Abstract
Key points
Mice reared in an enriched environment are demonstrated to have larger hippocampal gamma oscillations than those reared in isolation, thereby confirming previous observations in rats.
To test whether astrocytic Ca2+ surges are involved in this experience‐dependent LFP pattern modulation, we used inositol trisphosphate receptor type 2 (IP3R2)‐knockout (KO) mice, in which IP3/Ca2+ signalling in astrocytes is largely diminished.
We found that this experience‐dependent gamma power alteration persists in the KO mice.
Interestingly, hippocampal ripple events, the synchronized events critical for memory consolidation, are reduced in magnitude and frequency by both isolated rearing and IP3R2 deficiency.
Abstract
Rearing in an enriched environment (ENR) is known to enhance cognitive and memory abilities in rodents, whereas social isolation (ISO) induces depression‐like behaviour. The hippocampus has been documented to undergo morphological and functional changes depending on these rearing environments. For example, rearing condition during juvenility alters CA1 stratum radiatum gamma oscillation power in rats. In the present study, hippocampal CA1 local field potentials (LFP) were recorded from bilateral CA1 in urethane‐anaesthetized mice that were reared in either an ENR or ISO condition. Similar to previous findings in rats, gamma oscillation power during theta states was higher in the ENR group. Ripple events that occur during non‐theta periods in the CA1 stratum pyramidale also had longer intervals in ISO mice. Because astrocytic Ca2+ elevations play a key role in synaptic plasticity, we next tested whether these changes in LFP are also expressed in inositol trisphosphate receptor type 2 (IP3R2)‐knockout (KO) mice, in which astrocytic Ca2+ elevations are largely diminished. We found that the gamma power was also higher in IP3R2‐KO‐ENR mice compared to IP3R2‐KO‐ISO mice, suggesting that the rearing‐environment‐dependent gamma power alteration does not necessarily require the astrocytic IP3/Ca2+ pathway. By contrast, ripple events showed genotype‐dependent changes, as well as rearing condition‐dependent changes: ISO housing and IP3R2 deficiency both lead to longer inter‐ripple intervals. Moreover, we found that ripple magnitude in the right CA1 tended to be smaller in IP3R2‐KO. Because IP3R2‐KO mice have been reported to have depression phenotypes, our results suggest that ripple events and the mood of animals may be broadly correlated.
Keywords: astrocyte, enriched environment, hippocampal EEG, itpr2, mouse, social isolation
Key points
Mice reared in an enriched environment are demonstrated to have larger hippocampal gamma oscillations than those reared in isolation, thereby confirming previous observations in rats.
To test whether astrocytic Ca2+ surges are involved in this experience‐dependent LFP pattern modulation, we used inositol trisphosphate receptor type 2 (IP3R2)‐knockout (KO) mice, in which IP3/Ca2+ signalling in astrocytes is largely diminished.
We found that this experience‐dependent gamma power alteration persists in the KO mice.
Interestingly, hippocampal ripple events, the synchronized events critical for memory consolidation, are reduced in magnitude and frequency by both isolated rearing and IP3R2 deficiency.
Abbreviations
- ENR
enriched environment
- IP3R2
inositol trisphosphate receptor type 2
- ISO
isolated rearing
- KO
knockout
- LFP
local field potential
- REM
rapid‐eye‐movement
- RMS
root‐mean‐square
- WT
wild‐type
Introduction
Enriched environment (ENR) rearing is known to enhance cognitive and memory abilities. In the rodent brain, ENR has been demonstrated to enhance neurogenesis and induce morphological complexity of neurons (van Praag et al. 2000; Hirase & Shinohara, 2014). Many of these changes have been reported in the hippocampus, which is known to be critical for spatial navigation and episodic memory formation (Andersen et al. 2006). The hippocampus operates in distinct network states characterized by distinct local field potential (LFP) patterns. For example, theta and gamma oscillations appear during locomotion, vigilance and rapid‐eye‐movement (REM) sleep, whereas sharp wave‐associated ripples appear during awake immobility and slow wave sleep (Buzsáki, 2002, 2015). These LFP states also appear in urethane anaesthesia, whereby theta and non‐theta states spontaneously alternate, resembling sleep states. We have previously shown that ENR rearing after weaning increases the theta‐associated gamma oscillation power in the hippocampal CA1 than isolated rearing (ISO) in rats (Shinohara et al. 2013). This experience‐dependent enhancement of gamma oscillations is NMDA receptor‐dependent and is expressed most prominently in the stratum radiatum, where axons from bilateral CA3 pyramidal cells make synaptic connections.
Similar to all other parts of the brain, the hippocampus is populated with neurons and glia. Amongst glial cell types, astrocytes have been demonstrated to play an enabling role in synaptic plasticity in vitro (Yang et al. 2003; Henneberger et al. 2010) and in vivo (Takata et al. 2011; Chen et al. 2012; Navarrete et al. 2012; Monai et al. 2016) in the cortex and hippocampus. In these studies, astrocytic Ca2+ elevation has been suggested to be important to mediate synaptic plasticity. Large cytosolic Ca2+ elevations have indeed been observed in rodent hippocampal and cortical astrocytes in vivo (Stosiek et al. 2003; Hirase et al. 2004; Kuga et al. 2011). The pathway involving inositol trisphosphate receptor type 2 (IP3R2), the principal IP3 receptor in astrocytes, has been recognized as the principal mechanism for astrocytic Ca2+ elevations because IP3R2‐knockout (KO) mice display much compromised cytosolic Ca2+ elevations in astrocytes (Petravicz et al. 2008; Takata et al. 2011). The involvement of IP3R2‐mediated Ca2+ signalling in synaptic plasticity and learning, however, remains controversial (Agulhon et al. 2010; Petravicz et al. 2014).
