this situation, minimal incision of the temporal lobe dura is useful for preventing excessive displacement or retraction of the temporal lobe.
Of note, the information in this review is also useful for avoiding venous complications in the combined petrosal approach. In this context, neurosurgeons must understand the drainage direction of the petrosal vein via the SPS, and transect the SPS to preserve the petrosal vein drainage route through the SPS.
Surgery for anterior clinoidal meningioma is also influenced by drainage through the SMCV. Nagata et al. reported three venous drainage patterns in anterior clinoidal meningioma: (1) cortical type (63.6% of cases), where the Sylvian vein drains to the cortical veins only; (2) sphenobasal type (27.3% of cases), where the Sylvian vein drains into the pterygoid plexus; (3) cavernous type (9.1% of cases), where the Sylvian vein drains directly into the cavernous sinus. The authors concluded that a cortical-type venous structure does not restrict removal of the meningioma; however, neurosurgeons must preserve the venous drainage system in sphenobasal- or sphenoparietal-type cases using tailored a surgical strategy.68)
While many reports address the preservation of venous drainage routes, ligation of the temporal bridging vein has been argued to provide a safer and wider approach route. In one case series, ligation of the bridging veins from the temporal lobe to the middle cranial fossa floor was planned in the first stage, followed by tumor removal through the desired skull base approach in the second stage; of seven cases using this strategy, only one resulted in asymptomatic mild temporal edema.69) Based on accumulating knowledge about venous thrombosis, this technique may be useful in cases where other skull base approaches are contraindicated.
A thorough knowledge of venous anatomy and collateral routes is important for minimizing complications during skull base surgery for meningioma; to this end, the insufficient preoperative evaluation of the venous drainage route can lead to post-operative venous complications. To improve surgical results, the accumulation of knowledge about venous drainage patterns in skull base meningioma and implementation of a scoring system for the preoperative evaluation of procedural difficulty level in skull base meningioma70) are urgently needed.
Conclusion
In the present article, we reviewed the venous anatomy in the context of microsurgical skull base approaches, discussed changes in venous drainage patterns in skull base meningioma, and identified operative approaches that minimize the likelihood of venous complications after surgery for skull base meningioma. Venous drainage routes are gradually disturbed with the progression of meningioma, such that congested venous flow is rerouted to drain through alternative developmental routes. Neurosurgeons should carefully evaluate and understand these drainage routes preoperatively and take care to preserve these drainage routes intraoperatively by using a suitable approach. Of note, we have not discussed the condyle emissary vein, marginal sinus, or basilar plexus given that associations between these venous structures and meningioma have not yet been studied. Future studies should address venous drainage patterns in relevant pathologies such as foramen magnum meningioma.
Footnotes
Conflicts of Interest Disclosure
All authors who are members of the Japan Neurosurgical Society (JNS) have registered online self-reported COI Disclosure Statement Forms through the website for JNS members. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1).Rhoton AL, Jr: The cerebral veins. Neurosurgery 51: S159–S205, 2002 [PubMed] [Google Scholar]
