Abstract
Aconiti Lateralis Radix Praeparata (Fuzi) is obtained from processed daughter roots of Aconitum carmichaeli, a toxic plant with a high medical value well known in Chinese medicine. In addition to the known toxic alkaloids (aconitine, mesaconitine, and hypaconitine) and bioactive alkaloids (benzoylaconine, benzoylmesaconine, and benzoylhypaconine), three rarely found alkaloids have been previously reported in Fuzi, i.e., yunaconitine, 8-deacetyl-yunaconitine, and crassicauline A, and they were reported in recent years to cause potential risk to patients who took Fuzi or related products. To better control the quality of this herb and its related products and ensure safe use, developing a method to simultaneously determine these 9 alkaloids is important. In this research, sensitive and accurate ultra-high-performance liquid chromatography coupled with triple quadrupole mass spectrometry method was established and used to examine 51 Fuzi and 27 Fuzi-containing products. Unexpectedly, 8-deacetyl-yunaconitine was detected in 17 Fuzi samples (33.3%) and 3 Fuzi-containing products (11.1%); yunaconitine in 10 Fuzi samples (19.6%) and 10 Fuzi-containing products (37.0%); and crassicauline A in 3 Fuzi samples (5.8%). Industry and clinics should be aware of the unusually high detection rate of these three toxic alkaloids in the Fuzi herb and its related products and take the necessary precautions to protect patients from any potential risk.
Introduction
Aconiti Lateralis Radix Praeparata (Fuzi in Pinyin), the processed daughter root of Aconitum carmichaeli Debx. (Family Ranunculaceae), is a well-known Chinese medical material recorded in the Chinese Pharmacopoeia (CHP)1 and other traditional Chinese medicine (TCM) texts. Fuzi has been used for over 2000 years as an analgesic and anti-inflammatory agent in clinics2. According to TCM theories, Fuzi is very toxic2. Modern experimental studies have shown that diester-diterpenoid alkaloids (DDAs) are the active toxic ingredients in Fuzi. These toxic DDAs have cardiac, analgesic, and anti-inflammatory activities3–5. To reduce the toxicity, crude Fuzi must be processed by heating or steaming, and processed Fuzi must be boiled before oral administration according to TCM theories and practice. During these processes, aconitine (AC), mesaconitine (MA), and hypaconitine (HA), which are the main toxic DDAs in Fuzi, degrade into less toxic but still active monoester-diterpenoid alkaloids (MDAs), such as benzoylaconine (BAC), benzoylmesaconine (BMA), and benzoylhypaconine (BHA), respectively6. Therefore, 6 aconitum alkaloids have been selected in the CHP as the target elements for the quality control of aconitum herbs. According to the CHP (2015 Edition), the total amount of AC, MA and HA in Fuzi must be less than 0.020%, and the total amount of BAC, BMA and BHA must be higher than 0.010%. Nevertheless, poisoning cases are still occasionally reported. From 1989 to 2010, 140 cases of aconitum poisoning, including one fatal case, were reported in Hong Kong7. Additionally, 17 cases were reported in Taiwan from 1990–1999, 2017 cases in China from 1989–2008, and 121 cases in Korea from 1995–20078. Multiple reasons for aconitum poisoning exist and include over doses, inadequate processing, aconitum contamination in other herbs, dispensing and management errors, and hidden risk factors7. In the 17 cases reported in Hong Kong, yunaconitine (YAC), crassicauline A (CCA), and 8-deacetyl-yunaconitine (DYA) were detected instead of AC, MA and HA in the urine samples of the aconitum poisoning patients7,9. Although YAC and CCA are two DDAs with toxicities similar to AC10, they are mainly isolated from A. forrestii, A. crassicaule, and other aconitum species9, not from A. carmichaeli. Therefore, the surveillance of toxic alkaloids from aconitum herbs always neglects YAC and CCA. DYA is the hydrolytic product of yunaconitine. Although DYA is less toxic than YAC, it still presents a safety risk because of the conversion between the two compounds11. Because YAC, DYA and CCA were detected in the urine of the aconitum poisoning patients, these alkaloids are considered to be hidden risk factors and should be covered in laboratory screenings for toxic compounds9. Therefore, a method to simultaneously determine the levels of these 9 alkaloids is needed for quality control of the herb and its products and to ensure the safe use of these medical materials.
Although the high-performance liquid chromatography (HPLC) and liquid chromatography hyphenated with mass spectrometry (LC/MS) methods have been broadly applied for the quantification of aconitum alkaloids in herb or herbal products and body fluids over the past few years12–18, the maximum number of aconitum alkaloids simultaneously determined using these methods is only 7, i.e., AC, MA, HA, BAC, BMA, BHA, and YAC. The challenge in simultaneously determining the 9 aconitum alkaloids is that the level of some these alkaloids are significantly lower than the detection limits of the current methods. In the present study, ultra-high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (UHPLC–QQQ–MS/MS) was used to simultaneously determine the contents of 9 aconitum alkaloids in Fuzi and Fuzi-containing products. The method was rapid, sensitive, accurate and fully validated and was used to analyze 51 Fuzi samples and 27 Fuzi-containing product samples Table 1. In addition to quality control for Fuzi and its preparations, this method is also valuable for toxicological and forensic studies of related samples.
Table 1.
Sample information for the Fuzi samples and the related products.
| Sample number | Sample name | Source (region) |
|---|---|---|
| FZ01-FZ13 | Aconiti Lateralis Radix Praeparata | Hong Kong |
| FZ14-FZ15 | Aconiti Lateralis Radix Praeparata | Jiangxi Province |
| FZ16-FZ20 | Aconiti Lateralis Radix Praeparata | Hong Kong |
| FZ21-FZ23 | Aconiti Lateralis Radix Praeparata | Changsha, Hunan Province |
| FZ24-FZ26 | Aconiti Lateralis Radix Praeparata | Gansu Province |
| FZ27-FZ28 | Aconiti Lateralis Radix Praeparata | Bozhou, Anhui Province |
| FZ29-FZ30 | Aconiti Lateralis Radix Praeparata | Zhuhai, Guangdong Province |
| FZ31-FZ32 | Aconiti Lateralis Radix Praeparata | Guangzhou, Guangdong Province |
| FZ33-FZ35 | Aconiti Lateralis Radix Praeparata | Yunnan Province |
| FZ36-FZ38 | Aconiti Lateralis Radix Praeparata | Dalian, Liaoning Province |
| FZ39-FZ40 | Aconiti Lateralis Radix Praeparata | Macau |
| FZ41-FZ43 | Aconiti Lateralis Radix Praeparata | Hong Kong |
| FZ44-FZ45 | Aconiti Lateralis Radix Praeparata | Butuo, Sichuan Province |
| FZ46 | Aconiti Lateralis Radix | Butuo, Sichuan Province |
| FZ47 | Aconiti Lateralis Radix | Butuo, Sichuan Province |
| FZ48-FZ50 | Aconiti Lateralis Radix Praeparata | Taiwan |
| FZ51 | Aconiti Lateralis Radix Praeparata | Hong Kong |
| HSP01-HSP11 | Heishunpian granule | Hong Kong |
| DFP01-DFP03 | Danfupian granule | Shenzhen |
| BFZ | Baifuzi granule | Shenzhen |
| PFP | Paofupian granule | Shenzhen |
| YGW01-YGW02 | You-gui-wan | Shenzhen |
| SNT | Si-ni-tang | Shenzhen |
| MFXT01-MFXT02 | Ma-huang-fu-zi-xi-xin-tang | Shenzhen |
| ZWT | Zhen-wu-tang | Shenzhen |
| FLT | Fu-zi-li-zhong-tang | Shenzhen |
| JGSQ01-JGSQ02 | Jin-gui-shen-qi-wan | Shenzhen |
| XSLJT01-XSLJT02 | Xiang-sha-liu-jun-zi-tang | Shenzhen |
Results
UHPLC-MS optimization
Different solvents and gradient profiles of the mobile phase were compared to achieve a good resolution within 15 min. Acetonitrile and an acidic aqueous solution significantly improved the resolution and the symmetry of the target compounds. Acetonitrile-0.1% formic acid was used as the mobile phase with the gradient mentioned in the Materials and methods section at a flow rate of 0.35 mL/min.
