Abstract
AIM
To investigate the neuroprotective effect of gastrodin on retinal ganglion cells (RGCs) in an acute ocular hypertension (AOH) rat model and to identify its possible mechanism.
METHODS
AOH rat model was performed in a randomly selected eye by anterior chamber perfusion and either received an intraperitoneal injection with various concentrations of gastrodin or normal saline. After 2wk, the rats were sacrificed. FluoroGold was used to label survival RGCs. Immunostaining with anti-Iba1 in the retinal flat mounts to calculate the microglia density in the ganglion cell layer (GCL). Changes in microglial cytokines, tumour necrosis factor-alpha (TNF-α) and inducible NO synthase (iNOS) were examined with Western blot and reverse transcription-quantitative polymerase chain reaction. Expression levels of total and phosphorylated p38 mitogen activated protein kinase (MAPK) were determined by Western blot.
RESULTS
Results showed that AOH induced significant loss of RGCs and severe microglia activation in the GCL. Besides, AOH increased the phosphorylation of p38 MAPK and promoted the release of microglial cytokines in the retinas. Intraperitoneal injection with dose-dependent gastrodin significantly reduced the loss of RGCs and inhibited retinal microglia activation, accompanied with the decreased expression levels of microglial cytokines and p38 MAPK phosphorylation.
CONCLUSION
Gastrodin exerts a neuroprotective effect on RGCs in an acute glaucoma animal model via inhibiting microglia activation and microglial-mediated neuroinflammation. The finding demonstrates the potential application of gastrodin in the neuroprotective therapy of acute glaucoma and other retinal neurodegenerative diseases characterized by microglia activation and RGCs death.
Keywords: gastrodin, retina ganglion cells, microglia, neuroinflammation, acute ocular hypertension
INTRODUCTION
Acute angle closure glaucoma, which is characterized by a sudden and substantial increase in intraocular pressure (IOP), commonly occurs among Asians and presents acute loss of vision and pain[1]–[3]. Even with the available medical and surgical treatment, the rapid and elevated IOP can cause permanent and irreversible loss of vision due to the death of retinal ganglion cells (RGCs). The mechanisms by which elevated IOP leads to RGCs apoptosis are complicated and not well understood[4]–[5]. RGCs death and upregulation of microglia reactivity induced by acute ocular hypertension (AOH) have been described in detail in many studies[6]–[8]. In our previous study, we also characterized RGC loss and the activation of retina microglia in an AOH model in rat[8]. Accumulating evidence indicates that overactivated microglia can trigger neuroinflammation in the retina and subsequently lead to the death of RGCs. It is believed that overactivated microglia cells compose the majority of this inflammatory response and contribute to the neurodegenerative process, including the RGCs death. Suppression of microglial-mediated neuroinflammation will result in neuroprotection. Therefore, various strategies for inhibiting microglia activation, especially for the pharmacological inhibitors of microglial activation, have been developed[9].
In recent years, a number of herbals and its natural compound have become increasing popular and many herbal medicine have received considerable attention as alternative candidates for therapeutic purposes. The phenolic glucoside gastrodin, a natural product-based pharmacological inhibitor of microglial activation, has long been used for treating dizziness, epilepsy, stroke and dementia. Gastrodin is considered to specifically mediate microglial neurotoxicity and have potential neuroprotective effects, in particular to improve learning and facilitate memory consolidation and retrieval[10]. Recent studies strongly suggest that gastrodin has a protective action on neurons in different animal models[11]–[13]. RGCs are the only output neurons of the retina, however, to the best of our knowledge, no report has been conducted on the effect of gastrodin on RGCs. In the current study, we firstly elucidate the influence of gastrodin on the RGCs survival in an AOH rat model, and further investigated its possible mechanism.
