Abstract
The authors describe the surgical anatomy for the endoscopic endonasal approach (EEA) to the ventrolateral skull base. The ventrolateral skull base can be divided into two segments: the upper lateral and lower lateral skull base. The upper lateral skull base includes the cavernous sinus and the orbit, while the lower lateral skull base includes the petrous apex, Meckel’s cave, parapharyngeal space, infratemporal fossa, etc. To gain access to the upper lateral skull base, a simple opening of the ethmoid sinus provides sufficient exposure of this area. To reach the lower lateral skull base, a transpterygoid approach, following ethmoidectomy, is a key procedure providing wide exposure of this area. Understanding of surgical anatomy is mandatory for treating ventrolateral skull base lesions via EEA. An appropriate, less-invasive approach should be applied depending on the size, location, and type of lesion.
Keywords: endoscopic endonasal approach, cavernous sinus, orbit, petrous apex, Meckel’s cave, infratemporal fossa
Introduction
During the past decade, endoscopic endonasal approaches (EEAs) have been widely used to address various ventral cranial base lesions.1–7) As EEA provides the most direct access to the ventral skull base while obviating the need for retraction and manipulation of critical neurovascular structures, it seems to be a less invasive and more adequate approach than conventional open cranial base approaches to this deep surgical field. The ventral skull base can be classified into two planes—sagittal and coronal—with the sella turcica as the center.1–3) The coronal plane indicates the area located lateral to the sella turcica, i.e., the ventrolateral skull base. EEA to the ventrolateral skull base is relatively complicated due to its anatomical complexity.
The authors describe the step-by-step surgical anatomy for the EEA to the ventrolateral skull base.
Materials and Methods
Using cadaver heads, anatomical dissections were performed in the Anatomy Laboratory Toward Visuospatial Surgical Innovations in Otolaryngology and Neurosurgery (ALT-VISION) at The Ohio State University and the Anatomical Laboratory for Skull Base Surgery at Lariboisière Hospital. Fresh cadaver heads, without obvious intracranial disease and injected with blue and red latex into the venous and arterial systems, respectively, were used for anatomic dissection. The heads were rigidly fastened with standard cranial fixation pins in a position similar to that used during live operative approaches. An endoscope (Karl Storz GmbH, Tuttlingen, Germany) was used for dissections and photography. We used 18-cm-long, 4-mm-diameter endoscopes with 0°, 30°, and 70° lenses, connected to a light source via a fiber optic cable and to a camera fitted with 3-charge-coupled device, high-definition sensors. Images were recorded and stored using the Karl Storz Aida system (Karl Storz GmbH, Tuttlingen, Germany).
Surgical technique
Nasal phase
Nasal manipulations are performed through either one nostril or both nostrils. Both middle turbinates are mobilized laterally, instead of sacrificing them. The middle turbinate can be removed to make the surgical field wider if necessary. For the unilateral approach, the septal mucosa on one side is cut vertically at the level of the anterior margin of the middle turbinate (Fig. 1A) and dissected subperiosteally to reach the vomer bone (Fig. 1B). For the bilateral approach, the septal mucosa in the nares are cut horizontally starting from the natural ostium toward the point of the anterior margin of the middle turbinate (Fig. 1C), preserving the olfactory mucosa above the cutting line. Subsequently, the septal mucosa on both sides are dissected downward to expose the vomer bone (Fig. 1D). The vascularized nasoseptal flap should be prepared in advance or created as necessary for a skull base reconstruction if either postoperative cerebrospinal fluid leak or exposure of the internal carotid artery is anticipated.8,9) The septal bone is fractured and shifted laterally for the unilateral approach or removed for the bilateral approach, creating a binarial corridor to allow increased freedom of movement.
Fig. 1.