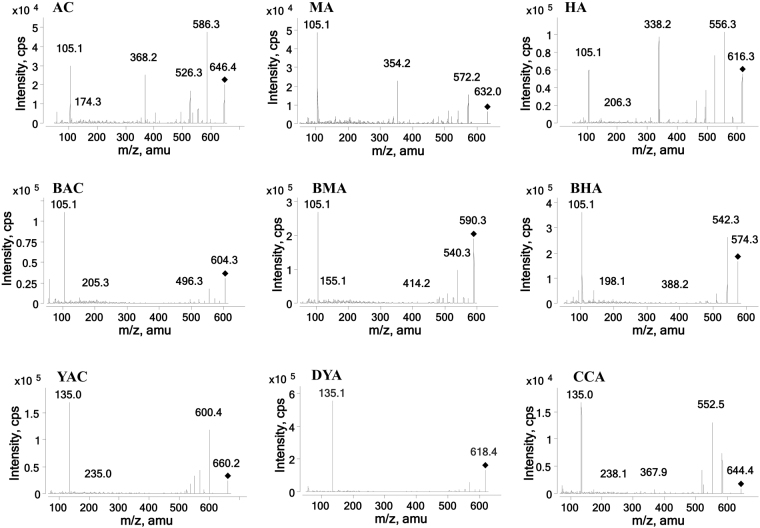
The negative and positive ion modes were compared for the MS analysis. The positive mode resulted in a higher sensitivity and cleaner mass spectral background than the negative mode. The collision energy and fragmentor voltage parameters were optimized to obtain the highest relative abundance of the exclusive ions and production in the multiple reaction monitoring (MRM) optimization conditions. The final conditions for the collision energy and fragmentor voltage are shown in the Instruments and UHPLC-MS conditions section. The MS/MS ion spectra of the 9 alkaloids are shown in Fig. 1.
Figure 1.
Full scan product ion mass spectra of the 9 compounds.
Method validation
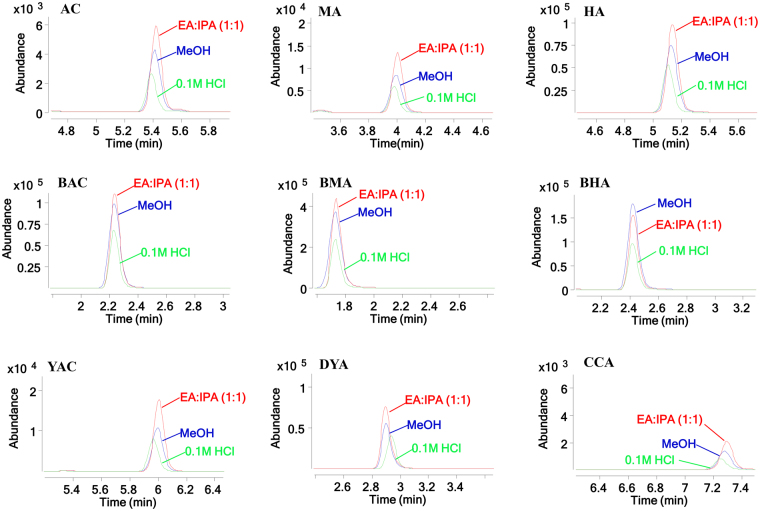
The extraction method, including the extraction solvent and extraction times, was optimized to effectively extract the 9 alkaloids with a large difference in the content levels for the UHPLC-MS analysis. A 0.1 M hydrochloric acid solution (50 mL), 50 mL of methanol with 3 mL of the ammonia test solution (ammonia TS, prepared by adding water to 400 mL of a 28% ammonia solution to make 1000 mL, according to the CHP), and 50 mL of isopropanol (IPA) and ethyl acetate (EA) (1:1) with 3 mL of the ammonia TS were compared as the extraction solvents. Ethyl acetate and isopropanol (1:1) with 3 mL of ammonia TS were the most efficient for extracting the alkaloids (Figure 2 and Table 2). The extraction times were also compared. One, two, and three times for the repeated extraction resulted in insignificant differences. Therefore, a single extraction was selected for this analysis. The optimal sample preparation method was extracting 2 g of sample with 50 mL ethyl acetate and isopropanol (1:1) and 3 mL ammonia TS in an ultrasonicator for 30 min.
Figure 2.
Extraction efficiency of the 9 compounds in the Fuzi samples using different solvents.
Table 2.
The peak areas of the 9 aconitum alkaloids in the Fuzi sample (FZ06) using the extraction method.
| Extraction methods | AC | MA | HA | BAC | BMA | BHA | YAC | DYA | CCA |
|---|---|---|---|---|---|---|---|---|---|
| EA:IPA (1:1) | 30906 | 68567 | 552857 | 524633 | 2171283 | 750078 | 92461 | 357995 | 12834 |
| MeOH | 23947 | 50014 | 460300 | 504798 | 2070036 | 988550 | 60098 | 255022 | 8767 |
| 0.1 M HCl | 13272 | 28606 | 284949 | 311592 | 1116647 | 465404 | 41436 | 191209 | 4919 |
The calibration curves with at least 5 different concentrations were analyzed. Table 3 lists the linear calibration curves with the R value, linear range, LOQ, LOD, and repeatability. Table 4 lists the precision, stability and recovery of the 9 alkaloids. A signal-to-noise ratio (S/N) of approximately 3 was set as the LOD, and a S/N of 10 was set as the LQD. All the calibration curves showed good linear ranges (R > 0.999) within the respective ranges and were adequate for the determinations of the 9 alkaloids in the samples.
Table 3.
Linearity, range and limits of determination and quantification.
| Analytes | Linearity | R | Range (ng/mL) | LOD (ng/mL) | LOQ (ng/mL) |
|---|---|---|---|---|---|
| AC | Y = 20447X + 6476 | 0.9997 | 0.656–6560 | 0.131 | 0.656 |
| MA | Y = 19008X + 4958 | 0.9997 | 0.546–5460 | 0.109 | 0.546 |
| HA | Y = 18548X + 8882 | 0.9995 | 0.789–7890 | 0.158 | 0.789 |
| BAC | Y = 22899X + 9210 | 0.9994 | 0.565–5650 | 0.113 | 0.565 |
| BMA | Y = 19125X + 31020 | 0.9990 | 1.65–16500 | 0.330 | 1.650 |
| BHA | Y = 24881X + 10680 | 0.9993 | 0.591–5910 | 0.118 | 0.591 |
| YAC | Y = 385584X + 5933 | 0.9998 | 0.495–4950 | 0.099 | 0.495 |
| DYA | Y = 42795X + 8856 | 0.9998 | 0.508–5080 | 0.102 | 0.508 |
| CCA | Y = 45812X − 1659 | 1.0000 | 0.253–2530 | 0.051 | 0.253 |
Table 4.