In the present study, we first confirmed that ENR rearing enhances hippocampal gamma oscillation power in mice. We then investigated whether this gamma oscillation enhancement is dependent on astrocytic Ca2+ signalling by using IP3R2‐KO mice. We found that hippocampal gamma oscillations of IP3R2‐KO are also larger in ENR than in ISO. Although IP3R2 deficiency did not display an obvious phenotype in gamma oscillations, IP3R2‐KO mice had impacts in the occurrence and magnitude of sharp wave‐associated ripple events that occur during non‐theta periods.
Methods
Ethical approval
The procedures involving animal care, surgery and sample preparation were approved by the Animal Experimental Committee of RIKEN Brain Science Institute [Animal: H27‐2‐230(6); DNA: 2016‐038(1)] and were performed in accordance with the guidelines of the Animal Experimental Committee of RIKEN Brain Science Institute. All efforts were made to minimize the animals’ pain and suffering and to reduce the number of animals used in the study. We understand the ethical principles under which The Journal of Physiology operates and our work complies with the journal's animal ethics checklist (Grundy, 2015).
Animals and rearing condition
Adult IP3R2‐KO mice (Futatsugi et al. 2005) (background strain: C57BL/6J) and littermates (wild‐type; WT) were used. Genotyping was made by PCR using a mixture of three primers (5′‐ to 3′): B2: AGAGACACGATGTCCCCAATGTAG; Z2: GATGTGCTGCAAGGCGATTAAG; F2: CCAGGAACAGGAAACCTACTTCTG. A 237 bp band (B2–F2) is amplified in the WT allele, whereas a 311 bp band (Z2–F2) is amplified in the IP3R2‐KO allele. After weaning (postnatal 20 days), mice were subjected to either ISO or ENR rearing for the following 6–7 weeks (Fig. 1 A). In the ISO condition, mice were kept singly in a standard cage (length 30 cm, width 20 cm, height 18 cm) without further enrichment. In the ENR condition, three to five mice were kept in a 3D‐enhanced cage (length 32 cm, width 23 cm, height 25 cm) with a ladder, two running wheels, and tunnels (Fig. 1 B). In addition, one novel object with different texture, shape or colour was replaced with a familiar object in the ENR cage every 2 days, with its location changed on the second day. In either ISO or ENR condition, animals were given access to food and water ad libitum. Body weight was measured before conducting the electrophysiological recording.
Figure 1. Local field potential measurement in mice reared in an ENR or ISO condition.

A, schedule of experiment. B, photographs for the ENR (upper) and ISO (lower) used in the current experiment. C, typical example of PCR‐based genotyping for WT and IP3R2‐KO mice. Middle: heterozygous IP3R2‐KO mouse (not used in the present study). D, body weight of mice before LFP recording in each combination of genotype (WT, IP3R2‐KO) and rearing environment (ENR, ISO). E, schematics of bilateral LFP recording from hippocampal CA1. Below: example of histological verification of electrode location for each hippocampal hemisphere. Inset: magnified to indicate the silicon probe tip location in CA1. F, simultaneous LFP recording example traces from the CA1 strata pyramidale (purple) and radiatum (navy). Statistics for spontaneously occurring theta periods are computed for single theta period (G), proportion of theta periods over all recording time (H), frequency of theta state (I). * P < 0.05, ** P < 0.01.
Electrophysiology
LFP recording was performed as reported previously (Sakatani et al. 2007; Shinohara et al. 2013). Briefly, mice were anaesthetized with an i.p. injection of urethane (1.2–1.4 g kg−1) and fixed in a stereotaxic frame. The depth of anaesthesia was determined by movement of the animal when ear bars were inserted and additional urethane was injected if necessary. A craniotomy was performed above each side of the dorsal hippocampus (Bregma: mediolaterial 1.8 mm, anteroposterior −1.8 mm). The dura was surgically removed and a 16‐channel linear silicon probe (inter‐channel distance = 50 μm; Alx15‐5 mim‐50‐177‐A16; NeuroNexus, Ann Arbor, MI, USA) was slowly inserted to the hippocampal CA1 so that the middle channel was located in the stratum pyramidale. A melted mixture of paraffin and paraffin oil was cooled down to ∼35°C (Mishima et al. 2007) and applied on the skull and craniotomy to maintain the moisture and temperature of the brain surface. Wideband (0.1–9000 Hz) extracellular field potentials were recorded continuously with a sampling rate of 31 kHz (Digital Lynx; Neuralynx, Bozeman, MT, USA). The body temperature was kept at ∼37°C throughout the surgery and recording sessions by a heat pad with rectal temperature feedback. Mice were supplemented with 0.2 ml of 5% glucose every hour (i.p.).
LFP data analysis
Data analyses were carried out using MATLAB (MathWorks Inc., Natick, MA, USA). For analyses of theta‐associated gamma oscillations, we employed the same procedure as reported previously (Shinohara et al. 2013). Briefly, raw LFP data were resampled to 1.25 kHz and theta periods were automatically detected by computing the spectrogram and finding the periods that satisfy two criteria: (i) the ratio of the peak powers of the theta band (3.5–7 Hz) and the delta (2–3 Hz) band in each bin exceeds 0.6 and (ii) the length of the period is at least 15 s long. The detection was carried out on the LFP recorded from the right CA1 stratum radiatum (150 μm below the stratum pyramidale). Next, the power spectral density was estimated by the Welch periodogram method for the detected theta periods. Theta, and low‐ and high‐gamma powers were calculated by integrating the power spectral densities for 3–6, 30–45 and 55–90 Hz, respectively. The spectral powers were averaged per animal over multiple recording sessions.