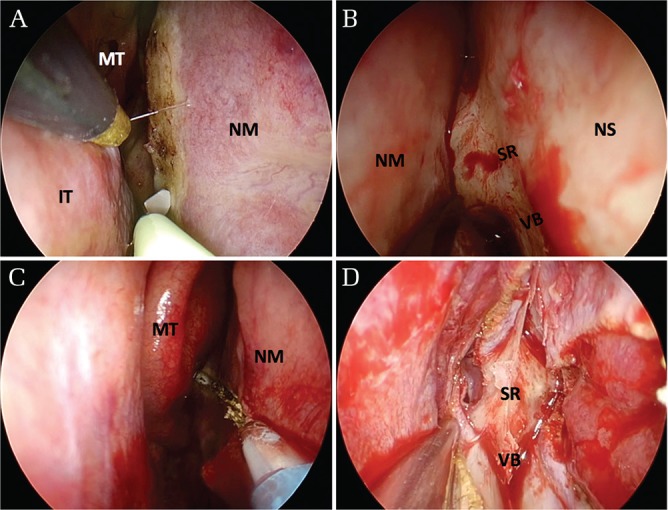
Surgical view of nasal phase. A and B. For the unilateral approach, the septal mucosa on one side is cut vertically at the level of the anterior margin of the middle turbinate (A) and dissected subperiosteally to reach the vomer bone (B). (C, D) For the bilateral approach without preparation of a nasoseptal flap, the septal mucosa in the nares are cut horizontally starting from the natural ostium toward the point of the anterior margin of the middle turbinate (C) and then dissected downward to expose the vomer bone (D). A nasoseptal flap can be made with antero-superior extension of mucosal cut, if necessary. IT: inferior turbinate, MT: middle turbinate, NM: nasoseptal mucosa, NS: nasal septum, SR: sphenoidal rostrum, VB: vomer bone.
Ethmoidectomy
Ethmoidectomy widens a surgical corridor to the ventrolateral skull base. The extent of ethmoidectomy (partial or complete ethmoidectomy) should be arranged based on the region being accessed. Complete ethmoidectomy starts with an uncinectomy. The uncinate process is excised to visualize the hiatus semilunaris leading to the ethmoidal bulla. Subsequently, anterior and posterior ethmoidectomy can be performed using cutting forceps or a microdebrider. During the ethmoidectomy, care should be taken not to unnecessarily injure the lamina papyracea or the intraorbital fat, as the lateral edge of the ethmoid sinuses is attached to the lamina papyracea.
Approach to the upper lateral skull base
The ventrolateral skull base can be divided into two segments: upper lateral and lower lateral. The upper lateral skull base includes the cavernous sinus and the orbit, and the lower lateral skull base includes the petrous apex, Meckel’s cave, parapharyngeal space, infratemporal fossa, etc. To gain access to the upper lateral skull base, a simple opening of the ethmoid sinus provides sufficient exposure of this area.
Cavernous sinus approach
After wide sphenoidotomies, several important surgical landmarks, including the medial and lateral optico–carotid recesses (OCRs) and carotid protuberances, can be observed (Fig. 2A).10,11) The lateral OCR is a bony depression that corresponds intracranially to the optic strut, a bony extension inferior to the anterior clinoid process; thus, it is an important surgical landmark for the cavernous sinus exposure, indicating the junction between the optic nerve and the internal carotid artery (Figs. 2B and 2C).10) If the sphenoid sinus is not well-pneumatized, drilling is required to remove the cancellous bone to expose the compact bone of the skull base. To access the cavernous sinus, posterior ethmoidectomy is usually sufficient in most cases.12,13) A bony window in front of the cavernous sinus is opened using a high-speed microdrill and a Kerrison rongeur. A Doppler ultrasonic probe or a fluorescence imaging system using indocyanine green is useful for indicating the position of the internal carotid artery (ICA) inside the cavernous sinus.14) Opening the dura mater covering the medial and anterior surfaces of the cavernous sinus exposes the carotid arteries and the optic, trochlear, abducens, and trigeminal nerves within the cavernous sinus (Fig. 2D). During the maneuver in the cavernous sinus, cranial nerve monitoring is essential for avoiding cranial nerve injuries.13,15,16)
Fig. 2.
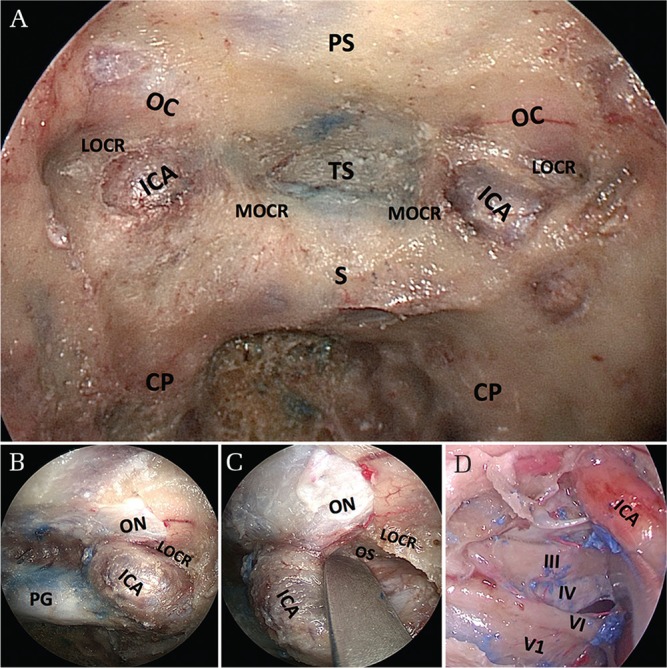
Stepwise views of surgical anatomy for the cavernous sinus approach. (A) Endonasal view inside the sphenoid sinus with 0° endoscope. Wide sphenoidotomy revealing several important surgical landmarks, including the medial and lateral optico–carotid recesses and carotid protuberances. (B) The lateral optico–carotid recess indicates the junction between the internal carotid artery and the optic nerve. (C) The lateral optico–carotid recess corresponds intracranially to the optic strut, a bony extension inferior to the anterior clinoid process. (D) Opening of the dura of the cavernous sinus on the right side revealing the contents of the sinus. CP: carotid protuberance, ICA: internal carotid artery, LOCR: lateral optico–carotid recess, MOCR: medial optico–carotid recess, OC: optic canal, ON: optic nerve (covered with dural sheath), PS: planum sphenoidale, S: sella turcica, V1: first division of the trigeminal nerve, V2: second division of the trigeminal nerve, III: oculomotor nerve, IV: trochlear nerve, VI: abducens nerve.