MATERIALS AND METHODS
All the animal procedures were approved by the Committee of Animal Care of Sun Yat-Sen University (Guangzhou, China) and all the Use of Animals were performed in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement. Adult female SD rats (age, 8wk; weight, 200-250 g) were used for all the experiments and every possible measure was performed in order to minimize animal suffering. The animals were caged individually in an environmentally controlled room (22°C-26°C) with an alternating 12h/12h light/dark cycle and enough food and water. The rats were divided into 4 groups: 1) control group; 2) normal saline (NS) group who were exposed to AOH and received intraperitoneal injection of 0.9% NS; 3) G10 group who were exposed to AOH and received intraperitoneal injection of 10 mg/kg gastrodin; 4) G50 group who were exposed to AOH and received intraperitoneal injection of 50 mg/kg gastrodin. Significant loss of RGCs is known to take place in a delayed fashion after the acute high IOP. The number of RGCs decreased to less than 50%, meanwhile, the retina microglial cells also increased significantly in number and displayed an activated morphology, as revealed by Iba1-positive cell on day 14 after acute high IOP[6]. Therefore, the loss of RGCs and the number of Iba1-positive retina microglia were determined at 2wk after rapid ocular hypertension. Gastrodin (Sigma Company), dissolved in NS, was administered intraperitoneally for 1d at the respective doses before the induction of AOH model.
Induction of Acute Ocular Hypertension
The detailed methodology used to generate AOH animal model was already described in our published material[8]. All the operations were performed under general anesthesia with intraperitoneal injection of chloral hydrate (400 mg/kg) and additional topical anesthesia (0.5% proparacaine hydrochloride eye drops, Alcon). The anterior chamber of a randomly chosen eye was cannulated with a 30-Gauge infusion needle connected with a 500 mL saline-filled bottle. By keeping the bottle to a height of 1500 mm above the eye, the IOP could rise to 110 mm Hg. The IOP was monitored using a Tono-Pen (Medtronic Co., Dublin, Ireland) and continuously maintained for 60min. Care was taken to keep the lens and the iris intact and only the rats with unimpaired lenses and corneas were included for the further experiment. The rats with only anterior chamber penetration and without IOP elevation were served as the control group. After 60min, the needle was removed from the anterior chamber and the rats were intraperitoneally injected with gastrodin at 10 mg/kg (G10 group), 50 mg/kg (G50 group) and 0.9% NS (NS group), respectively, once a day for the next 2wk.
Quantification of Survival Retinal Ganglion Cells
Retrograde FluoroGold labeling was used to quantify the number of survival RGCs in the retina. The procedures of FluoroGold labeling was described in detail elsewhere[14]. To avoid confounding the RGCs with dye engulfing microglia or macrophages, 2% FluoroGold (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was injected into both superior colliculi of each hemisphere at 9d after the induction of AOH model. After 5d, the rats were sacrificed under deep anaesthesia and their globes were harvested. Each retina was detached carefully from the eye cup and fixed with 4% paraformaldehyde for 1h. After wishing with phosphate buffer saline (PBS), the retinas were prepared as a flat mount and divided into four quadrants (superior and inferior, nasal and temporal) by four radial cuts. The retinas were photographed with a fluorescent microscope (Carl Zeiss Imaging, Inc., Jena, Germany), images were acquired at 1.0 mm to 2.0 mm from the optic disc center in each quadrant. There random rectangular areas (0.36×0.24 mm2) were examined in each quadrant and subsequently the numbers of RGCs were counted automatically and manually by two operators using one commercial software (Image Pro Plus 6.0, Cybernetics, Bethesda, MD, USA). The values were averaged over the total twelve square areas to get the average number of RGCs. The density of the RGCs was dividing the average number of RGCs by the area of the square areas.
Immunofluorescence and Cell Counting of Retinal Microglia
Immediately after photographing, the retinas were extensively washed with PBS three times and then incubated with 5% Tween, 5% Triton-X100 in PBS overnight. Non-specific binding was blocked by incubation with dried milk solution. Subsequently, the retinal flat mounts were incubated with rabbit anti-Iba1 antibody (1:400, Wako) at 4°C overnight. After a washing step at room temperature, the retinas were incubated with secondary Alexa Fluor-conjugated antibodies (Cell Signaling Technology, USA) for one hour in the dark at room temperature. After washing three times with PBS, the retinas were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) and mounted in fluorescent mounting medium. A confocal laser scanning microscope (Zeiss LSM 510 Meta, Zeiss Co., Jena, Germany) was used to take the pictures of the retinas, and then the images were analyzed by Image Pro Plus 6.0 software. All the images were acquired at 1.0 mm to 2.0 mm from the optic disc center, the same as the quantification of RGCs. The number of microglia in each quadrant was counted and averaged to calculate the density of microglia in the ganglion cell layer (GCL) of the retina.