For analyses of ripple events, LFP signals in the stratum pyramidale were first resampled to 20 kHz. Then, ripple events were detected automatically. The LFP was band‐pass filtered for the ripple frequency band (80–250 Hz) and the resultant signal was squared and then smoothed with a Hamming window of length 19.2 ms. In the first screening, ripples events were detected as the periods where the smoothed signal exceeds the mean value by seven times the SD with an inter‐ripple interval of 100 ms. In the second screening, the local minima within ± 35 ms of each detected point (i.e. ripple trough) was assigned as the ripple timing and 200 ms ripple‐filtered waveform centered around the ripple timing was extracted for further analyses. After automatic detection, detected ripples were manually confirmed by the coincidence of a sharp wave in the stratum radiatum using NeuroScope (Hazan et al. 2006) that marked the detected ripple timings on LFP traces. The peak ripple amplitude was defined as the amplitude of the largest trough of each extracted ripple event and expressed in absolute value. The root‐mean‐square (RMS) amplitude was computed for the ± 25 ms interval from the ripple peak. Cumulative inter‐ripple‐interval distributions were calculated by binning inter‐ripple intervals of less than 20 s into bins of 100 ms width. Ripple occurrence frequency was computed for non‐theta periods, which are complementary to the detected theta‐periods for gamma analyses. Detected times ripples are compared between the left and right hemispheres, and the ripples observed within ± 35 ms on both hemispheres are termed as ‘co‐ripples’.
For all analyses, the amplifier gains were calibrated with sinusoidal signals of various frequencies produced by a signal generator. Data are presented as the mean ± SEM. Unless otherwise noted, statistical comparisons of two population means were performed via a t test and multiple population comparisons were performed with one‐way ANOVA followed by a Tukey–Kramer test.
Histology
In a set of experiments, electrodes were coated with DiI and electrode tracks were visualized by fluorescence. After electrophysiological recording, the mouse brain was fixed by transcardial perfusion with 4% paraformaldehyde in 0.1% phosphate buffer after the animal was deeply anaesthetized by additional i.p. injection of urethane (1.5 g kg−1). Following post‐fixation (overnight or longer in the fixative), the fixed brain was sliced by a microtome (Pro‐7; Dosaka, Kyoto, Japan) with the thickness set to 60 μm and the sections were mounted on a slide glass. The slide was semi‐dried and sealed with a coverslip using Vectashield mounting medium (H‐1200; Vector Laboratories, Burlingame, CA, USA) as the mounting medium. Sections were examined within 2 days using a standard fluorescence microscope (BX‐60; Olympus, Tokyo, Japan).
Results
To investigate whether rearing environment affects neuronal activity, we reared mice in two distinct rearing environments for 6–7 weeks after weaning: isolated (ISO) and enriched (ENR) environments (Fig. 1 A and B). In addition to WT mice, we performed experiments with IP3R2‐KO mice to examine whether astrocytic IP3/Ca2+ signalling plays a role in rearing experience‐dependent neural activity changes (Fig. 1 C). The numbers of mice used in the present study are: WT‐ENR, n = 9; WT‐ISO, n = 9; IP3R2‐KO‐ENR, n = 7; and IP3R2‐KO‐ISO, n = 6. Body weight was significantly larger in ENR mice regardless of IP3R2 deficiency (Genotype: F = 0.75, P = 0.39; Environment: F = 24.28, P < 0.0001) (Fig. 1 C).
We then recorded LFPs from bilateral dorsal hippocampal CA1 with 16‐channel silicon probes (Fig. 1 E) under anaesthesia. As reported previously (Wolansky et al. 2006), the hippocampal LFP spontaneously alternated between theta and non‐theta patterns (Fig. 1 F). Theta states were automatically detected by an algorithm based on the theta/delta power ratio of stratum radiatum LFP (see Methods). The average duration of a theta state was similar across genotypes and rearing environments (Genotype: F = 3.56, P = 0.07; Environment: F = 1.63, P = 0.21; Interaction: F = 1.06, P = 0.31) (Fig. 1 G), although there was a trend for longer theta state duration in IP3R2‐KO‐ISO (P = 0.152, post‐hoc Tukey–Kramer test against WT‐ENR) (Fig. 1 G). The proportion of theta states also showed similar tendencies across genotypes and rearing environments as single theta state duration (Genotype: F = 1.98, P = 0.17; Environment: F = 0.87, P = 0.36; Interaction: F = 3.05, P = 0.09) (Fig. 1 H). On the other hand, the frequencies of LFP state transition in all groups were similar (P > 0.4 for post‐hoc Tukey–Kramer tests of all combinations) (Fig. 1 I).
Gamma power enhancement by environmental enrichment
We first analysed gamma oscillations during theta states. As shown by the example traces in Fig. 2 A, stratum radiatum theta LFPs from WT‐ENR mice encompass gamma oscillations. In WT‐ISO mice, gamma oscillations appear to be diminished. Slow and fast gamma oscillation (30–45 Hz and 55–90 Hz, respectively) power was computed by spectral analyses. Comparison of the mean power spectral density between WT‐ENR and WT‐ISO mice showed general increases of spectral power around the slow gamma range (Fig. 2 B) in WT‐ENR mice. On the other hand, the interhemispheric gamma coherence was low (<0.2) and was not significantly affected by rearing condition (Fig. 2 C). Spectral powers of WT mice were compared between WT‐ENR and WT‐ISO for slow gamma and fast gamma (Fig. 2 D and E). There was a general tendency that slow gamma power was elevated in ENR group in both hemispheres; however, the increase reached statistical significance only on the right side (P = 0.0157). Fast gamma power did not show obvious changes depending on rearing condition in either hemisphere.
Figure 2. Comparisons of theta‐associated gamma oscillations in CA1 stratum radiatum.

A, example CA1 stratum radiatum LFP traces during theta periods from three individual WT‐ENR mice (upper, purple) and three WT‐ISO (lower, brown) mice. B, power spectral densities of CA1 stratum radiatum LFP of WT‐ENR and WT‐ISO mice. Shaded areas represent the frequency ranges for slow (35–45 Hz) and fast (55–90 Hz) gamma oscillations. C, interhemispheric coherence spectrum of CA1 stratum radiatum LFP. D and E, slow and fast gamma powers are compared in WT mice in the left (blue) and right (red) hemispheres. F and G, gamma power comparisons in IP3R2‐KO mice. H, comparison of slow gamma power by rearing condition in the left and right CA1 stratum radiatum. * P < 0.05.