- 2).Delion M, Dinomais M, Mercier P: Arteries and veins of the cerebellum. Cerebellum 2016 [DOI] [PubMed] [Google Scholar]
- 3).Tanoue S, Kiyosue H, Okahara M, et al. : Para-cavernous sinus venous structures: anatomic variations and pathologic conditions evaluated on fat-suppressed 3D fast gradient-echo MR images. AJNR Am J Neuroradiol 27: 1083–1089, 2006 [PMC free article] [PubMed] [Google Scholar]
- 4).Matsushima K, Matsushima T, Kuga Y, et al. : Classification of the superior petrosal veins and sinus based on drainage pattern. Neurosurgery 10 Suppl 2: 357–367, 2014 [DOI] [PubMed] [Google Scholar]
- 5).Suzuki Y, Matsumoto K: Variations of the superficial middle cerebral vein: classification using three-dimensional CT angiography. AJNR Am J Neuroradiol 21: 932–938, 2000 [PMC free article] [PubMed] [Google Scholar]
- 6).Wetzel SG, Law M, Lee VS, Cha S, Johnson G, Nelson K: Imaging of the intracranial venous system with a contrast-enhanced volumetric interpolated examination. Eur Radiol 13: 1010–1018, 2003 [DOI] [PubMed] [Google Scholar]
- 7).Adachi K, Hayakawa M, Ishihara K, et al. : Study of Changing Intracranial Venous Drainage Patterns in Petroclival Meningioma. World Neurosurg 92: 339–348, 2016 [DOI] [PubMed] [Google Scholar]
- 8).Oishi M, Fukuda M, Ishida G, Saito A, Hiraishi T, Fujii Y: Presurgical simulation with advanced 3-dimensional multifusion volumetric imaging in patients with skull base tumors. Neurosurgery 68: 188–199, 2011 [DOI] [PubMed] [Google Scholar]
- 9).Deda H, Erden I, Yagmurlu B: Evaluation of petrosal sinus patency with 3-dimensional contrast-enhanced magnetic resonance venography in petroclival meningiomas for surgical strategy. Surgical Neurology 64 Suppl 2: S67–S71, 2005 [DOI] [PubMed] [Google Scholar]
- 10).Casey SO, Alberico RA, Patel M, et al. : Cerebral CT venography. Radiology 198: 163–170, 1996 [DOI] [PubMed] [Google Scholar]
- 11).Morisako H, Goto T, Chokyu I, Ishibashi K, Ohata K: Preoperative evaluation of the petrosal vein with contrast-enhanced PRESTO imaging in petroclival meningiomas to establish surgical strategy. Neurol Med Chir (Tokyo) 53: 490–495, 2013 [DOI] [PubMed] [Google Scholar]
- 12).Gharabaghi A, Rosahl SK, Feigl GC, et al. : Surgical planning for retrosigmoid craniotomies improved by 3D computed tomography venography. Eur J Surg Oncol 34: 227–231, 2008 [DOI] [PubMed] [Google Scholar]
- 13).Hacker H: Normal Supratentorial veins and dural sinuses. St Louis, Mosby, 1974, pp. 1851–77 [Google Scholar]
- 14).Guppy KH, Origitano TC, Reichman OH, Segal S: Venous drainage of the inferolateral temporal lobe in relationship to transtemporal/transtentorial approaches to the cranial base. Neurosurgery 41: 615–620, 1997 [DOI] [PubMed] [Google Scholar]
- 15).Gray H, Lewis WH: Anatomy of the Human Body, 20th. Philadelphia and New York, Lea & Febiger, 1918 [Google Scholar]
- 16).Kazumata K, Kamiyama H, Ishikawa T, et al. : Operative anatomy and classification of the sylvian veins for the distal transsylvian approach. Neurol Med Chir (Tokyo) 43: 427–434, 2003 [DOI] [PubMed] [Google Scholar]
- 17).Bisaria KK: The superficial sylvian vein in humans: with special reference to its termination. Anat Rec 212: 319–325, 1985 [DOI] [PubMed] [Google Scholar]
- 18).Bonneville JF, Cattin F, Racle A, et al. : Dynamic CT of the laterosellar extradural venous spaces. AJNR Am J Neuroradiol 10: 535–542, 1989 [PMC free article] [PubMed] [Google Scholar]
- 19).Umansky F, Nathan H: The lateral wall of the cavernous sinus. With special reference to the nerves related to it. J Neurosurg 56: 228–234, 1982 [DOI] [PubMed] [Google Scholar]