Precision, repeatability, stability and recovery of the 9 compounds.
| Analyte | Precision RSD (%) (n = 6) | Repeatability RSD (%) (n = 6) | Stability RSD (%) (n = 6) | Recovery | ||||
|---|---|---|---|---|---|---|---|---|
| Original (μg) | Spiked (μg) | Found (μg) | Recovery (%) | RSD (%) | ||||
| AC | 2.90 | 1.52 | 1.95 | 1.72 | 1.73 | 3.42 | 98.2 | 2.54 |
| MA | 1.80 | 1.75 | 2.11 | 3.42 | 3.56 | 7.04 | 101.7 | 2.49 |
| HA | 2.35 | 1.79 | 1.55 | 50.8 | 50.3 | 100 | 98.9 | 3.30 |
| BAC | 1.39 | 1.36 | 1.54 | 52.4 | 52.3 | 104 | 99.1 | 2.65 |
| BMA | 0.818 | 1.38 | 1.04 | 222 | 220 | 442 | 100.1 | 2.35 |
| BHA | 2.55 | 1.47 | 0.369 | 83.2 | 83.1 | 165 | 99.2 | 1.71 |
| YAC | 1.32 | 1.05 | 1.38 | 1.08 | 1.12 | 2.19 | 98.8 | 3.32 |
| DYA | 1.29 | 1.36 | 1.77 | 5.66 | 5.66 | 11.3 | 100.1 | 2.73 |
| CCA | 1.36 | 1.86 | 1.67 | 0.584 | 0.592 | 1.17 | 99.5 | 2.92 |
The precision was determined by measuring one concentration level six times in the same day. The repeatability was tested using six samples that were prepared using the same sample and method. The stability was determined over periods of 0, 2, 4, 6, 8 and 12 h in one day. The results (Table 4) indicated that the method for the investigated samples had good precision and reproducibility. In addition, the compounds were sufficiently stable and could be routinely analyzed within 12 h at room temperature.
The recovery was examined by spiking a certain amount (1 g) of a Fuzi sample with a known amount of the mixed standards (n = 6). The recoveries were calculated using the equation below:
Recovery (%) = (total amount detected−amount original)/amount spiked × 100%
As shown in Table 4, the recoveries of the 9 alkaloids ranged from 98.2% to 101.7%, and the RSD values were less than 4.0%, which showed the high quantification accuracy.
Sample determination
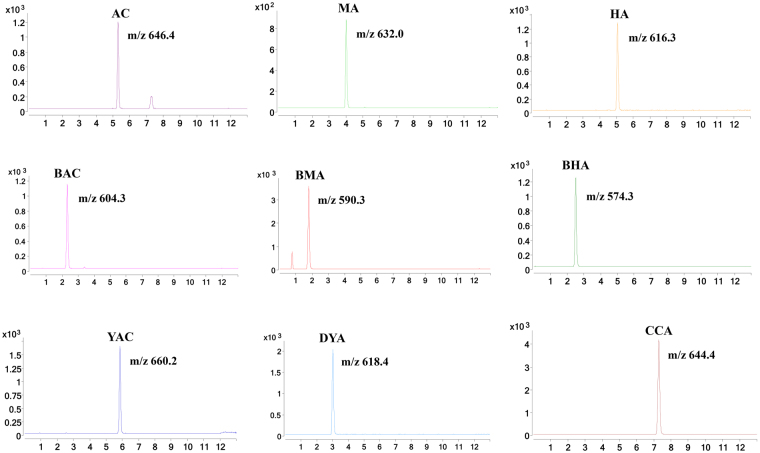
The established method was successfully used for the simultaneous determination of 9 alkaloids in 51 Fuzi samples and 27 Fuzi-containing products. The representative MRM chromatograms of the reference standard mixture and the Fuzi sample are shown in Fig. 3. The contents of the 9 alkaloids are summarized in Table 5, and the content distribution of the 9 alkaloids in different regions of China is shown in Fig. 4.
Figure 3.
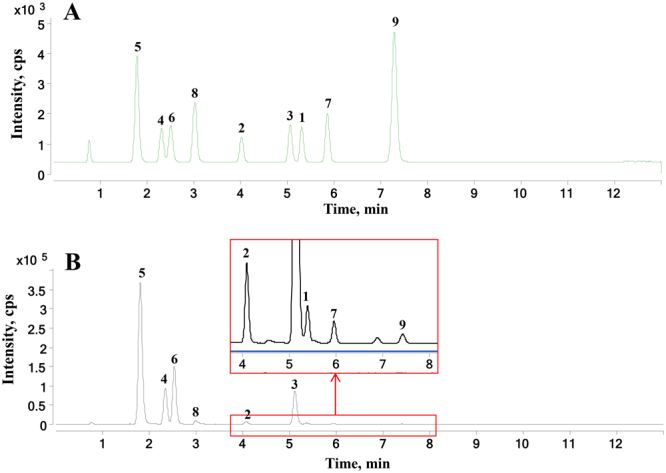
Representative MRM chromatograms of the reference standard mixture (A) and Fuzi samples (B). 1, AC; 2, MA; 3, HA; 4, BAC; 5, BMA; 6, BHA; 7, YAC; 8, DYA; 9, CCA.
Table 5.
The contents of the 9 aconitum alkaloids in the Fuzi samples and Fuzi-containing products.