Next, the same analyses were performed for IP3R2‐KO mice (Fig. 2 F and G). We find that slow gamma power also tended to increase by ENR rearing on both sides. Group comparison by two‐way ANOVA for slow gamma power on the right side shows that the main effect is the rearing environment (Environment: F = 6.35, P = 0.018; Genotype: F = 0.07, P = 0.7984) (Fig. 2 G). A similar outcome was computed for the left‐side slow gamma power (Environment: F = 4.98, P = 0.0342; genotype: F = 0.21, P = 0.6472) (Fig. 2 G). Rearing condition‐induced fast gamma power changes were not remarkable in IP3R2‐KO mice, resembling WT. These results suggest that slow gamma oscillations have higher power (i.e. magnitude) in ENR mice than in ISO mice regardless of IP3R2.
Hippocampal ripples are modulated by rearing condition and IP3R2
Large irregular activities are observed in CA1 stratum radiatum LFPs during non‐theta periods. Transient ripple oscillations appear in the stratum pyramidale as sharp waves occur in the stratum radiatum (Fig. 1 F). We next investigated whether ripple occurrence and magnitude are influenced by rearing environment or by IP3R2. As shown in Fig. 3 A, ripples appear multiple times during 5 s of non‐theta periods in WT and IP3R2‐KO mice. To examine ripple occurrence, we plotted the group‐averaged inter‐ripple interval cumulative distribution for each combination of the rearing environment and genotype (Fig. 3 B). Strikingly, the distribution for IP3R2‐KO‐ISO was shifted to the right, indicating a relative abundance of longer inter‐ripple intervals. Indeed, the upper quartile (75th percentile) value of the inter‐ripple‐intervals was significantly affected by both the genotype and rearing environment (Genotype: F = 4.50, P = 0.008; Environment: F = 3.10, P = 0.0239, for ripples detected in the right CA1) (Fig. 3 C) and IP3R2‐KO‐ISO mice had significantly higher upper quartile inter‐ripple interval values than WT‐ENR mice (Tukey–Kramer post hoc test, P = 0.005749).
Figure 3. Comparisons of ripple occurrence in CA1 stratum pyramidale.
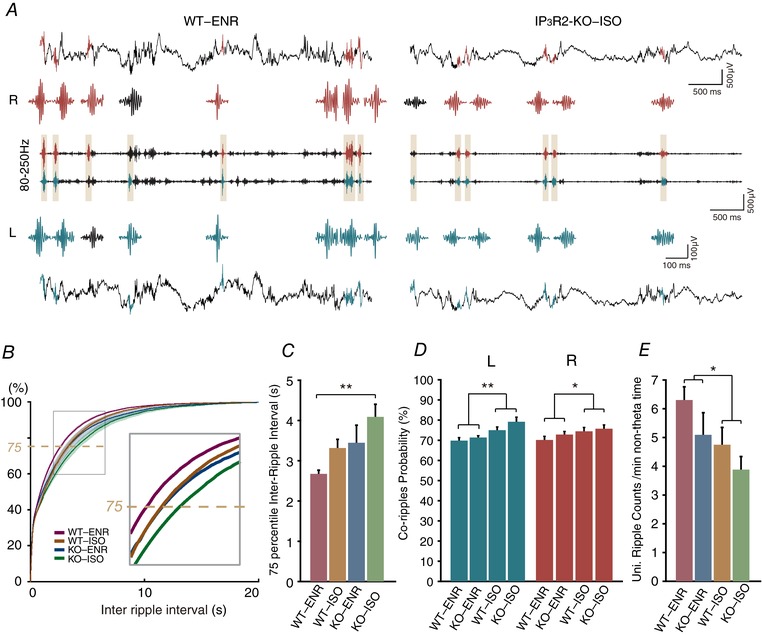
A, example CA1 stratum pyramidale LFP traces during non‐theta periods from a WT‐ENR mouse (left) and a IP3R2‐KO‐ISO mouse (right) from the right (upper) and left (lower) hemispheres. Bandpass filtered traces (80–250 Hz) for ripple detection is displayed in the middle. Detected ripples are highlighted in the filtered trace and coloured according to the detected hemisphere (right: red, left: blue). The ripple waveforms are extracted are magnified in the vicinity. B, cumulative plot for inter‐ripple intervals for WT‐ENR (red), WT‐ISO (brown), IP3R2‐KO‐ENR (blue) and IP3R2‐KO‐ISO (green). C, comparison of inter‐ripple‐interval of 75% quartile. D, proportion of bilaterally detectable co‐ripples are compared. E, occurrence of unilaterally significant ripples is increased in WT‐ENR compared to IP3R2‐KO‐ISO. * P < 0.05, ** P < 0.01.
Most hippocampal ripples were observed synchronously in both hemispheres, satisfying ripple detection criteria on each side (‘co‐ripples’; see Methods). Occasionally, ripples on the contralateral side did not satisfy the detection criteria even though high‐frequency oscillations were discernible. We refer to these ripple events as unilaterally significant ripples. The proportion of co‐ripple occurrence over all detected ripple events on the right hemisphere was 0.70, 0.74, 0.73 and 0.76 for WT‐ENR, WT‐ISO, IP3R2‐KO‐ENR and IP3R2‐KO‐ISO, respectively. Similar values were calculated for the left hemisphere. Two‐way ANOVA analysis showed that there is a significant effect for rearing environment (right: P = 0.042; left: P = 0.0005) (Fig. 3 D), whereas there is a weak effect for genotype (right: P = 0.25; left: P = 0.099). These analyses showed that ripples are probably more bilaterally detectable in ISO mice. Conversely, the likelihood of unilaterally significant ripple occurrence was significantly higher in WT‐ENR mice (right: 6.30 ± 0.46 events min–1) than in IP3R2‐KO‐ISO (3.88 ± 0.45 events min–1, Tukey–Kramer post hoc test, P = 0.04245) (Fig. 3 E). We have varied the ripple detection threshold from 6 SD to 8 SD and found that ripple occurrence and magnitude have similar statistical tendency as the default threshold of 7 SD (see Supporting information, Fig. S1).