Transorbital approach
A transorbital approach begins with complete anterior and posterior ethmoidectomies to expose the medial wall of the orbit (Fig. 3A). The lamina papyracea is drilled until only a layer of paper-thin bone remains over the periorbita; it is then removed with a Cottle elevator to complete the decompression of the medial orbital wall (Fig. 3B). The procedure can be followed by decompression of the medial aspect of the optic canal. The optic canal can be easily identified from the orbita even if the sphenoid sinus is not well-pneumatized, and is not visible inside the sphenoid sinus; thus, the drilling over the optic canal should proceed from the orbital side toward the proximal side (Figs. 3C and 3D). If needed, the approach can be extended into the intraconal compartment opening the periorbita and exposing the medial and inferior rectus muscles.
Fig. 3.
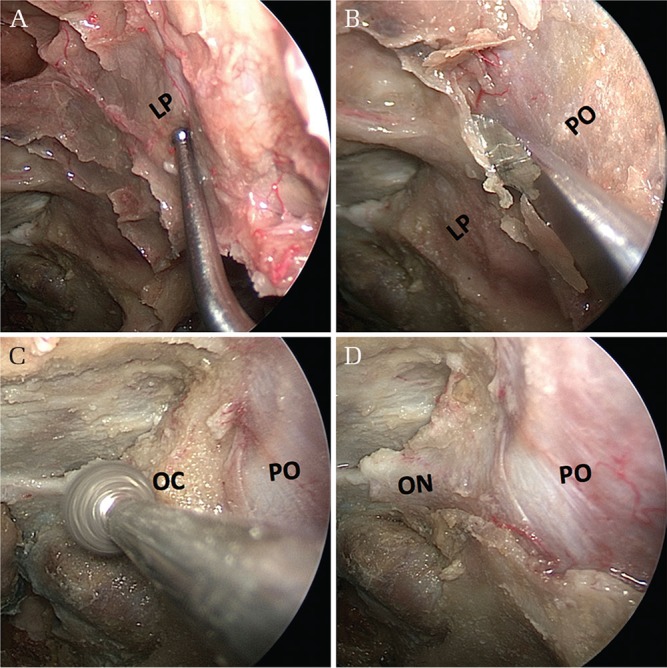
Stepwise views of surgical anatomy for the transorbital approach. (A) Following complete anterior and posterior ethmoidectomies on the left side, the lamina papyracea is exposed. (B) The lamina papyracea can be drilled until only a layer of paper-thin bone remains over the periorbita, and then removed with a Cottle elevator to complete the decompression of the medial orbital wall. (C) Drilling over the optic canal to decompress the medial aspect of the canal. (D) Removal of bone over the orbit and optic canal showing the periorbita and the dural sheath of the optic nerve. LP: lamina papyracea, ON: optic nerve (covered with dural sheath), PO: periorbita.