| Sample number | AC (μg/g) | MA (μg/g) | HA (μg/g) | BAC (μg/g) | BMA (μg/g) | BHA (μg/g) | YAC (μg/g) | DYA (μg/g) | CCA (μg/g) | Total contents (%) AC + MA + HA | Total contents (%) BAC + BMA + BHA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FZ01 | 2.52 | 11.5 | 62.1 | 53.7 | 296 | 80.6 | — | 1.66 | — | 0.00761 | 0.0430 |
| FZ02 | 1.41 | 7.03 | 52.0 | 48.4 | 259 | 71.3 | — | 1.64 | — | 0.00604 | 0.0379 |
| FZ03 | 0.170 | 0.710 | 8.53 | 83.8 | 22.5 | 41.0 | — | — | — | 0.000941 | 0.0147 |
| FZ04 | 15.5 | 59.3 | 79.9 | 30.0 | 199 | 23.6 | — | 0.0106 | — | 0.0155 | 0.0253 |
| FZ05 | 0.0200 | 0.690 | 14.4 | 25.3 | 95.9 | 52.3 | — | — | — | 0.00151 | 0.0174 |
| FZ06 | 0.630 | 5.25 | 71.2 | 57.7 | 244 | 82.6 | 0.838 | 3.57 | 0.0454 | 0.00771 | 0.0384 |
| FZ07 | 5.16 | 17.7 | 82.6 | 63.1 | 279 | 91.1 | 0.0530 | 1.64 | — | 0.0105 | 0.0433 |
| FZ08 | 20.8 | 75.2 | 9.98 | 36.7 | 211 | 29.1 | — | 0.0677 | — | 0.0106 | 0.0277 |
| FZ09 | 0.963 | 22.0 | 79.9 | 38.1 | 115 | 64.7 | — | — | — | 0.0103 | 0.0218 |
| FZ10 | 0.0100 | 0.160 | 47.6 | 44.8 | 196 | 60.1 | 0.277 | 1.34 | 0.152 | 0.00478 | 0.0301 |
| FZ11 | 26.0 | 16.4 | 123 | 45.4 | 233 | 37.2 | — | 0.0256 | — | 0.0165 | 0.0316 |
| FZ12 | 22.6 | 43.8 | 330 | 390 | 1800 | 382 | — | 0.233 | — | 0.0396 | 0.257 |
| FZ13 | 1.44 | 3.04 | 22.1 | 32.9 | 162 | 33.1 | — | — | — | 0.00266 | 0.0228 |
| FZ14 | 3.21 | 8.50 | 58.9 | 51.6 | 196 | 56.2 | — | — | — | 0.00706 | 0.0304 |
| FZ15 | 6.64 | 24.2 | 81.2 | 43.6 | 258 | 45.5 | — | — | — | 0.0112 | 0.0347 |
| FZ16 | 0.127 | 0.153 | 5.04 | 16.2 | 91.8 | 22.6 | — | — | — | 0.000532 | 0.0131 |
| FZ17 | 0.519 | 0.870 | 12.5 | 25.5 | 171 | 38.9 | — | — | — | 0.00139 | 0.0235 |
| FZ18 | 0.314 | 0.335 | 11.0 | 29.6 | 103 | 51.1 | — | — | — | 0.00116 | 0.0184 |
| FZ19 | 0.899 | 1.52 | 8.32 | 70.8 | 242 | 172 | — | — | — | 0.00107 | 0.0485 |
| FZ20 | 1.47 | 0.28 | 5.31 | 90.4 | 499 | 139 | — | 0.0262 | — | 0.000706 | 0.0728 |
| FZ21 | 18.6 | 38.6 | 169 | 31.2 | 125 | 44.7 | 0.0455 | 0.0191 | — | 0.0226 | 0.0201 |
| FZ22 | 26.6 | 85.7 | 129 | 48.1 | 291 | 45.9 | — | 0.00483 | — | 0.0241 | 0.0385 |
| FZ23 | 22.9 | 75.0 | 149 | 51.8 | 331 | 52.9 | — | — | — | 0.0247 | 0.0436 |
| FZ24 | 40.5 | 33.7 | 77.6 | 44.8 | 146 | 50.7 | — | 0.0105 | — | 0.0152 | 0.0242 |
| FZ25 | 5.59 | 11.9 | 114 | 41.8 | 158 | 80.5 | — | — | — | 0.0131 | 0.0280 |
| FZ26 | 144 | 314 | 98.2 | 66.8 | 299 | 11.4 | — | — | — | 0.0556 | 0.0377 |
| FZ27 | 4.76 | 14.5 | 127 | 98.1 | 379 | 257 | — | — | — | 0.0146 | 0.0734 |
| FZ28 | 6.79 | 10.3 | 113 | 136 | 522 | 265 | — | 0.00708 | — | 0.0130 | 0.0923 |
| FZ29 | 2.06 | 8.13 | 14.7 | 13.5 | 72.3 | 19.6 | — | — | — | 0.00249 | 0.0105 |
| FZ30 | 5.24 | 15.0 | 94.3 | 52.4 | 329 | 59.9 | — | — | — | 0.0115 | 0.0441 |
| FZ31 | 29.4 | 69.5 | 164 | 21.4 | 99.4 | 25.19 | — | — | — | 0.0263 | 0.0146 |
| FZ32 | 22.0 | 84.5 | 98.2 | 34.6 | 247 | 24.45 | 0.00711 | 0.00648 | — | 0.0205 | 0.0306 |
| FZ33 | 24.0 | 78.6 | 133 | 25.6 | 164 | 104 | — | — | — | 0.0236 | 0.0294 |
| FZ34 | 4.13 | 10.5 | 36.7 | 43.2 | 133 | 44.6 | — | — | — | 0.00513 | 0.0221 |
| FZ35 | 4.68 | 11.3 | 84.4 | 26.3 | 141 | 42.6 | — | — | — | 0.0100 | 0.0210 |
| FZ36 | 1.81 | 0.770 | 82.2 | 119 | 649 | 255 | 0.0151 | — | — | 0.00848 | 0.102 |
| FZ37 | 49.9 | 6.34 | 243 | 123 | 505 | 102 | 0.0988 | — | — | 0.0299 | 0.0730 |
| FZ38 | 89.4 | 12.3 | 227 | 69.0 | 226 | 37.3 | 0.0824 | — | — | 0.0329 | 0.0332 |
| FZ39 | 0.779 | 1.49 | 54.2 | 23.3 | 103 | 56.9 | 0.290 | 0.0102 | — | 0.00565 | 0.0183 |
| FZ40 | 13.4 | 34.2 | 73.7 | 34.6 | 165 | 33.8 | — | — | — | 0.0121 | 0.0233 |
| FZ41 | 2.59 | 4.79 | 34.8 | 74.0 | 256 | 208 | — | — | — | 0.00422 | 0.0538 |
| FZ42 | 0.290 | 0.560 | 8.78 | 20.0 | 100 | 24.9 | — | — | — | 0.000963 | 0.0145 |
| FZ43 | — | — | 6.39 | 30.0 | 119 | 135 | — | — | — | 0.000639 | 0.0284 |
| FZ44 | 0.585 | 0.949 | 6.47 | 37.4 | 212 | 13.9 | — | — | — | 0.000800 | 0.0263 |
| FZ45 | 0.726 | 1.27 | 5.53 | 36.3 | 213 | 11.0 | — | — | — | 0.000753 | 0.0260 |
| FZ46 | 170 | 490 | 312 | 4.33 | 31.6 | 5.89 | — | — | — | 0.0972 | 0.00418 |
| FZ47 | 47.3 | 258 | 184 | 3.32 | 38.7 | 3.34 | — | — | — | 0.0489 | 0.00454 |
| FZ48 | 3.67 | 5.28 | 68.0 | 194 | 679 | 84.9 | — | — | — | 0.00770 | 0.0958 |
| FZ49 | 16.0 | 65.9 | 37.0 | 10.7 | 66.5 | 3.43 | — | — | — | 0.0119 | 0.00807 |
| FZ50 | 5.50 | 25.8 | 101 | 75.0 | 674 | 37.4 | — | — | — | 0.0133 | 0.0786 |
| FZ51 | 0.762 | 0.851 | 36.8 | 89.5 | 428 | 131 | 2.25 | 0.665 | 0.337 | 0.0038 | 0.0649 |
| Minimum | 0.0100 | 0.153 | 5.04 | 3.32 | 22.5 | 3.34 | 0.00711 | 0.00483 | 0.0454 | ||
| Maximum | 170 | 490 | 330 | 390 | 1800 | 382 | 0.838 | 3.57 | 0.152 | ||
| Average | 17.8 | 42.2 | 84.2 | 57.3 | 263 | 74.3 | 0.190 | 0.642 | 0.099 | ||