Examination of the extracted ripple waveforms suggested variable profiles (Fig. 3 A). We computed two measures that represent the magnitude of a ripple: the peak ripple amplitude and the RMS amplitude. We found that they are positively correlated with correlation coefficients of >0.8 in all groups (WT‐ENR = 0.85; WT‐ISO = 0.80; IP3R2‐KO‐ENR = 0.82; IP3R2‐KO‐ISO = 0.85) (Fig. 4 A and B). Distributions of RMS ripple amplitude for all mice are shown in box plots in Fig. 4 C. We investigated ripple amplitudes and whether their variability is affected by the environmental or genetic factors. We compared ripple amplitudes by taking the median of RMS amplitude values as the representative value for each mouse. RMS amplitudes were plotted separately for co‐ripples and unilaterally significant ripples for the right hemisphere in Fig. 4 D and E. Interestingly, the RMS ripple amplitude was affected by the genetic deletion of IP3R2 (co‐ripples: P = 0.082, unilaterally significant ripples: P = 0.035). Of note, neither rearing environment, nor genotype had significant effects in RMS ripple amplitudes in the left hemisphere. To address the variability of ripple amplitudes, the coefficient of variation was calculated for each mouse. Accordingly, we found that neither rearing environment, nor genotype had a significant effect on the variability of ripple amplitude.
Figure 4. Comparisons of ripple magnitude in CA1 stratum pyramidale.

A, scatter plot to show a positively correlated relationship between ripple RMS amplitude and ripple peak amplitude in a WT‐ENR mouse. B, contour plots representing the distributions of ripple RMS amplitude vs. ripple peak amplitude for WT‐ENR, WT‐ISO, IP3R2‐KO‐ENR and IP3R2‐KO‐ISO groups. C, box plots showing individual variabilities of ripple RMS amplitude. Dashed lines represent the range for upper and lower quartile. The median of each distribution is marked by the horizontal line in the box. D and E, RMS amplitude is separately analysed for co‐ripples (D) and unilaterally significant ripples (E). * P < 0.05.
Discussion
ENR rearing has been reported to enhance cognitive and memory abilities. In rodents, it has been demonstrated that ENR rearing enhances learning and memory (Nithianantharajah & Hannan, 2006), as well as facilitates hippocampal LTP and neuronal excitability (Malik & Chattarji, 2012). In the present study, we first aimed to confirm the relative increase of hippocampal gamma power in ENR mice compared to that in ISO mice, which we have previously reported in rats (Shinohara et al. 2013). Second, we investigated whether astrocytic IP3/Ca2+ signalling is involved in such experience‐dependent LFP changes. Although urethane affects multiple neurotransmitter systems (Hara & Harris, 2002) and potentially elicits quasi‐natural oscillatory patterns, we performed LFP recordings under urethane anaesthesia because: (i) both synchronized (theta) and de‐synchronized (non‐theta) states are expressed during urethane anaesthesia, resembling slow wave and rapid‐eye‐movement sleep states, respectively (Wolansky et al. 2006; Clement et al. 2008); (ii) a silicon probe implant that lasts for 6 weeks from the onset of environmental exposure (i.e. at weaning) for chronic recording would be technically difficult; and (iii) identical recording conditions can be imposed for all mice.
The gamma oscillations that we investigated were recorded during theta states. Theta states are associated with medial septal cholinergic activity that projects to the hippocampus (Green & Arduini, 1954; King et al. 1998; Yoder & Pang, 2005). We did not observe statistically significant changes in the proportion of theta state periods by rearing condition or by IP3R2 deficiency, which is consistent with the study by Cao et al. (2013), who reported similar sleep patterns in WT and IP3R2 KO. A recent study by Foley et al. (2017) reported longer REM sleep in transgenic mice in which astrocytic IP3 signalling is attenuated by overexpression of an IP3‐degrading enzyme. Such an approach to block IP3 signalling is potentially more powerful because it also interferes the signalling through IP3R1 and IP3R3 receptors. Indeed, sparse yet discernible IP3R3‐dependent astrocytic Ca2+ elevations have been reported recently (Sherwood et al. 2017). Nevertheless, we found trends for prolonged theta state period and larger portion of theta states over a recorded period for IP3R2‐KO‐ISO mice (Fig. 1 G). These results lend credence to the notion that IP3R2 phenotypes are expressed in stressful conditions. Notably, abnormal REM sleep patterns, including a shorter latency from the onset of slow wave sleep and longer period, have been reported in depressed patients (Berger & Riemann, 1993). Given these connections, further investigation of astrocytic IP3/Ca2+ signalling may lead to potential therapeutic interventions for mood disorders caused by external environmental stress.
We found that slow gamma oscillation power is higher in ENR mice than in ISO mice (Fig. 2 B), showing a similar tendency to our previous study with rats (Shinohara et al. 2013). Indeed, there is a broad enhancement of spectral power density over the slow gamma range in ENR mice compared to the power spectral density distribution of ISO mice. The higher gamma powers in ENR mice are consistent with previously reported associations between gamma oscillations and memory or cognitive abilities in rodents and humans (Sederberg et al. 2006; Tort et al. 2009; Lu et al. 2011; Igarashi et al. 2014; Staresina et al. 2016). We note that the relative increase of slow gamma power of ENR to that of ISO gamma power was more modest in mice (left: 33%; right: 44%) than in rats (left: 51%; right: 103%) (Shinohara et al. 2013). Recently, Valero‐Aracama et al. (2015) reported a critical time window of 40 days for ENR‐induced facilitation of dorsal CA1 neuronal excitability in C56BL/6 female mice. It is possible that the ENR rearing length used in the current experiment (6 weeks) was close to the end of this plastic window, and that some of the ENR‐induced (or ISO‐induced) circuit plasticity had already reverted by the time of recording.