Western Blot Analysis
Fresh-dissected retinas were immediately homogenized in RIPA lysis buffer (Beyotime Co., Jiangsu Province, China) and centrifuged for 15min to collect the supernatant. The protein concentration in each sample was detected by the BCA method using one commercial Protein Assay Kit (Beijing Co Win; Bioscience Co., Beijing, China) and the protein integrity was verified using standard silver staining of typical SDS gels. Each sample containing equal amount of protein was separated in a 10% SDS-polyacrylamide gel and transferred onto PVDF membranes. After incubation with 5% non-fat dry milk in TBST [10 mmol/L Tris-HCl (pH 7.6), 150 mmol/L NaCl and 0.1% Tween-20] for 1h at room temperature, the transferred membranes were then incubated overnight at 4°C with goat anti-tumour necrosis factor-alpha (TNF-α) (1:500, Santa Cruz Biotechnology), rabbit anti-inducible NO synthase (iNOS, 1:800, Santa Cruz Biotechnology), rabbit anti phosphorylated-p38 mitogen activated protein kinase (MAPK, Thr180/Tyr182, 1:500, Santa Cruz Biotechnology), rabbit anti total p38 MAPK (1:500, Santa Cruz Biotechnology) and β-actin (1:500, Santa Cruz Biotechnology). After washing three times with TBST, the membranes were incubated with horseradish-conjugated secondary antibodies (1:5000 or 1:10000, Beijing Biosynthesis Biotechnology) for 1h at room temperature. Blots were washed again three times in TBST and developed by an enhanced chemiluminescence detection system (Millipore, Jaffrey, NH, USA). Membranes were exposed to Fuji medical X-ray films (Fuji Photo Film Co., Ltd, Karagawa, Japan) and the densitometric analysis was performed by Quality One software (Bio-Rad, Philadelphia, PA, USA).
Reverse Transcription-quantitative Polymerase Chain Reaction
Retinas were collected at day 14 postoperatively, following treatment with NS or various doses of gastrodin. The mRNA expression levels of TNF-α and iNOS were determined by reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Total RNA was isolated using Trizol reagent (Invitrogen Co., Carlsbad, CA, USA) and the first strand cDNA was generated by reverse transcription using one commercial reagent (Takara Bio Inc., Shiga, Japan), according to the manufacturer's instructions. Quantitative PCR was performed using Fast Start Universal SYBR Green Master (Roche Life Science) on Lightcycler 480 system (Roche Life Science). The primers used were as follows: TNF-α primers: forwad (5′-CAGGTTCCGTCCCTCTCATA-3′) and reverse (5′-TGCCAGTTCCACATCTCG-3′), iNOS primers: forwad (5′-GCAAGCCCTCACCTACTTCC-3′) and reverse (5′-AACCTCTGCCTGTGCGTCT-3′), β-actin was used as an internal control. Rat β-actin endogenous reference genes primers (NO. B661202, Sangon Biotech) and the other primers were all purchased from Sangon Biotech (Shanghai, china). All reactions were performed in triplicate and average threshold cycle (Ct) values >35 were considered to be negative. The relative expression levels of mRNAs were calculated using comparative Ct method. Each group was normalized to the control group and then set at 100%.
Statistical Analysis
SPSS software (version 21.0; IBM-SPSS Inc., Chicago, IL, USA) and GRAPHPAD PRISM (version 5.0; GraphPad Inc., La Jolla, CA, USA) were used for statistical analysis and graphics. The summarized data were expressed as mean±standard deviation (SD) obtained from three independent experiments. The differences between different groups were calculated by one-way analysis of variance (ANOVA) or the Student's t-test. Statistical significance was defined as P<0.05.