| Median | 4.76 | 11.5 | 75.65 | 43.4 | 205 | 48.3 | 0.0824 | 0.0259 | 0.0987 | ||
| HSP01 | — | — | 0.566 | 45.2 | 259 | 110 | 3.00 | — | — | ||
| HSP02 | — | — | 1.05 | 63.7 | 336 | 147 | 4.26 | — | — | ||
| HSP03 | 0.794 | 2.65 | 31.7 | 24.0 | 152 | 40.1 | — | — | — | ||
| HSP04 | — | — | 0.067 | 43.4 | 241 | 103 | — | — | — | ||
| HSP05 | 0.0481 | 0.140 | 6.11 | 14.6 | 70.3 | 46.8 | — | — | — | ||
| HSP06 | 0.266 | — | — | 37.7 | 312 | 121 | — | — | — | ||
| HSP07 | — | 0.00660 | 0.272 | 54.7 | 333 | 92.8 | 0.141 | — | — | ||
| HSP08 | — | 0.375 | 11.2 | 74.3 | 389 | 199 | 1.34 | — | — | ||
| HSP09 | 0.130 | 1.21 | 59.2 | 77.3 | 299 | 112 | — | — | — | ||
| HSP10 | — | 0.191 | 6.95 | 108 | 197 | 144 | 1.64 | — | — | ||
| HSP11 | — | — | 0.551 | 37.6 | 280 | 35.7 | — | — | — | ||
| DFP01 | — | — | 0.655 | 33.3 | 211 | 63.2 | — | — | — | ||
| DFP02 | — | 0.482 | — | 0.081 | — | 0.591 | — | — | — | ||
| DFP03 | 0.368 | 0.289 | — | 0.0876 | 0.919 | 1.27 | — | — | — | ||
| BFZ | — | 0.0658 | 0.164 | 0.256 | 2.17 | 0.404 | — | — | — | ||
| PFP | — | — | 0.340 | 10.7 | 37 | 17.7 | — | — | — | ||
| YGW01 | — | — | 0.122 | 0.758 | 5.8 | 1.47 | 0.0302 | — | — | ||
| YGW02 | 0.0258 | 1.97 | 0.400 | 2.19 | 13.6 | 4.15 | 0.388 | 0.0328 | — | ||
| SNT | — | — | 1.04 | 7.04 | 45.1 | 13.8 | 0.0981 | — | — | ||
| MFXT01 | — | 0.0145 | 1.79 | 14.6 | 83.9 | 27.8 | 0.276 | 0.0912 | — | ||
| MFXT02 | — | 0.0214 | 2.35 | 11.6 | 70.1 | 21.9 | 0.402 | 0.0886 | — | ||
| ZWT | — | 0.0798 | 1.09 | 5.69 | 31.1 | 14.5 | — | — | — | ||
| FLT | — | 0.0811 | 2.35 | 11.6 | 70.1 | 5.72 | — | — | — | ||
| JGSQ01 | — | — | 0.0324 | 0.677 | 0.317 | 1.31 | — | — | — | ||
| JGSQ02 | 0.374 | 1.10 | 1.75 | 1.60 | 9.69 | 1.97 | — | — | — | ||
| XSLJT01 | — | — | — | — | — | — | — | — | — | ||
| XSLJT02 | — | — | — | — | — | — | — | — | — | ||
| Minimum | 0.0258 | 0.00660 | 0.0324 | 0.0810 | 0.317 | 0.404 | 0.0302 | 0.0328 | |||
| Maximum | 0.794 | 2.65 | 59.2 | 108 | 389 | 199 | 4.26 | 0.0912 | |||
| Average | 0.287 | 0.578 | 5.90 | 27.2 | 144 | 53.1 | 1.16 | 0.0709 | |||
| Median | 0.266 | 0.191 | 1.045 | 14.6 | 77.1 | 27.8 | 0.395 | 0.0886 |
Notes:
“—” under the lower limit of detection.
FZ, Aconiti Lateralis Radix Praeparata; HPS: Heishunpian granule; DFP: Danfupian; BFZ: Baifuzi; YGW: You-gui-wan; SNT: Si-ni-tang; MFXT: Ma-huang-fu-zi-xi-xin-tang; ZWT: Zhen-wu-tang; FLT: Fu-zi-li-zhong-wan; JGSQ: Jing-gui-shen-qi-wan.
AC: aconitine; MA: mesaconitine; HA: hypaconitine; BAC: benzoylaconine; BMA: benzoylmesaconine; BHA: benzoylhypaconine; YAC: yunaconitine; CCA: crassicauline A; DYA: 8-deacetyl-yunaconitine.
Figure 4.
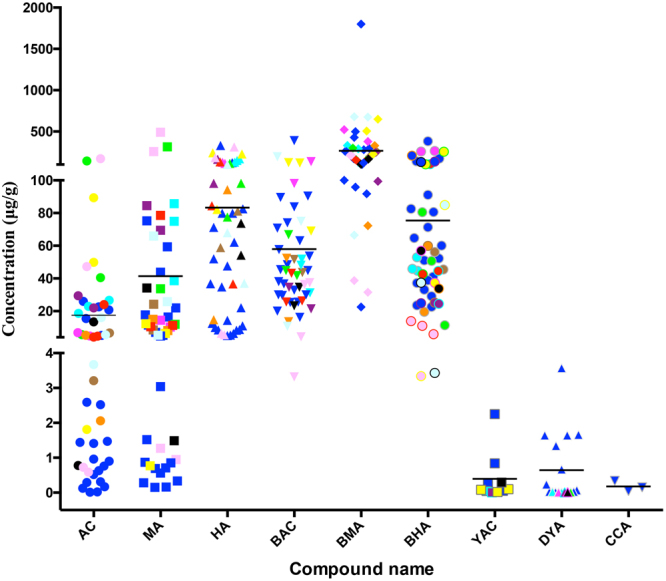
Distribution of the content of the 9 compounds in the Fuzi samples from different regions of P.R. China. Blue: Hong Kong, brown: Jiangxi, cyan: Changsha, green: Gansu, magenta: Bozhou, orange: Zhuhai, purple: Guangzhou, red: Yunnan, yellow: Dalian, black: Macao, pale magenta: Butuo, pale cyan: Taiwan. The black line represents the mean concentration of each compound.
Discussion
An optimized method for quantifying the main constituents in pharmaceutical preparations is important for quality control standards to ensure the safety, quality and effectiveness of Chinese herbal products. In this study, 3 active alkaloids and 6 toxic alkaloids in Fuzi were selected as chemical markers for quality control. A total of 51 samples, 49 processed Fuzi and 2 unprocessed Fuzi samples, were collected from 10 areas in China, including 8 provinces and 2 special administrative regions, namely, Guangdong (Zhuhai, Guangzhou), Hunan (Changsha), Anhui (Bozhou), Jiangxi, Gansu, Liaoning (Dalian), Yunnan, and Taiwan Provinces, as well as Hong Kong and Macao, providing a good geographical distribution of the samples.
The total concentrations of BAC, BMA, and BHA in the 49 processed Fuzi samples complied with the CHP criteria except for one sample (no. FZ49) that was below the CHP limits. In these 49 samples, the contents of AC, MA and HA were beyond the CHP limits in 10 batches; i.e., approximately 20.4% of the processed Fuzi samples did not meet the requirements of the national pharmacopoeia of China, which implies a quality control matter for processed Fuzi. The contents of the six aconitum alkaloids (AC, MA, HA, BAC, BMA and BHA) in the two unprocessed aconitum roots (No. FZ46 and FZ47) were above the CHP limits, showing that aconitum roots need to be processed to ensure safety. The unqualified Fuzi samples were collected from Hong Kong (one batch, No. F12), Changsha (3 batches, No. F21, F22 and F23), Gansu Province (one batch, No. F26), Guangzhou (2 batches, No. F31 and F32), Yunnan Province (one batch, No. F33) and Dalian (2 batches, No. F37 and F38) (Fig. 5).