We also noted that the interhemispheric coherence at gamma frequencies is considerably lower in mice than in rats. Furthermore, gamma coherence was generally low (<0.1) under the urethane‐anaesthetized condition. The low interhemispheric gamma coherence recorded during anaesthesia was similar between ISO and ENR mice. This result was in contrast to our previous rat experiments, in which marked gamma coherence increases were observed in a urethane‐anaesthetized condition (Shinohara et al. 2013). The low gamma coherence is probably related to the anaesthesia because highly coherent interhemispheric gamma activity has been reported in mice running in a wheel (Buzsaki et al. 2003). It is not clear how interhemispheric gamma desynchronization occurs in urethane‐anaesthetized mice, whereas urethane‐anesthetized rats, especially those raised in an enriched environment, display a significant gamma coherence (∼0.5) (Shinohara et al. 2013). Indeed, it appears to be counterintuitive because the relative sizes of both cortical and hippocampal commissures are larger in the mouse than the rat (Bishop & Wahlsten, 1999). The exact mechanism of urethane anaesthesia remains to be clarified; however, urethane has been known to enhance GABAA‐, glycine‐ and nACh‐ receptor‐mediated currents and inhibit NMDA‐ and AMPA‐receptor‐mediated currents (Hara & Harris, 2002). Although we are unaware of a comprehensive transcriptome database that allows inter‐species comparison of receptors and channels in distinct brain regions, it is speculated that differential expression of these urethane‐sensitive receptors might help understand the gamma coupling differences.
As noted earlier, theta states are associated with cholinergic input to the hippocampus. Hippocampal astrocytes have been shown to respond to synaptically released acetylcholine with intracellular Ca2+ elevations (Araque et al. 2002; Navarrete et al. 2012; Pabst et al. 2016). Prior studies have shown cholinergically activated astrocytes play a role in glutamatergic synaptic plasticity (Takata et al. 2011; Chen et al. 2012; Navarrete et al. 2012; Papouin et al. 2017). The presence of ENR‐dependent gamma enhancement in IP3R2‐KO mice suggests that astrocytic IP3/Ca2+ signalling is not an essential component of this modulation. The results of the present study, however, do not exclude the possibility that residual Ca2+ astrocytic activities observable in IP3R2‐KO mice (Kanemaru et al. 2014; Srinivasan et al. 2015; Agarwal et al. 2017; Stobart et al. 2017), albeit with reduced occurrence, have a role.
Although we could not find obvious LFP phenotypes in IP3R2‐KO mice in theta‐associated gamma oscillations, we found differences in ripple events that occur during non‐theta periods. Ripple occurrence was decreased by both IP3R2 deficiency and by ISO rearing. These effects appear to be additive because the two‐way ANOVA does not suggest an interaction of the two factors. IP3R2‐KO mice have been reported for higher susceptibility to depressive behaviour after social defeat stress as a result of decreased extracellular ATP levels, presumably because of a lack of Ca2+‐induced gliotransmission (Cao et al. 2013). Susceptibility to stress in IP3R2‐KO mice has also been demonstrated in a chronic restraint stress model of depression (Monai et al. 2016). In addition to ripple occurrence frequency, we found paralleling decreases of ripple magnitude by ISO‐rearing and by IP3R2 deficiency on the right side. Hippocampal ripples have been implied in memory consolidation by transferring information representation to the cortex (Buzsáki, 1989; Girardeau et al. 2009; Ego‐Stengel & Wilson, 2010; Maingret et al. 2016). The attenuation of ripple events in IP3R2‐KO‐ISO mice with respect to both occurrence and magnitude predicts compromised hippocampus‐dependent memory consolidation. In rats, the right hippocampus has been reported to have learning‐associated transcriptomic changes compared to the left (Klur et al. 2009). Moreover, in a mouse ‘split brain model’, the right hippocampus appears to be more involved in maintaining spatial memory (Shinohara et al. 2012). It is thus tempting to speculate that the ripple changes on the right side in IP3R2‐KO‐ISO mice reflect some aspect of memory processing. A previous study reported an absence of outstanding behavioural phenotype in IP3R2‐KO mice in standard behavioural test battery (Petravicz et al. 2014). Perhaps behavioural phenotypes for IP3R2 are more apparent under chronic stress, such as the aforementioned depression models.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
HH, YS, MT and KM have conceived and designed the work. MT and XW acquired the data. LFP analysis codes were developed by YS. Data analysis was performed by XW, MT and YS. HH, MT, XW and YS wrote the manuscript. All authors have approved the final version of the manuscript submitted for publication and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the RIKEN Brain Science Institute, KAKENHI grants (26117520, 16H01888 to HH, 25221002 to KM, 26282222, 17H02221 to YS) and HFSP (RGP0036/2014).
Supporting information
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Figure S1. Effect of varying ripple detection threshold. Ripple detection threshold was varied from 6 × SD to 8 × SD. The occurrence (A) and RMS amplitude (B) of detected ripples are plotted for each combination of genotype (WT or IP3R2‐KO) and housing condition (enriched or isolated). These analyses were made for recordings from the right CA1 stratum pyramidale. The relationship among the experiment groups remains generally similar across the range of threshold for both ripple occurrence and RMS amplitude.
Acknowledgements
We thank Ms Etsuko Ebisui and Dr Chihiro Hisatsune for mouse supply and genotyping, as well as Dr Kentaroh Takagaki and members of the laboratory for comments on earlier versions of the manuscript.
Linked articles This article is highlighted by a Perspective by Wittner. To read this Perspective, visit https://doi.org/10.1113/JP275055.