- 20).San Millán Ruiz D, Gailloud P, de Miquel Miquel MA, et al. : Laterocavernous sinus. Anat Rec 254: 7–12, 1999 [DOI] [PubMed] [Google Scholar]
- 21).Matsushima T, Rhoton AL, de Oliveira E, Peace D: Microsurgical anatomy of the veins of the posterior fossa. J Neurosurg 59: 63–105, 1983 [DOI] [PubMed] [Google Scholar]
- 22).Tanriover N, Abe H, Rhoton AL, Kawashima M, Sanus GZ, Akar Z: Microsurgical anatomy of the superior petrosal venous complex: new classifications and implications for subtemporal transtentorial and retrosigmoid suprameatal approaches. J Neurosurg 106: 1041–1050, 2007 [DOI] [PubMed] [Google Scholar]
- 23).Watanabe T, Igarashi T, Fukushima T, Yoshino A, Katayama Y: Anatomical variation of superior petrosal vein and its management during surgery for cerebellopontine angle meningiomas. Acta Neurochir (Wien) 155: 1871–1878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Duvernoy HM: The superficial veins of the human brain. Veins of the brain stem and of the base of the brain. Springer, Berlin Heidelberg New York, 1975 [Google Scholar]
- 25).Mizutani K, Toda M, Yoshida K: The analysis of the petrosal vein to prevent venous complications during the anterior transpetrosal approach in the resection of petroclival meningioma. World Neurosurg 93: 175–182, 2016 [DOI] [PubMed] [Google Scholar]
- 26).Chen HJ, Lui CC: Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: report of a case. J Formos Med Assoc 94: 503–505, 1995 [PubMed] [Google Scholar]
- 27).Inamasu J, Shiobara R, Kawase T, Kanzaki J: Haemorrhagic venous infarction following the posterior petrosal approach for acoustic neurinoma surgery: a report of two cases. European Archives of Otorhinolaryngology 259: 162–165, 2002 [DOI] [PubMed] [Google Scholar]
- 28).Koerbel A, Gharabaghi A, Safavi-Abbasi S, et al. : Venous complications following petrosal vein sectioning in surgery of petrous apex meningiomas. Eur J Surg Oncol 35: 773–779, 2009 [DOI] [PubMed] [Google Scholar]
- 29).Koerbel A, Wolf SA, Kiss A: Peduncular hallucinosis after sacrifice of veins of the petrosal venous complex for trigeminal neuralgia. Acta Neurochir (Wien) 149: 831–833, 2007 [DOI] [PubMed] [Google Scholar]
- 30).Masuoka J, Matsushima T, Hikita T, Inoue E: Cerebellar swelling after sacrifice of the superior petrosal vein during microvascular decompression for trigeminal neuralgia. J Clin Neurosci 16: 1342–1344, 2009 [DOI] [PubMed] [Google Scholar]
- 31).Nakase H, Shin Y, Nakagawa I, Kimura R, Sakaki T: Clinical features of postoperative cerebral venous infarction. Acta Neurochir (Wien) 147: 621–626, 2005 [DOI] [PubMed] [Google Scholar]
- 32).Rhoton AL: The posterior fossa veins. Neurosurgery 47: S69–S92, 2000 [DOI] [PubMed] [Google Scholar]
- 33).Singh D, Jagetia A, Sinha S: Brain stem infarction: a complication of microvascular decompression for trigeminal neuralgia. Neurol India 54: 325–326, 2006 [DOI] [PubMed] [Google Scholar]
- 34).Strauss C, Neu M, Bischoff B, Romstöck J: Clinical and neurophysiological observations after superior petrosal vein obstruction during surgery of the cerebellopontine angle: case report. Neurosurgery 48: 1157–1161, 2001 [DOI] [PubMed] [Google Scholar]
- 35).Tsukamoto H, Matsushima T, Fujiwara S, Fukui M: Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: case report. Surg Neurol 40: 31–34, 1993 [DOI] [PubMed] [Google Scholar]
- 36).Zhong J, Li ST, Xu SQ, Wan L, Wang X: Management of petrosal veins during microvascular decompression for trigeminal neuralgia. Neurol Res 30: 697–700, 2008 [DOI] [PubMed] [Google Scholar]