Approach to the lower lateral skull base
Transpterygoid approach
The pterygopalatine fossa is bound by the pterygoid process posteriorly, the palatine bone anteromedially, and the maxilla anterolaterally. A transpterygoid approach is a key procedure to reach the lower lateral skull base and provides a wide exposure of this area.17,18) A transpterygoid approach starts with an antrostomy (Fig. 4A). The terminal branches of the internal maxillary artery, the sphenopalatine and posterior nasal arteries are identified and dissected at the sphenopalatine foramen (Fig. 4B). This foramen is located posterior to the superior third of the posterior wall of the antrum. A wide nasomaxillary window is opened and the posterior wall of the antrum is removed with either a Kerrison rongeur or a high-speed drill. This exposes the pterygopalatine fossa (Fig. 4C). While exposing the pterygopalatine fossa, subperiosteal dissection is useful to keep the contents of the fossa, especially fat tissue, in place and to make the dissection easier. The sphenopalatine and posterior nasal arteries can be divided and mobilized laterally thorough coagulation using a bipolar electrocautery, which exposes the vidian canal (Fig. 4D). The location of the vidian canal is usually at the insertion of the medial pterygoid plate on the pterygoid process of the sphenoid bone. The vidian nerve and artery inside the canal can be coagulated and divided, allowing for the subsequent lateralization of the contents of the pterygopalatine fossa and identification of the pterygoid base as well as the foramen rotundum (Fig. 4E).17,19,20) Image guidance may be helpful to confirm the adequacy of the exposure.
Fig. 4.
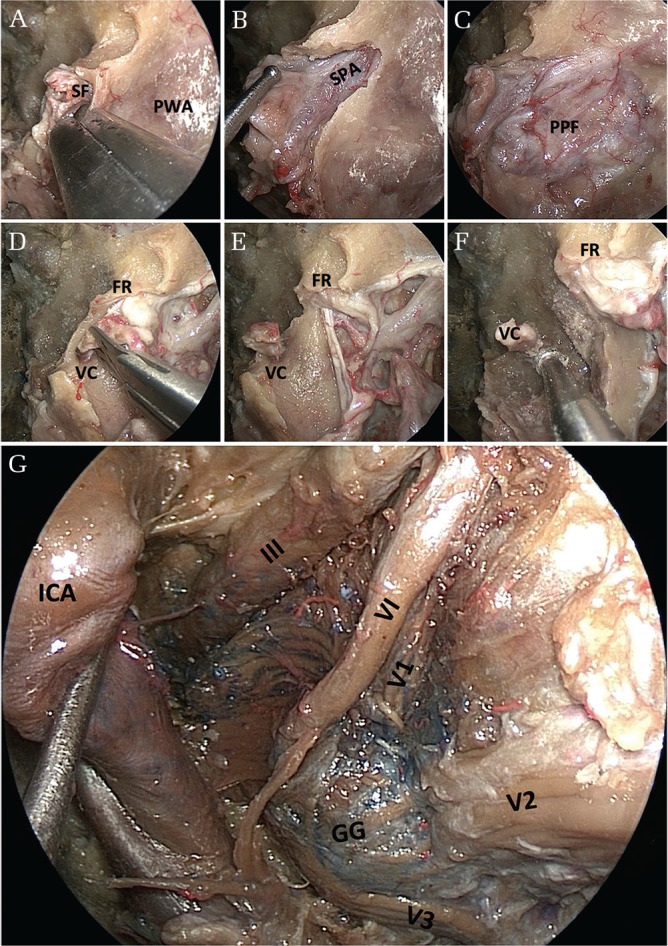
Stepwise views of surgical anatomy for the transpterygoid and Meckel’s cave approaches. (A) An antrostomy revealing the posterior wall of the antrum. Removal of the bone can be started from the sphenopalatine foramen, which is located in the superomedial margin of the pterygopalatine fossa. (B) Partial removal of the posterior wall of the antrum revealing a bulging of the sphenopalatine artery inside the pterygopalatine fossa. (C) Removal of the posterior wall of the antrum revealing the contents of the pterygopalatine fossa covered with periosteum. (D) Lateral mobilization of the contents of the pterygopalatine fossa exposing the vidian canal and the foramen rotundum. (E) Cutting the vidian canal allowing for the subsequent lateralization of the contents of the pterygopalatine fossa. (F) Drilling the bone below the vidian canal, as well as the bone between V2 and the vidian canal, to get to the petrous apex. (G) Opening the periosteal dural layer revealing all contents around the cavernous sinus and the Meckel’s cave. FR: foramen rotundum, GG: Gasserian ganglion, ICA: internal carotid artery, PPF: pterygopalatine fossa, PWA: posterior wall of the antrum, VC: vidian canal, V1: first division of the trigeminal nerve, V2: second division of the trigeminal nerve, V3: third division of the trigeminal nerve, III: oculomotor nerve, IV: trochlear nerve.