References
- Agarwal A, Wu P‐H, Hughes EG, Fukaya M, Tischfield MA, Langseth AJ, Wirtz D & Bergles DE (2017). Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93, 587–605.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C, Fiacco TA & McCarthy KD (2010). Hippocampal short‐ and long‐term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327, 1250–1254. [DOI] [PubMed] [Google Scholar]
- Andersen P, Morris R, Amaral D, Bliss T. & O'Keefe J. eds. (2006). The Hippocampus Book. Oxford University Press, Oxford. [Google Scholar]
- Araque A, Martin ED, Perea G, Arellano JI & Buno W (2002). Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci 22, 2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M & Riemann D (1993). REM sleep in depression‐an overview. J Sleep Res 2, 211–223. [DOI] [PubMed] [Google Scholar]
- Bishop KM & Wahlsten D (1999). Sex and species differences in mouse and rat forebrain commissures depend on the method of adjusting for brain size. Brain Res 815, 358–366. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2002). Theta oscillations in the hippocampus. Neuron 33, 325–340. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (1989). Two‐stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. [DOI] [PubMed] [Google Scholar]
- Buzsáki G (2015). Hippocampal sharp wave‐ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Buhl DL, Harris KD, Csicsvari J, Czeh B & Morozov A (2003). Hippocampal network patterns of activity in the mouse. Neuroscience 116, 201–211. [DOI] [PubMed] [Google Scholar]
- Cao X, Li L‐P, Wang Q, Wu Q, Hu H‐H, Zhang M, Fang Y‐Y, Zhang J, Li S‐J, Xiong W‐C, Yan H‐C, Gao Y‐B, Liu J‐H, Li X‐W, Sun L‐R, Zeng Y‐N, Zhu X‐H & Gao T‐M (2013). Astrocyte‐derived ATP modulates depressive‐like behaviors. Nat Med 19, 773–777. [DOI] [PubMed] [Google Scholar]
- Chen N, Sugihara H, Sharma J, Perea G, Petravicz J, Le C & Sur M (2012). Nucleus basalis‐enabled stimulus‐specific plasticity in the visual cortex is mediated by astrocytes. Proc Natl Acad Sci USA 109, E2832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement EA, Richard A, Thwaites M, Ailon J, Peters S & Dickson CT (2008). Cyclic and sleep‐like spontaneous alternations of brain state under urethane anaesthesia. PLoS ONE 3, e2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ego‐Stengel V & Wilson MA (2010). Disruption of ripple‐associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J, Blutstein T, Lee S, Erneux C, Halassa MM & Haydon P (2017). Astrocytic IP3/Ca2+ signaling modulates theta rhythm and REM sleep. Front Neural Circuits 11, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, Kitaguchi T, Takahashi‐Iwanaga H, Noda T, Aruga J & Mikoshiba K (2005). IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science 309, 2232–2234. [DOI] [PubMed] [Google Scholar]
- Girardeau G, Benchenane K, Wiener SI, Buzsáki G & Zugaro MB (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12, 1222–1223. [DOI] [PubMed] [Google Scholar]
- Green JD & Arduini AA (1954). Hippocampal electrical activity in arousal. J Neurophysiol 17, 533–537. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K & Harris RA (2002). The anesthetic mechanism of urethane: the effects on neurotransmitter‐gated ion channels. Anesth Analg 94, 313–318. [DOI] [PubMed] [Google Scholar]
- Hazan L, Zugaro M & Buzsaki G (2006). Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J Neurosci Methods 155, 207–216. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH & Rusakov DA (2010). Long‐term potentiation depends on release of D‐serine from astrocytes. Nature 463, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Qian L, Bartho P & Buzsaki G (2004). Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol 2, E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H & Shinohara Y (2014). Transformation of cortical and hippocampal neural circuit by environmental enrichment. Neuroscience 280, 282–298. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Lu L, Colgin LL, Moser M‐B & Moser EI (2014). Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature 510, 143–147. [DOI] [PubMed] [Google Scholar]
- Kanemaru K, Sekiya H, Xu M, Satoh K, Kitajima N, Yoshida K, Okubo Y, Sasaki T, Moritoh S, Hasuwa H, Mimura M, Horikawa K, Matsui K, Nagai T, Iino M & Tanaka KF (2014). In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. Cell Rep 8, 311–318. [DOI] [PubMed] [Google Scholar]
- King C, Recce M & O'Keefe J (1998). The rhythmicity of cells of the medial septum/diagonal band of Broca in the awake freely moving rat: relationships with behaviour and hippocampal theta. Eur J Neurosci 10, 464–477. [DOI] [PubMed] [Google Scholar]
- Klur S, Muller C, Pereira de Vasconcelos A, Ballard T, Lopez J, Galani R, Certa U & Cassel J‐CC (2009). Hippocampal‐dependent spatial memory functions might be lateralized in rats: an approach combining gene expression profiling and reversible inactivation. Hippocampus 19, 800–816. [DOI] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N & Ikegaya Y (2011). Large‐scale calcium waves traveling through astrocytic networks in vivo. J Neurosci 31, 2607–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CB, Jefferys JG, Toescu EC & Vreugdenhil M (2011). In vitro hippocampal gamma oscillation power as an index of in vivo CA3 gamma oscillation strength and spatial reference memory. Neurobiol Learn Mem 95, 221–230. [DOI] [PubMed] [Google Scholar]
- Maingret N, Girardeau G, Todorova R, Goutierre M & Zugaro M (2016). Hippocampo‐cortical coupling mediates memory consolidation during sleep. Nat Neurosci 19, 959–964. [DOI] [PubMed] [Google Scholar]
- Malik R & Chattarji S (2012). Enhanced intrinsic excitability and EPSP‐spike coupling accompany enriched environment‐induced facilitation of LTP in hippocampal CA1 pyramidal neurons. J Neurophysiol 107, 1366–1378. [DOI] [PubMed] [Google Scholar]