Figure 5.
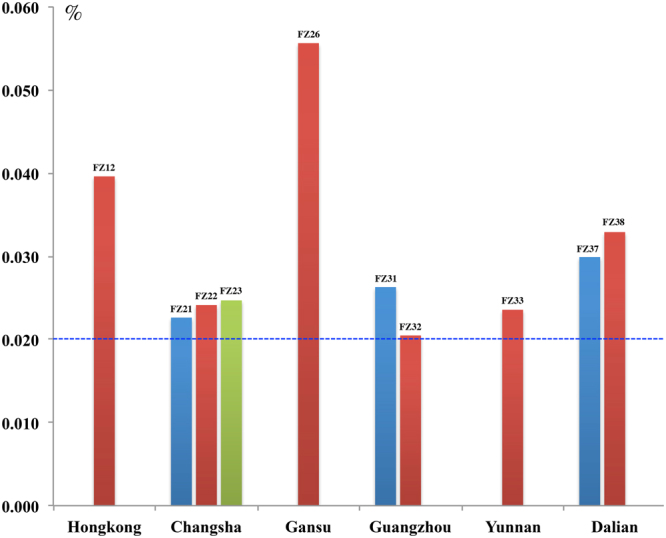
The AC, MA and HA contents in 10 batches from five places that were beyond the limits set by the Chinese Pharmacopoeia. Blue dotted line: the upper limit of the total amounts of AC, MA and HA according to the Chinese Pharmacopoeia, i.e., less than 0.020%.
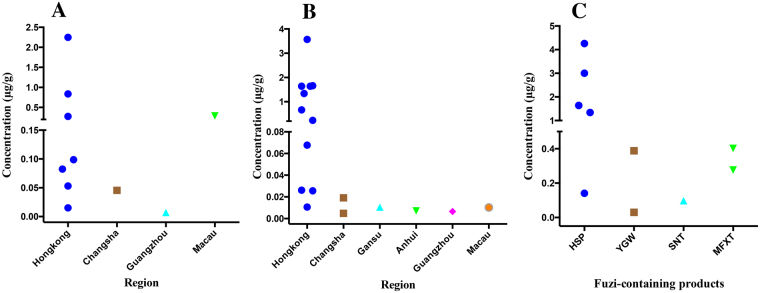
YAC was detected in 10 of the 51 Fuzi samples at concentrations ranging from 0.00711 to 2.25 μg/g. These samples were collected from five places, including Hong Kong, Changsha, Guangzhou, Dalian and Macao. Meanwhile, DYA was detected in 17 Fuzi samples at concentrations ranging from 0.00483 to 3.57 μg/g. Figures 6A and B show the contents of YAC and DYA in Fuzi samples from different areas, and the contents were highest in the samples from Hong Kong. CCA was detected in 3 samples (No. FZ06, FZ10 and FZ51 from Hong Kong) at concentrations of 0.0454, 0.152 and 0.337 μg/g, respectively. Three Fuzi samples (approximately 5.88%, no. FZ06, FZ10 and FZ51) had all three alkaloids (YAC, DYA and CCA). Four samples (approximately 7.84%, No. FZ07, FZ21, FZ32 and FZ39) contained trace amounts of both YAC and DYA.
Figure 6.
The contents of the three toxic alkaloids (YAC, DYA and CCA) in some of the Fuzi samples and Fuzi-containing products. (A) The YAC content in the Fuzi samples and the distribution area, (B) the DYA content in the Fuzi samples and the distribution area, (C) the YAC content in Fuzi-containing products from Hong Kong.
In addition, 27 samples of Fuzi-containing products were also analyzed. YAC was found in 10 samples (Fig. 6C), and a trace amount of DYA was detected in 3 samples (No. YGW02, MFXT01 and MFXT02). In addition, the distributions of the contents of the 9 components in all the Fuzi-containing products are shown in Fig. 7.
Figure 7.
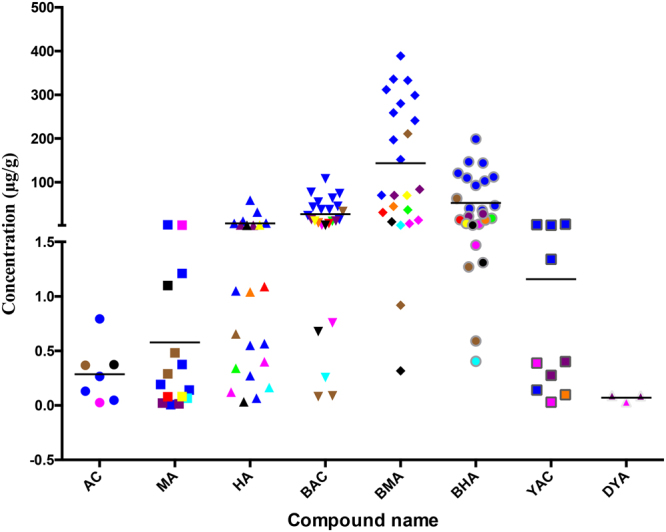
Distribution of the content of the 9 compounds in the Fuzi-containing products from Hong Kong. Blue: HPS, brown: DFP, cyan: BFZ, green: PFP, magenta: YGW, orange: SNT, purple: MFT, red: ZWT, yellow: FLT, black: JGSQ. The black line represents the mean concentration of each compound.
YAC, DYA and CCA were detected in one third of the Fuzi samples (17/51) and 37% of the Fuzi-containing products (10/27). DYA was the most frequently detected alkaloid in the Fuzi samples, and YAC was the most frequently detected alkaloid in the Fuzi-containing products. Both compounds have a high detection rate, which has not been reported before. YAC, DYA and CCA are toxic aconitum alkaloids, and the acute toxicity of YAC is comparable to that of aconitine18. The detection of the three alkaloids at such a high rate implies that there may be unknown risks for patients who consume Fuzi or Fuzi-containing products. One of the major alkaloids in A. vilmorinianum, A. hemsleyanum and A. nagarum is YAC19. The roots of the above species are occasionally misrepresented as Radix Aconiti Kusnezoffii and used as “Caowu” (in Chinese) in Chinese herbal medicine. In addition, the detection of YAC was reported in some herbs that are closely related to Fuzi, such as Aconiti Radix Preparata, Aconiti Kusnezoffii Radix, and Aconiti Kusnezoffii Radix Preparata, and products containing these herbs, but not in Fuzi14. The previously published literature also implies that the determination of YAC, DYA and CCA contents in Fuzi and Fuzi-containing products is a safety issue when Fuzi and its products are used in clinics.
In recent years, the frequency of aconitum poisoning has increased, and this could be a result of the YAC, DYA and CCA contents in processed Fuzi, as discovered by the current study. Currently, the YAC or CCA contents in aconitum are unknown, and only two studies have reported the isolation of YAC and DYA from Fuzi20,21. However, the Fuzi source was not clearly presented in the reports, and the evidence was insufficient to draw a conclusion. Therefore, a possible reason for the detection of YAC, DYA and CCA in Fuzi could be contamination. YAC and CCA, which are mainly found in certain aconitum roots from Southwest China, were most commonly detected22. Fuzi may be contaminated by aconitum roots via accidental contamination, misidentification or even intentional adulteration. In the production, storage, packaging and transport of herbal medicines, unwanted introduction of foreign substances, including toxic plants or weeds, can occur (World Health Organization, 2007). Determining how non-toxic herbs are contaminated with aconitum roots is not easy. Thus, strengthening the source control through good agricultural and supply practices and appropriate quality assurance is important.