RESULTS
Gastrodin Treatment Attenuated Retinal Ganglion Cells Loss After Acute Ocular Hypertension
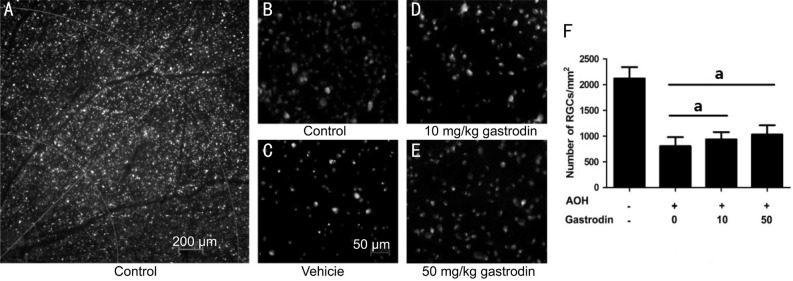
As gastrodin dramatically protects neuronal cells in different models, we investigated whether it had a neuroprotective effect on RGCs. The survival RGCs was evaluated by retrograde FluoroGold labeling. There was a significant RGCs loss in the AOH retinas due to the acute IOP elevation. Only 37.9% RGCs were labeled by FluoroGold in the NS group after 2wk (Figure 1). Intraperitoneal injection with gastrodin 10 mg/kg or 50 mg/kg once daily for 15d significantly inhibited the loss of RGCs because of AOH damage (Figure 1D, 1E). Compared to the NS group, the density of the RGCs increased to 937.4±141.7 cells/mm2 and 1031±179.2 cells/mm2, respectively (Figure 1F). These results indicated that systemic gastrodin had a neuroprotective effect on RGCs in the AOH animal model.
Figure 1. Neuroprotective effect of gastrodin on RGCs with AOH damage.
FluoroGold (FG)-labeled RGCs in whole-mount retina (A); Representative photograph of FG-labeled RGCs in the control group (B) and in the rats either receiving an intraperitoneal injection with 0.9% NS (C) and various concentrations of gastrodin (10 mg/kg, D; 50 mg/kg, E) at 2wk after AOH. Quantitative analysis of the number of FG-labeled RGCs at 2wk in the four groups (F). n=6 in each group. Data represent the mean±standard deviation (SD). aP<0.01.
Gastrodin Treatment Inhibited Tetinal Microglia Accumulation Caused by Acute Ocular Hypertension
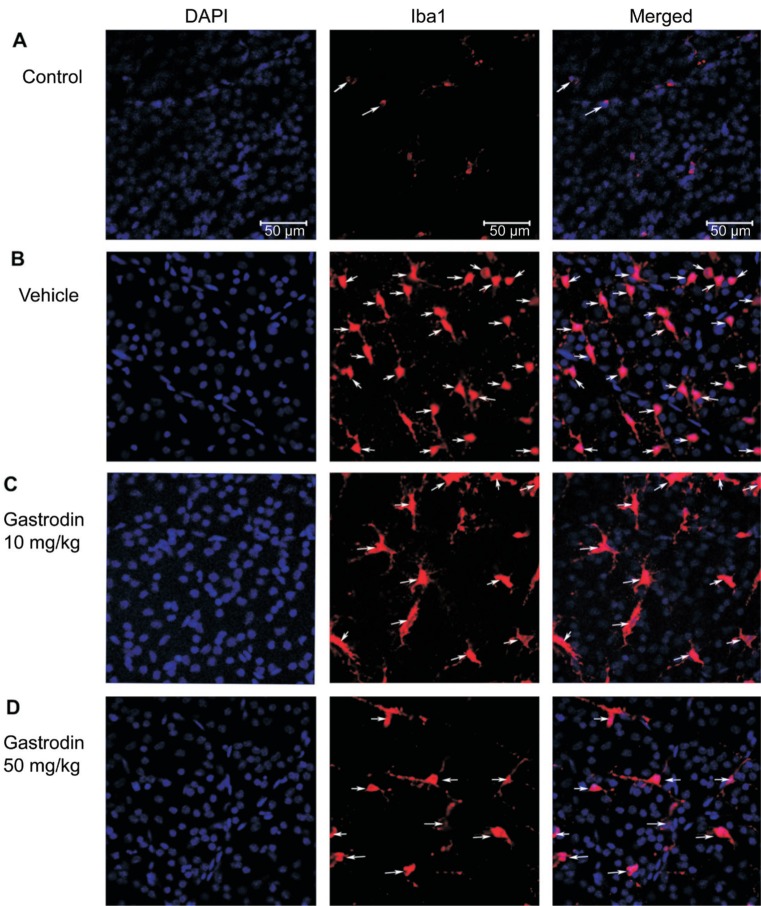
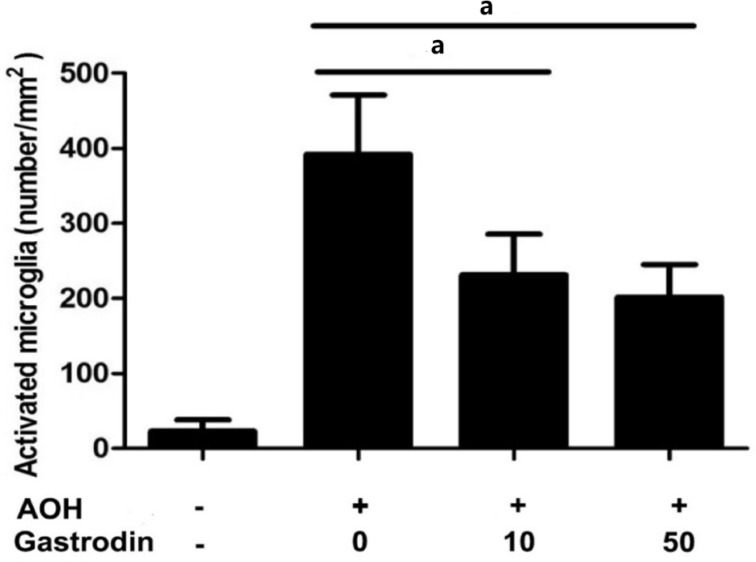
Previous analysis showed that gastrodin is a natural product-based inhibitor of microglial activation. To verify whether gastrodin could inhibit microglial activation in the AOH rat model, we immediately tested the density of microglia in the retinal GCL layer using immunofluorescence. The results showed that acute IOP elevation induced severe accumulation of microglia in the GCL layer of the retina, as detected by retinal flat-mount immunostaining for the microglia marker Iba1. In the control group, the