- Mishima T, Sakatani S & Hirase H (2007). Intracellular labeling of single cortical astrocytes in vivo. J Neurosci Methods 166, 32–40. [DOI] [PubMed] [Google Scholar]
- Monai H, Ohkura M, Tanaka M, Oe Y, Konno A, Hirai H, Mikoshiba K, Itohara S, Nakai J, Iwai Y & Hirase H (2016). Calcium imaging reveals glial involvement in transcranial direct current stimulation‐induced plasticity in mouse brain. Nat Commun 7, 11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M, Perea G, Fernandez de Sevilla D, Gómez‐Gonzalo M, Núñez A, Martín ED & Araque A (2012). Astrocytes mediate in vivo cholinergic‐induced synaptic plasticity. PLoS Biol 10, e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J & Hannan AJ (2006). Enriched environments, experience‐dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7, 697–709. [DOI] [PubMed] [Google Scholar]
- Pabst M, Braganza O, Dannenberg H, Hu W, Pothmann L, Rosen J, Mody I, van Loo K, Deisseroth K, Becker AJ, Schoch S & Beck H (2016). Astrocyte intermediaries of septal cholinergic modulation in the hippocampus. Neuron 90, 853–865. [DOI] [PubMed] [Google Scholar]
- Papouin T, Dunphy JM, Tolman M, Dineley KT & Haydon PG (2017). Septal cholinergic neuromodulation tunes the astrocyte‐dependent gating of hippocampal NMDA receptors to wakefulness. Neuron 94, 840–854.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Boyt KM & McCarthy KD (2014). Astrocyte IP3R2‐dependent Ca(2+) signaling is not a major modulator of neuronal pathways governing behavior. Front Behav Neurosci 8, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J, Fiacco TA & McCarthy KD (2008). Loss of IP3 receptor‐dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J Neurosci 28, 4967–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G & Gage FH (2000). Neural consequences of environmental enrichment. Nat Rev Neurosci 1, 191–198. [DOI] [PubMed] [Google Scholar]
- Sakatani S, Seto‐Ohshima A, Itohara S & Hirase H (2007). Impact of S100B on local field potential patterns in anesthetized and kainic acid‐induced seizure conditions in vivo. Eur J Neurosci 25, 1144–1154. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Schulze‐Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS & Kahana MJ (2006). Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cereb Cortex 17, 1190–1196. [DOI] [PubMed] [Google Scholar]
- Sherwood MW, Arizono M, Hisatsune C, Bannai H, Ebisui E, Sherwood JL, Panatier A, Oliet SHR & Mikoshiba K (2017). Astrocytic IP3Rs: contribution to Ca 2+ signalling and hippocampal LTP. Glia 65, 502–513. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Hosoya A & Hirase H (2013). Experience enhances gamma oscillations and interhemispheric asymmetry in the hippocampus. Nat Commun 4, 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Hosoya A, Yamasaki N, Ahmed H, Hattori S, Eguchi M, Yamaguchi S, Miyakawa T, Hirase H & Shigemoto R (2012). Right‐hemispheric dominance of spatial memory in split‐brain mice. Hippocampus 22, 117–121. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Huang BS, Venugopal S, Johnston AD, Chai H, Zeng H, Golshani P & Khakh BS (2015). Ca2+ signaling in astrocytes from Ip3r2−/− mice in brain slices and during startle responses in vivo. Nat Neurosci 18, 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Michelmann S, Bonnefond M, Jensen O, Axmacher N, Fell J, Tully M, Kahana M, Johnston D, Wilson M, Tonegawa S & Schulze‐Bonhage A (2016). Hippocampal pattern completion is linked to gamma power increases and alpha power decreases during recollection. Elife 5, 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart JL, Ferrari KD, Barrett MJP, Stobart MJ, Looser ZJ, Saab AS & Weber B (2017). Long‐term in vivo calcium imaging of astrocytes reveals distinct cellular compartment responses to sensory stimulation. Cereb Cortex; https://doi.org/10.1093/cercor/bhw366. [DOI] [PubMed] [Google Scholar]
- Stosiek C, Garaschuk O, Holthoff K & Konnerth A (2003). In vivo two‐photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100, 7319–7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E, Mikoshiba K & Hirase H (2011). Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31, 18155–18165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Komorowski RW, Manns JR, Kopell NJ & Eichenbaum H (2009). Theta‐gamma coupling increases during the learning of item‐context associations. Proc Natl Acad Sci USA 106, 20942–20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero‐Aracama MJ, Sauvage MM & Yoshida M (2015). Environmental enrichment modulates intrinsic cellular excitability of hippocampal CA1 pyramidal cells in a housing duration and anatomical location‐dependent manner. Behav Brain Res 292, 209–218. [DOI] [PubMed] [Google Scholar]
- Wolansky T, Clement EA, Peters SR, Palczak MA & Dickson CT (2006). Hippocampal slow oscillation: a novel EEG state and its coordination with ongoing neocortical activity. J Neurosci 26, 6213–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Ge W, Chen Y, Zhang Z, Shen W, Wu C, Poo M & Duan S (2003). Contribution of astrocytes to hippocampal long‐term potentiation through release of D‐serine. Proc Natl Acad Sci USA 100, 15194–15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder RM & Pang KCH (2005). Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus 15, 381–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclaimer: Supporting information has been peer‐reviewed but not copyedited.
Figure S1. Effect of varying ripple detection threshold. Ripple detection threshold was varied from 6 × SD to 8 × SD. The occurrence (A) and RMS amplitude (B) of detected ripples are plotted for each combination of genotype (WT or IP3R2‐KO) and housing condition (enriched or isolated). These analyses were made for recordings from the right CA1 stratum pyramidale. The relationship among the experiment groups remains generally similar across the range of threshold for both ripple occurrence and RMS amplitude.