In this study, a method for determining 9 aconitum alkaloids in Fuzi samples was established and validated, providing an important reference for the quality control of Fuzi and research on related toxic components, such as YAC. Does Fuzi contain YAC and other ingredients, and what content levels should be set for safe use? These questions should be further investigated in future pharmacological and toxicological studies. The existing information on the quality control of YAC, DYA and CCA in Fuzi and Fuzi-containing products is insufficient. In this work, these 3 alkaloids were all detected in commercial Fuzi samples and Fuzi-containing products, implying that YAC, DYA and CCA might be hidden toxic ingredients and should be used as quality control and toxicological monitoring indicators. In addition, limit levels for the three alkaloids should be set after careful and thorough studies on their safety and pharmacological effects. The current findings should indicate to manufacturers of Fuzi and Fuzi-containing products that strictly controlling the quality of these medicinal materials is important.
Materials and Methods
Chemicals, reagents and materials
Fuzi samples were purchased from local drug stores or herb markets in different places in P.R. China, including Guangdong, Liaoning, Hunan, Yunan, Jianxi, Anhui, Sichuan Provinces and Hong Kong, Macao, and Taiwan. All these materials were authenticated by Dr. Zhi-Feng Zhang (An expert of herbal authentication at Macau University of Science and Technology). Voucher specimens were deposited in the State Key Laboratory of Quality Research in Chinese Medicine (Macau University of Science and Technology). The Fuzi-containing products used in this study were Heishunpian granule (HSP), Danfupian granule (DFP), Baifuzi granule (BFZ), Paofupian granule (PFP), You-gui-wan (YGW), Si-ni-tang (SNT), Ma-huang-fu-zi-xi-xin-tang (MFXT), Zhen-wu-tang (ZWT), Fu-zi-li-zhong-tang (FLT), and Jin-gui-shen-qi-wan (JGSQ) and were purchased from local pharmacies in Shenzhen and Hong Kong P.R. China. Xiang-sha-liu-jun-zi-tang (XSLJZT) that is free of Fuzi was used as a negative control. The sample information, including the sample number, sample name and sample source, is shown in Table 1.
The reference standard compounds (purity ≥ 98%), aconitine, hypaconitine, mesaconitine, benzoylaconine, benzoylhypaconine, benzoylmesaconine, and crassicauline A, were purchased from the Chengdu Must Bio-technology Co., Ltd. (Chengdu, P.R. China). Yunaconitine (purity ≥ 98%) was purchased from the Testing Laboratory for Chinese Medicine (Hong Kong, P.R. China). 8-Deacetyl-yunaconitine (purity ≥ 98%) was kindly provided by Prof. Xiao-Xia Ma of Yunnan University of TCM (P.R. China). The structures are given in Fig. 8. Formic acid (St. Louis, MO, USA), acetonitrile and methanol (Houston, TX, USA) were all HPLC grade. Other chemicals and reagents were of analytical grade.
Figure 8.
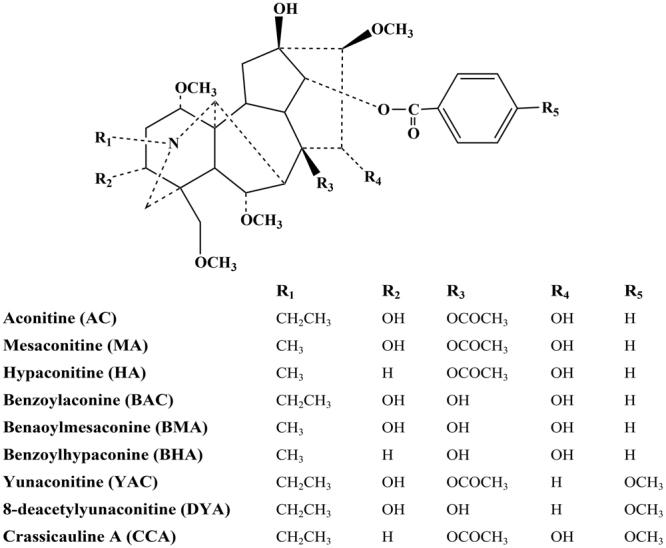
Chemical structures of the 9 standard reference compounds.
Instruments and UHPLC-MS conditions
An UHPLC system (1290 series, Agilent Technologies, Santa Clara, CA, USA) coupled with an Agilent 6460 Triple Quadrupole Mass Spectrometer (QQQ-MS, Agilent Technologies, Santa Clara, CA, USA) was used to quantitatively detect the 9 aconitum alkaloids. Chromatographic separations were performed on a Waters Acquity UPLC C18 column (1.7 μm, 2.1 mm × 100 mm, Waters, Milford, MA, USA) at 30 °C. The mobile phase consisted of 0.1% acetic acid (A) and acetonitrile (B), and the gradient elution was as follows: 20–25% B from 0-4 min, 25% B from 4–10 min, 90% B from 10.01–12 min, and 20% B from 12.01–15 min. An aliquot of 2 μL was injected, and the flow rate was 350 μL/min. The detection of the 9 alkaloids was performed using MRM and an ESI source in a positive ion mode. The transitions of the 9 compounds were m/z 646.4 → 586.3 (Frag 184, CE33) for AC, m/z 632.0 → 105.1 (Frag 184, CE 50) for MA, m/z 616.3 → 556.3 (Frag 184, CE 29) for HA, m/z 604.3 → 105.1 (Frag 242, CE 50) for BAC, m/z 590.3 → 105.1 (Frag 184, CE 49) for BMA, m/z 574.3 → 105.1 (Frag 184, CE 50) for BHA, m/z 660.2 → 135.0 (Frag 205, CE 64) for YCA, m/z 618.4 → 135.1 (Frag 235, CE 52) for DYA, and m/z 644.4 → 135.0 (Frag 220, CE 68) for CCA. The other parameters were as follows: drying gas (N2) flow rate, 11.0 L/min; drying gas temperature, 300 °C; nebulizer, 15 psig; and capillary voltage, 4000 V.
Standard solution preparation
An appropriate amount of the 9 reference standards was dissolved with methanol to prepare stock solutions, which were stored at 4 °C. The standard mixture solution was obtained by accurately mixing the 9 stock solutions and diluting them with 50% methanol. The final concentrations of AC, MA, HA, BAC, BMA, BHA, YAC, DYA, and CCA in the mixture solution were 6.56, 5.46, 7.89, 5.65, 16.5, 5.91, 4.95, 5.08 and 2.53 μg/mL, respectively. The different concentrations of the standard solutions in Table 3 were obtained by diluting the mixture solution. Two microliters of the standard solution were injected into the UHPLC-MS system, and the total ion chromatogram charts of the 9 reference standards are shown in Fig. 9.
Figure 9.
UHPLC–QQQ–MS/MS EIC of the 9 reference standard compounds.
Method validation and preparation of the sample solutions
The method was validated in accordance with the Guidelines for the Validation of Quality Standard of TCM (CHP, 2015 Edition, Volume 1)1 and the US Food and Drug Administration bioanalytical method validation (US Food and Drug Administration, 2001)23,24.