Meckel’s cave approach
The vidian canal is a curved bony canal running through the floor of the sphenoid sinus from the pterygopalatine fossa posteriorly to the foramen lacerum. The vidian nerve and artery run inside the canal toward the point of transition from the horizontal petrous ICA to the vertical paraclival segment at the level of the foramen lacerum; therefore, the vidian canal can serve as an opening into the petroclival junction by safely allowing the identification of the petrous ICA.20) Once the vidian canal is identified, drilling can then proceed cautiously along its inferior and medial aspect toward the petrous ICA (Fig. 4F). This allows you to drill the bone safely, avoiding the ICA injury. The bone between V2 and the vidian canal can also be removed with a high-speed drill and a rongeur to expose the periosteal dural layer overlying the anterior surface of Meckel’s cave.21) Pneumatization of the sphenoid bone and sphenoid sinus creates a natural corridor, allowing the surgeon to approach various neurovascular structures with minimal drilling or manipulation of intracranial structures.22,23) Dural opening should start from the foramen rotundum and follow the V2 toward the Meckel’s cave in order to avoid injury to the abducens nerve, which runs superolaterally above the superior margin of the V2.21) After opening the periosteal dura, the Gasserian ganglion and all trigeminal branches, as well as the cranial nerves (III, IV, VI) in the cavernous sinus, can be visualized (Fig. 4G).
Infratemporal fossa approach
The infratemporal fossa is an anatomic space located under the floor of the middle cranial fossa and posterior to the maxilla that contains the parapharyngeal and masticator spaces. For the transpterygoid infratemporal fossa dissection, the antrostomy is extended anteriorly until the nasolacrimal duct with a backbiter or a microdebrider and the base of the pterygoid plates are exposed (Figs. 5A–5D). The nasolacrimal duct should be cut sharply to keep the aperture of the duct intact, maintaining its function (Fig. 5B). Although the presence of the medial and lateral pterygoid muscles makes the dissection difficult, subperiosteal dissection along the lateral pterygoid plates will guide you to the infratemporal fossa and the foramen ovale and the mandibular nerve (Figs. 5E–5G). Image guidance would be helpful to confirm the position of the foramen ovale and the mandibular nerve. The approach can be extended to the foramen spinosum and then to the parapharyngeal carotid artery.24,25) The foramen spinosum as well as the middle meningeal artery are posterior and lateral to the foramen ovale. The parapharyngeal carotid artery enters the carotid canal posterior to the foramen spinosum and foramen ovale.
Fig. 5.
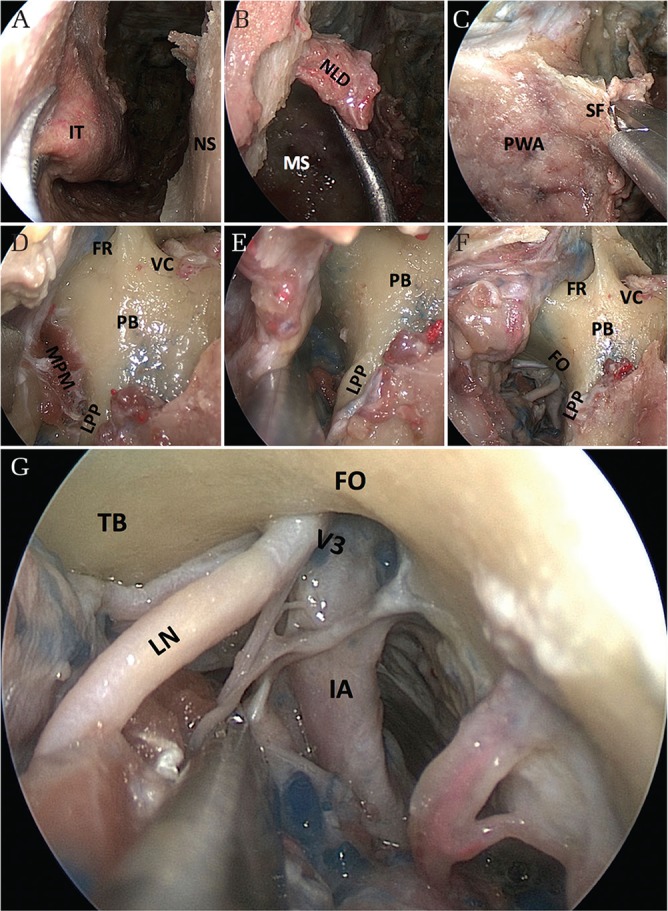
Stepwise views of surgical anatomy for the infratemporal fossa approach. (A) A maxillary antrostomy starting with a sacrifice of the inferior turbinate. (B) Nasolacrimal duct located in the anterior margin of the antrum. The duct should be cut sharply to keep its function intact. (C) Opening the pterygopalatine fossa. (D) Transpterygoid approach exposing the base of the pterygoid plates. (E, F) Subperiosteal dissection along the lateral pterygoid plate to find the foramen ovale. (G) Final view of the infratemporal fossa approach showing the contents around the foramen ovale. FO: foramen ovale, FR: foramen rotundum, IT: inferior turbinate, LPP: lateral pterygoid plate, MS: maxillar sinus, NLD: nasolacrimal duct, NS: nasal septum, PB: pterygoid body, PWA: posterior wall of the antrum, SF: sphenopalatine foramen, TB: temporal base, VC: vidian canal, V3: third division of the trigeminal nerve, V3br: branch of third division of the trigeminal nerve.