- 37).Ebner FH, Roser F, Shiozawa T, et al. : Petrosal vein occlusion in cerebello-pontine angle tumour surgery: an anatomical study of alternative draining pathways. Eur J Surg Oncol 35: 552–556, 2009 [DOI] [PubMed] [Google Scholar]
- 38).Anichini G, Iqbal M, Rafiq NM, Ironside JW, Kamel M: Sacrificing the superior petrosal vein during microvascular decompression. Is it safe? Learning the hard way. Case report and review of literature. Surg Neurol Int 7: S415–420, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).McLaughlin MR, Jannetta PJ, Clyde BL, Subach BR, Comey CH, Resnick DK: Microvascular decompression of cranial nerves: lessons learned after 4400 operations. J Neurosurg 90: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 40).Haq IB, Susilo RI, Goto T, Ohata K: Dural incision in the petrosal approach with preservation of the superior petrosal vein. J Neurosurg 124: 1074–1078, 2016 [DOI] [PubMed] [Google Scholar]
- 41).Hasegawa M, Yamashita J, Yamashima T: Anatomical variations of the straight sinus on magnetic resonance imaging in the infratentorial supracerebellar approach to pineal region tumors. Surg Neurol 36: 354–359, 1991 [DOI] [PubMed] [Google Scholar]
- 42).Suzuki Y, Ikeda H, Shimadu M, Ikeda Y, Matsumoto K: Variations of the basal vein: identification using three-dimensional CT angiography. AJNR Am J Neuroradiol 22: 670–676, 2001 [PMC free article] [PubMed] [Google Scholar]
- 43).Yaşargil MG, Wieser HG, Valavanis A, von Ammon K, Roth P: Surgery and results of selective amygdala-hippocampectomy in one hundred patients with nonlesional limbic epilepsy. Neurosurg Clin N Am 4: 243–261, 1993 [PubMed] [Google Scholar]
- 44).Shibao S, Fujiwara H, Borghei-Razavi H, Yoshida K: Encountering a basal vein of rosenthal variant during the anterior transpetrosal approach. World Neurosurg 86: 513 e19–22, 2016 [DOI] [PubMed] [Google Scholar]
- 45).DiMeco F, Li KW, Casali C, et al. : Meningiomas invading the superior sagittal sinus: surgical experience in 108 cases. Neurosurgery 55: 1263–1274, 2004 [DOI] [PubMed] [Google Scholar]
- 46).Morisako H, Goto T, Ohata K: Petroclival meningiomas resected via a combined transpetrosal approach: surgical outcomes in 60 cases and a new scoring system for clinical evaluation. J Neurosurg 122: 373–380, 2015 [DOI] [PubMed] [Google Scholar]
- 47).Cho CW, Al-Mefty O: Combined petrosal approach to petroclival meningiomas. Neurosurgery 51: 708–716; discussion 708–716, 2002 [PubMed] [Google Scholar]
- 48).Almefty R, Dunn IF, Pravdenkova S, Abolfotoh M, Al-Mefty O: True petroclival meningiomas: results of surgical management. J Neurosurg 120: 40–51, 2014 [DOI] [PubMed] [Google Scholar]
- 49).Bambakidis NC, Kakarla UK, Kim LJ, et al. : Evolution of surgical approaches in the treatment of petroclival meningiomas: a retrospective review. Neurosurgery 61: 202–209; discussion 209–211, 2007 [DOI] [PubMed] [Google Scholar]
- 50).Kawase T, Shiobara R, Toya S: Middle fossa transpetrosal-transtentorial approaches for petroclival meningiomas. Selective pyramid resection and radicality. Acta Neurochir (Wien) 129: 113–120, 1994 [DOI] [PubMed] [Google Scholar]
- 51).Little KM, Friedman AH, Sampson JH, Wanibuchi M, Fukushima T: Surgical management of petroclival meningiomas: defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery 56: 546–559, 2005 [DOI] [PubMed] [Google Scholar]
- 52).Mathiesen T, Gerlich A, Kihlström L, Svensson M, Bagger-Sjöbäck D: Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas. Neurosurgery 60: 982–991, 2007 [DOI] [PubMed] [Google Scholar]
- 53).Al-Mefty O, Fox JL, Smith RR: Petrosal approach for petroclival meningiomas. Neurosurgery 22: 510–517, 1988 [DOI] [PubMed] [Google Scholar]