density of microglia in the retinal GCL was 23.1±15.2 cells/mm2 (Figure 2A). After 2wk in the NS group, the density of microglia markedly increased to 392.3±79.0 cells/mm2 and most of the microglia cells displayed a typical amoeboid morphology with large round cell bodies (Figure 2B). Whereas 2wk after AOH, the numbers of Iba1 positive retinal microglia obviously reduced to 231.3±54.3 cells/mm2 and 201.9±43.1 cells/mm2 in the rats intraperitoneally injected with gastrodin at 10 mg/kg and 50 mg/kg (Figures 2C, 2D and 3).
Figure 2. Effect of gastrodin on retinal microglia accumulation caused by AOH.
Staining for Iba1-positive microglia (red) in the whole-mount retina. In the control group, only few microglia cells displayed a typical amoeboid morphology with large round cell bodies (A). At 2wk after AOH, there was severe accumulation of microglia in the GCL and most of the microglia showed as amoeboid morphology (B). Gastrodin treatment obviously inhibited the microglia accumulation in the GCL (C, D).
Figure 3. Quantitative analyses of the number of retinal microglia at 2wk post-AOH in rats receiving normal saline, 10 mg/kg and 50 mg/kg gastrodin.
n=6 in each group. Data represent the mean±standard deviation (SD). aP<0.01.
Gastrodin Treatment Suppressed Microglial-mediated Neuroinflammation Triggered by Acute Ocular Hypertension
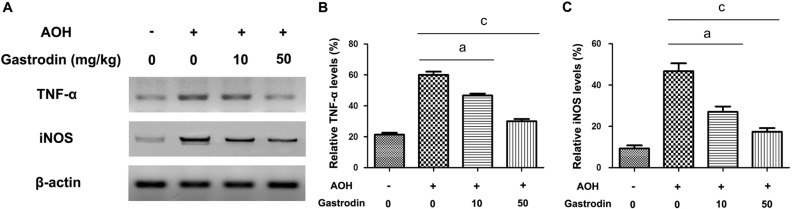
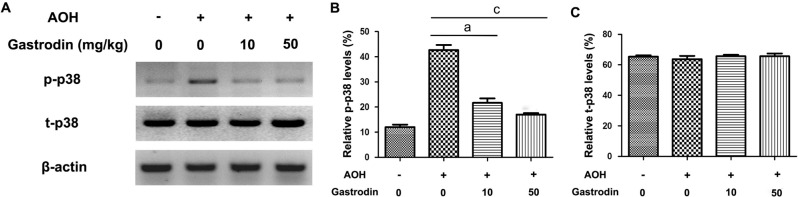
Given the important role of microglial-mediated neuroinflammation on the death of RGCs, the microglial cytokines, TNF-α and iNOS, were determined through Western blot (Figure 4A). As reported previously, acute IOP elevation activated the p38 MAPK signaling pathway in the retina. The levels of total p38 MAPK and phosphorylated-p38 MAPK were also detected with Western blot (Figure 5A). The protein expression levels of TNF-α, iNOS and p38 MAPK phosphorylation were significantly higher in the NS group compared with the other three groups. As expected, treatment with gastrodin significantly reduced the TNF-α and iNOS protein expression levels and phosphorylation of p38 MAPK (Figures 4, 5).