Discussion
EEAs have become an important alternative to traditional transcranial approaches in skull base surgery, especially for ventral skull base lesions. After nasal manipulation and sphenoidotomy, a wide and straight surgical corridor to the entire ventral skull base is provided under endoscopic view without brain retraction. Due to the technical and instrumental advancement in the past decade, the limits of EEA are constantly being reduced.4)
EEAs can be classified according to their anatomical orientation into the sagittal and coronal planes, having the sella turcica as the center.1–3) Approaches in the sagittal plane access the ventral skull base from the frontal sinus to the second cervical vertebra,1,2) and their surgical maneuvers in the nasal cavity are relatively simple with less obstructive structures compared to the coronal plane modules. Meanwhile, approaches in the coronal plane are relatively complicated due to anatomical complexity, and expose lesions that extend laterally to the midline of the roof of the orbit, the floor of the middle cranial fossa, and the jugular foramen.3,18,21,26,27) During dissection in the coronal plane, there are several obstructive structures, such as nasal turbinates, ethmoid and maxilla sinuses, pterygopalatine fossa, ICA, and cranial nerves. Therefore, understanding of surgical anatomy is mandatory for treating the ventrolateral skull base lesions via EEA. Laboratory hands-on training, using cadaver heads and/or training models, is an important and necessary step in the acquisition of anatomical knowledge and surgical skills for EEA.28,29)
The indications and limitations for EEAs to the ventrolateral skull base are summarized in Table 1. In the coronal plane, the most critical and defining structure is the ICA, and vascular injuries are more often associated with the treatment of ventrolateral lesions (lateral to the ICA).30) The vidian canal and the lateral OCR are two important surgical landmarks around and inside the sphenoid sinus to indicate the position of the ICA, allowing safe surgical maneuver near the ICA.10,20) Surgical instruments, such as a navigation system, a Doppler ultrasonic probe, a fluorescence imaging system, and cranial nerve monitoring, are also helpful for performing a safe procedure that avoids surgical complications during EEA.14–16)
Table 1.
Summary of indications and limitations for the endoscopic endonasal approach to the ventrolateral skull base
| Key landmarks | Indications | Limiting factors | |
|---|---|---|---|
| Cavernous sinus approach | Posterior ethmoid sinus LOCR Carotid protuberance | Cavernous sinus tumor | Cranial nerves (III, IV, V, VI) ICA (C2, C3, C4) |
| Transorbital approach | LOCR Ethmoid sinus Lamina papyracea | Orbital tumor Traumatic ophthalmopathy Graves ophthalmopathy | Optic nerve |
| Transpterygoid approach | Sphenopalatine foramen Vidian canal Foramen rotundum | Meckel’s cave tumor Infratemporal fossa tumor | Cranial nerves (V, VI) ICA (C4, C5) |
| Infratemporal fossa approach | Lateral pterygoid plate Foramen ovale | Infratemporal fossa tumor | LPtM LVP CCA |
CCA: cervical carotid artery, ICA: internal carotid artery, LOCR: lateral optico-carotid recess, LPtM: lateral pterygoid muscle, LVP: levator veli palatini muscle.
As EEA uses the sinonasal tract as the surgical corridor to the skull base, sinonasal trauma is a primary source of postoperative morbidity in many of the patients.31–33) Though most sinonasal morbidities are transient and resolve within several months, symptoms can persist for longer with more complex surgeries. Specifically, expanded EEA with harvesting of a nasoseptal flap is related to the impairment of olfaction and mucociliary clearance during the immediate postoperative period,34) which can occasionally be permanent morbidities and lower the patient’s quality of life. Therefore, preoperative surgical planning based on the surgical anatomy is important to minimize endonasal manipulation as much as possible and to prevent sinonasal complications, resulting in good surgical outcomes.
Conclusion
We demonstrated surgical anatomy for EEA to the ventrolateral skull base. Understanding of surgical anatomy is mandatory for treating ventrolateral skull base lesions via EEA. An appropriate, less-invasive approach should be applied depending on the size, location, and type of lesion.