- 54).Chanda A, Nanda A: Partial labyrinthectomy petrous apicectomy approach to the petroclival region: an anatomic and technical study. Neurosurgery 51: 147–159; discussion 159–160, 2002 [DOI] [PubMed] [Google Scholar]
- 55).Couldwell WT, Fukushima T, Giannotta SL, Weiss MH: Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg 84: 20–28, 1996 [DOI] [PubMed] [Google Scholar]
- 56).Sekhar LN, Schessel DA, Bucur SD, Raso JL, Wright DC: Partial labyrinthectomy petrous apicectomy approach to neoplastic and vascular lesions of the petroclival area. Neurosurgery 44: 537–550, 1999 [DOI] [PubMed] [Google Scholar]
- 57).Spetzler RF, Daspit CP, Pappas CT: The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg 76: 588–599, 1992 [DOI] [PubMed] [Google Scholar]
- 58).Hafez A, Nader R, Al-Mefty O: Preservation of the superior petrosal sinus during the petrosal approach. J Neurosurg 114: 1294–1298, 2011 [DOI] [PubMed] [Google Scholar]
- 59).Shibao S, Borghei-Razavi H, Yoshida K: Anterior transpetrosal approach: epidural or subdural? Neurosurg Rev 39: 531–534, 2016 [DOI] [PubMed] [Google Scholar]
- 60).Ichimura S, Yoshida K, Kagami H, et al. : Epidural anterior petrosectomy with subdural visualization of sphenobasal vein via the anterior transpetrosal approach—technical case report. Neurosurg Rev 35: 609–613, 2012 [DOI] [PubMed] [Google Scholar]
- 61).Shibao S, Toda M, Orii M, Fujiwara H, Yoshida K: Various patterns of the middle cerebral vein and preservation of venous drainage during the anterior transpetrosal approach. J Neurosurg 124: 432–439, 2016 [DOI] [PubMed] [Google Scholar]
- 62).Fukushima T, Day JD, Hirahara K: Extradural total petrous apex resection with trigeminal translocation for improved exposure of the posterior cavernous sinus and petroclival region. Skull Base Surg 6: 95–103, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63).Zhao JC, Liu JK: Transzygomatic extended middle fossa approach for upper petroclival skull base lesions. Neurosurg Focus 25: E5; discussion E5, 2008 [DOI] [PubMed] [Google Scholar]
- 64).Kaku S, Miyahara K, Fujitsu K, et al. : Drainage pathway of the superior petrosal vein evaluated by CT venography in petroclival meningioma Surgery. J Neurol Surg B Skull Base 73: 316–320, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65).Rosenørn J, Diemer N: The risk of cerebral damage during graded brain retractor pressure in the rat. J Neurosurg 63: 608–611, 1985 [DOI] [PubMed] [Google Scholar]
- 66).Kaido T, Nakase H, Nagata K, Otsuka H, Sakaki T: Intermittent isometric exposure prevents brain retraction injury under venous circulatory impairment. Neurol Res 23: 739–744, 2001 [DOI] [PubMed] [Google Scholar]
- 67).Sakaki T, Matsuyama T, Nagata K, Nakase H, Hirabayashi H, Morimoto T: Delayed intracerebral haemorrhage after intracranial surgery. J Clin Neurosci 6: 54–57, 1999 [DOI] [PubMed] [Google Scholar]
- 68).Nagata T, Ishibashi K, Metwally H, et al. : Analysis of venous drainage from sylvian veins in clinoidal meningiomas. World Neurosurg 79: 116–123, 2013 [DOI] [PubMed] [Google Scholar]
- 69).Savardekar AR, Goto T, Nagata T, et al. : Staged ‘intentional’ bridging vein ligation: a safe strategy in gaining wide access to skull base tumors. Acta Neurochir (Wien) 156: 671–679, 2014 [DOI] [PubMed] [Google Scholar]
- 70).Adachi K, Kawase T, Yoshida K, Yazaki T, Onozuka S: ABC Surgical Risk Scale for skull base meningioma: a new scoring system for predicting the extent of tumor removal and neurological outcome. Clinical article. J Neurosurg 111: 1053–1061, 2009 [DOI] [PubMed] [Google Scholar]