The Fuzi powder (2.0 g) was accurately weighed and ultrasonically extracted for 30 min using 3 mL of ammonia TS and 50 mL of ethyl acetate-isopropanol (1:1, v/v) in a conical flask that was covered after the solution cooled to room temperature. The loss in weight was supplemented via the addition of more extraction solvent. The extraction was filtered, and 25 mL of the successive filtrate was evaporated to dryness below 40 °C. The residue was precisely dissolved in 3 mL of isopropanol- dichloromethane (1:1, v/v). The solution was then diluted with methanol in a 1:10 ratio and filtered with a 0.22 μm microporous membrane filter. Two microliters of the successive filtrate was injected into the UHPLC-MS system for the analysis, according to the method mentioned in the Instruments and UHPLC-MS conditions section.
Acknowledgements
This project was sponsored by the Macao Science and Technology Development Fund (092/2012/A3 to L. Liu).
Author Contributions
H.Z. and L.L. conceived the study. H.Z., Y.X. and Z.Q.L. designed the experiments. F.H., C.S.C., and C.J.W. performed the experiments and the data analysis. H.Z., F.H. and C.J.W. wrote the manuscript. All the authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Fan He and Can-Jian Wang contributed equally to this work.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liang Liu, Email: lliu@must.edu.mo.
Hua Zhou, Email: hzhou@must.edu.mo.
References
- 1.Pharmacopoeia Commission of PRC, Pharmacopoeia of the People’s Republic of China, vol. 1, China Medical Science Press, Beijing (2015).
- 2.Yan GL, et al. Rapid and global detection and characterization of aconitum alkaloids in Yin Chen Si Ni Tang, a traditional Chinese medical formula, by ultra performance liquid chromatography-high resolution mass spectrometry and automated data analysis. J. Pharm.Biomed. Anal. 2010;53:421–431. doi: 10.1016/j.jpba.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ameri A. The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 1998;56:211–235. doi: 10.1016/S0301-0082(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 4.Desai HK, Hart BP, Caldwell RW, Huang JZ, Pelletier SW. Certain norditerpenoid alkaloids and their cardiovascular action. J. Nat. Prod. 1998;61:743–748. doi: 10.1021/np970499j. [DOI] [PubMed] [Google Scholar]
- 5.Bisset NG. Arrow poisons in China. Part II. Aconitum-botany, chemistry, and pharmacology. J. Ethnopharmacol. 1981;4:247–336. doi: 10.1016/0378-8741(81)90001-5. [DOI] [PubMed] [Google Scholar]
- 6.Song L, et al. Rapid determination of yunaconitine and related alkaloids in aconites and aconite-containing drugs by ultra high-performance liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2012;26:1567–74. doi: 10.1002/bmc.2733. [DOI] [PubMed] [Google Scholar]
- 7.Chan, T.Y. Incidence and Causes of Aconitum Alkaloid Poisoning in Hong Kong from 1989 to 2010. Phytother Res. 29, 1107–1111 (2015). [DOI] [PubMed]
- 8.Chan TY. Causes and prevention of herb-induced aconite poisonings in Asia. Hum Exp Toxicol. 2011;30:2023–2026. doi: 10.1177/0960327111407224. [DOI] [PubMed] [Google Scholar]
- 9.Lai CK, Poon WT, Chan YW. Hidden aconite poisoning: identification of yunaconitine and related aconitum alkaloids in urine by liquid chromatography-tandem mass spectrometry. J Anal Toxico. 2006;30:426–433. doi: 10.1093/jat/30.7.426. [DOI] [PubMed] [Google Scholar]
- 10.Zhu DY, Bai DL, Tang XC. Recent studies on traditional Chinese medicinal plants. Drug Devel. Res. 1996;39:147–157. doi: 10.1002/(SICI)1098-2299(199610)39:2<147::AID-DDR6>3.0.CO;2-P. [DOI] [Google Scholar]
- 11.Guo ZJ, et al. A preliminary research on the efficacy and toxicity of Yunaconitine and 8-deacetylyunaconitine islated from the processed products of Aconiti Knsnezoffii Radix. Chinese Journal of Information of TCM. 2015;22:60–63. [Google Scholar]
- 12.Beike J, Frommherz L, Wood MB, Kohler H. Determination of aconitine in body fluids by LC-MSMS. Int J Legal Med. 2004;118:289–293. doi: 10.1007/s00414-004-0463-2. [DOI] [PubMed] [Google Scholar]
- 13.Beyer J, Peters FT, Kraemer T, Maurer HH. Detection and validated quantification of toxic alkaloids in human blood plasma- comparison of LC-APCI-MS with LC-ESI-MS/MS. J Mass Spectrom. 2007;42:621–633. doi: 10.1002/jms.1191. [DOI] [PubMed] [Google Scholar]
- 14.Hattori H, et al. Simultaneous analysis of aconitine, mesaconitine, hypaconitine and jesaconitine in whole blood by LC-MS/MS using a new polymer column. Forensic Toxicol. 2009;27:7–11. doi: 10.1007/s11419-008-0060-z. [DOI] [Google Scholar]
- 15.Song L, et al. Rapid determination of yunaconitine and related alkaloids in aconites and aconite-containing drugs by ultra high-performance liquid chromatography–tandem mass spectrometry. Biomed Chromatogr. 2012;12:1567–1574. doi: 10.1002/bmc.2733. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZH, Wang ZP, Wen J, He Y. Simultaneous determintation of three aconitum alkaloids in urine by LC-MS/MS. J. Pharm.Biomed. Anal. 2007;45:145–148. doi: 10.1016/j.jpba.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Jiang ZH, et al. Quantification of aconitum alkaloids in aconite roots by a modified RP-HPLC method. Phytochem. Anal. 2005;16:415–421. doi: 10.1002/pca.861. [DOI] [PubMed] [Google Scholar]
- 18.Xie Y, Jiang ZH, Zhou H, Liu L. Simultaneous determination of six Aconitum alkaloids in proprietary Chinese medicines by high-performance liquid chromatography. J. Chromatogr. A. 2005;1093:195–203. doi: 10.1016/j.chroma.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 19.Bello-Ramirez AM, Nava-Ocampo AA. A QSAR analysis of toxicity of Aconitum alkaloids. Fundam Clin Pharmacol. 2004;18:699–704. doi: 10.1111/j.1472-8206.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 20.Xiao PG, et al. A pharmacophylogenetic study of Aconitum L. (Ranunculaceae) from China. Acta Phytotaxon Sin. 2006;44:41–46. doi: 10.1360/aps050046. [DOI] [Google Scholar]
- 21.Yu HJ, Liang TT. A new alkaloid from the roots of Aconitum carmichaeli Debx. J. Chin Chem Soc. 2012;59:693–695. doi: 10.1002/jccs.201200077. [DOI] [Google Scholar]
- 22.Wu SL, Liu F. 8-O-Ethylyunaconitine from the roots of Aconitum carmichaeli Debx. Acta Cryst. 2012;68:o2238. doi: 10.1107/S1600536812026463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan TY. Aconitum Alkaloid Poisoning Because of Contamination of Herbs by Aconite Roots. Phytother Res. 2016;30:3–8. doi: 10.1002/ptr.5495. [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER), Guidance for Industry, Bioanalytical Method Validation, 2001 (May).