Footnotes
Funding
This study was performed with grant support from The Japanese Foundation for Research and Promotion of Endoscopy. Cadaveric dissections were performed at both the ALT-VISION lab at the Ohio State University and the Anatomical Laboratory for Skull Base Surgery at Lariboisière Hospital. The ALT-VISION lab receives unrestricted educational support from the following companies: Carl Zeiss Microscopy, Intuitive Surgical Corp., KLS Martin Corp., Karl Storz Endoscopy, Leica Microscopy, Medtronic Corp., Stryker Corp., and Vycor Medical. The lab at Lariboisière Hospital receives unrestricted educational support from the following companies: Carl Zeiss Microscopy and Karl Storz Endoscopy.
Conflicts of Interest Disclosure
The authors have no personal financial interest in any of the drugs, materials, or devices described in this article.
References
- 1).Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL: Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus 19: E3, 2005 [PubMed] [Google Scholar]
- 2).Kassam A, Snyderman CH, Mintz A, Gardner P, Carrau RL: Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus 19: E4, 2005 [PubMed] [Google Scholar]
- 3).Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R: Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus 19: E6, 2005 [PubMed] [Google Scholar]
- 4).Prevedello DM, Doglietto F, Jane JA, Jagannathan J, Han J, Laws ER: History of endoscopic skull base surgery: its evolution and current reality. J Neurosurg 107: 206–213, 2007 [DOI] [PubMed] [Google Scholar]
- 5).Chibbaro S, Cornelius JF, Froelich S, et al. : Endoscopic endonasal approach in the management of skull base chordomas—clinical experience on a large series, technique, outcome, and pitfalls. Neurosurg Rev 37: 217–224; discussion 224–225, 2014 [DOI] [PubMed] [Google Scholar]
- 6).Froelich S, Cebula H, Debry C, Boyer P: Anterior communicating artery aneurysm clipped via an endoscopic endonasal approach: technical note. Neurosurgery 68: 310–316, 2011 [DOI] [PubMed] [Google Scholar]
- 7).Oyama K, Ikezono T, Tahara S, Shindo S, Kitamura T, Teramoto A: Petrous apex cholesterol granuloma treated via the endoscopic transsphenoidal approach. Acta Neurochir (Wien) 149: 299–302; discussion 302, 2007 [DOI] [PubMed] [Google Scholar]
- 8).Kassam AB, Thomas A, Carrau RL, et al. : Endoscopic reconstruction of the cranial base using a pedicled nasoseptal flap. Neurosurgery 63: 44–53, 2008 [DOI] [PubMed] [Google Scholar]
- 9).Hadad G, Bassagasteguy L, Carrau RL, et al. : A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope 116: 1882–1886, 2006 [DOI] [PubMed] [Google Scholar]
- 10).Rhoton ALJ: The sellar region. Neurosurgery 51: S335–S374, 2002 [PubMed] [Google Scholar]
- 11).Labib MA, Prevedello DM, Fernandez-Miranda JC, et al. : The medial opticocarotid recess: an anatomic study of an endoscopic “key landmark” for the ventral cranial base. Neurosurgery 72: 66–76, 2013 [DOI] [PubMed] [Google Scholar]
- 12).Kitano M, Taneda M: Extended transsphenoidal approach with submucosal posterior ethmoidectomy for parasellar tumors. Technical note. J Neurosurg 94: 999–1004, 2001 [DOI] [PubMed] [Google Scholar]
- 13).Kitano M, Taneda M, Shimono T, Nakao Y: Extended transsphenoidal approach for surgical management of pituitary adenomas invading the cavernous sinus. J Neurosurg 108: 26–36, 2008 [DOI] [PubMed] [Google Scholar]
- 14).Hide T, Yano S, Shinojima N, Kuratsu J: Usefulness of the indocyanine green fluorescence endoscope in endonasal transsphenoidal surgery. J Neurosurg 122: 1185–1192, 2015 [DOI] [PubMed] [Google Scholar]
- 15).Kawamata T, Ishii N, Amano K, Namioka T, Hori T, Okada Y: A novel simple real-time electrooculographic monitoring system during transsphenoidal surgeries to prevent postoperative extraocular motor nerve dysfunction. Neurosurg Rev 36: 371–376, 2013 [DOI] [PubMed] [Google Scholar]
- 16).Oyama K, Kawana F, Suenaga K, Fukuhara N, Yamada S: A handmade eye movement monitor using a piezoelectric device during transsphenoidal surgery. Neurosurg Rev 37: 287–290, 2014 [DOI] [PubMed] [Google Scholar]
- 17).Cavallo LM, Messina A, Gardner P, et al. : Extended endoscopic endonasal approach to the pterygopalatine fossa: anatomical study and clinical considerations. Neurosurg Focus 19: E5, 2005 [PubMed] [Google Scholar]
- 18).de Lara D, Ditzel Filho LF, Prevedello DM, et al. : Endonasal endoscopic approaches to the paramedian skull base. World Neurosurg 82: S121–S129, 2014 [DOI] [PubMed] [Google Scholar]
- 19).Kasemsiri P, Solares CA, Carrau RL, et al. : Endoscopic endonasal transpterygoid approaches: anatomical landmarks for planning the surgical corridor. Laryngoscope 123: 811–815, 2013 [DOI] [PubMed] [Google Scholar]
- 20).Kassam AB, Vescan AD, Carrau RL, et al. : Expanded endonasal approach: vidian canal as a landmark to the petrous internal carotid artery. J Neurosurg 108: 177–183, 2008 [DOI] [PubMed] [Google Scholar]
- 21).Kassam AB, Prevedello DM, Carrau RL, et al. : The front door to meckel’s cave: an anteromedial corridor via expanded endoscopic endonasal approach-technical considerations and clinical series. Neurosurgery 64: 71–82, 2009 [DOI] [PubMed] [Google Scholar]
- 22).Magro F, Solari D, Cavallo LM, et al. : The endoscopic endonasal approach to the lateral recess of the sphenoid sinus via the pterygopalatine fossa: comparison of endoscopic and radiological landmarks. Neurosurgery 59: 237–243, 2006 [DOI] [PubMed] [Google Scholar]
- 23).Wang J, Bidari S, Inoue K, Yang H, Rhoton A: Extensions of the sphenoid sinus: a new classification. Neurosurgery 66: 797–816, 2010 [DOI] [PubMed] [Google Scholar]
- 24).Taniguchi M, Kohmura E: Endoscopic transnasal transmaxillary transpterygoid approach to the parapharyngeal space: an anatomic study. Minim Invasive Neurosurg 53: 255–260, 2010 [DOI] [PubMed] [Google Scholar]
- 25).Theodosopoulos PV, Guthikonda B, Brescia A, Keller JT, Zimmer LA: Endoscopic approach to the infratemporal fossa: anatomic study. Neurosurgery 66: 196–202; discussion 202–203, 2010 [DOI] [PubMed] [Google Scholar]
- 26).Prevedello DM, Ditzel Filho LF, Solari D, Carrau RL, Kassam AB: Expanded endonasal approaches to middle cranial fossa and posterior fossa tumors. Neurosurg Clin N Am 21: 621–635, vi, 2010 [DOI] [PubMed] [Google Scholar]
- 27).Taniguchi M, Akutsu N, Mizukawa K, Kohta M, Kimura H, Kohmura E: Endoscopic endonasal translacerum approach to the inferior petrous apex. J Neurosurg 124: 1032–1038, 2016 [DOI] [PubMed] [Google Scholar]
- 28).Oyama K, Ditzel Filho LF, Muto J, et al. : Endoscopic endonasal cranial base surgery simulation using an artificial cranial base model created by selective laser sintering. Neurosurg Rev 38: 171–178; discussion 178, 2015 [DOI] [PubMed] [Google Scholar]
- 29).Muto J, Carrau RL, Oyama K, Otto BA, Prevedello DM: Training model for control of an internal carotid artery injury during transsphenoidal surgery. Laryngoscope 127: 38–43, 2017 [DOI] [PubMed] [Google Scholar]
- 30).Kassam AB, Prevedello DM, Carrau RL, et al. : Endoscopic endonasal skull base surgery: analysis of complications in the authors’ initial 800 patients. J Neurosurg 114: 1544–1568, 2011 [DOI] [PubMed] [Google Scholar]
- 31).Awad AJ, Mohyeldin A, El-Sayed IH, Aghi MK: Sinonasal morbidity following endoscopic endonasal skull base surgery. Clin Neurol Neurosurg 130: 162–167, 2015 [DOI] [PubMed] [Google Scholar]
- 32).Little AS, Kelly D, Milligan J, et al. : Predictors of sinonasal quality of life and nasal morbidity after fully endoscopic transsphenoidal surgery. J Neurosurg 122: 1458–1465, 2015 [DOI] [PubMed] [Google Scholar]
- 33).Pant H, Bhatki AM, Snyderman CH, et al. : Quality of life following endonasal skull base surgery. Skull Base 20: 35–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Alobid I, Enseñat J, Mariño-Sánchez F, et al. : Impairment of olfaction and mucociliary clearance after expanded endonasal approach using vascularized septal flap reconstruction for skull base tumors. Neurosurgery 72: 540–546, 2013 [DOI] [PubMed] [Google Scholar]