Figure 4. Effect of gastrodin on protein expression of TNF-α and iNOS in the retina after AOH.
Representative Western blot showing TNF-α and iNOS expression at 2wk after AOH injury (A); Quantitative analyses of TNF-α and iNOS protein expression (B, C). β-actin is used as a loading control. n=6 in each group. Data represent the mean±standard deviation (SD). aP<0.01. cP<0.001.
Figure 5. Effect of gastrodin on protein expression of total p38 (t-p38) and phosphorylated-p38 (p-p38) in the retina after AOH.
n=6 in each group. Data represent the mean±standard deviation (SD). aP<0.01. cP<0.001.
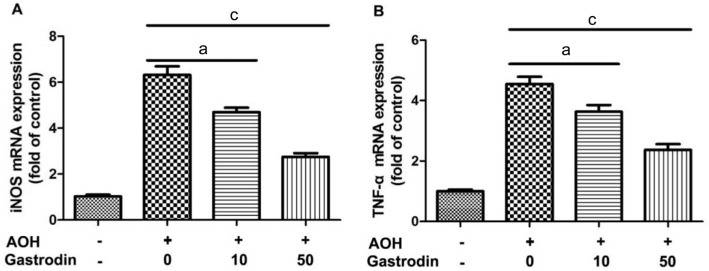
Gastrodin Treatment Reduced the mRNA Expression Levels of TNF-α and iNOS in the Retinas of Acute Ocular Hypertension
The effect of gastrodin on the regulation of TNF-α and iNOS was investigated by RT-qPCR. As shown in Figure 6, AOH induced the upregulation of TNF-α and iNOS mRNAs in the retinas of NS group. The further results revealed that both genes significantly decreased in G10 and G50 groups compared with the NS group.
Figure 6. Effect of gastrodin on expression levels of TNF-α and iNOS mRNA in the retina after AOH.
n=6 in each group. Data represent the mean±standard deviation (SD). aP<0.01. cP<0.001.
DISCUSSION
Gastrodin, a main constituent of a Chinese herbal medicine Tianma, has been known to display anti-inflammatory effects. More importantly, gastrodin can breach the blood-brain barrier and enter into the central nervous system. Therefore, gastrodin has been studied intensively in various experimental models, including Parkinson's disease[15], stroke[16], inflammatory pain[17], among others. The latest studies also suggest that gastrodin exhibits neuroprotection in neurons under a pathological situation[11]–[13]. For the first time, the current study observed that gastrodin significantly inhibited the AOH-induced microglia activation and the production of pro-inflammatory mediators (TNF-α and iNOS) and then concomitantly reduced the loss of RGCs in a dose-dependent manner. These results suggested that gastrodin has a neuroprotective effect on RGCs.
As the only resident immune cells of the retina, microglial cells become readily activated during trauma, inflammation, or immunologic stimuli. Abnormal microglia reactivity was reported in both glaucoma patients and animal models[18]–[19]. In the current project, we used the AOH rat model to simulate the activation of retinal microglia in acute glaucoma and again confirmed short-term ocular hypertension lead to severe retinal microglia activation[8]. Accumulating evidence shows that the over-activated microglia can secrete a large number of neurotoxic factors, which magnify and perpetuate the local inflammation and immunological reactions to induce RGCs death[20]. Therefore, inhibition of microglial activation has been found to be a beneficial therapeutic strategy to reduce RGCs death.
Our data showed that AOH upregulated the mRNA and protein levels of TNF-α and iNOS in the retina. The rapid upregulation of the proinflammatory cytokines can promote RGCs death after the damage of AOH. It has been proposed that activated microglia are the main cellular source of TNF-α and iNOS[21]. This study investigated whether gastrodin treatment inhibits AOH-induced microglia activation and production of TNF-α and iNOS. Our resluts suggest that gastrodin obviously inhibited the microglia accumulation in the GCL and thus decreased the protein expression levels of TNF-α and iNOS. Interestingly, gastrodin could also inhibit the TNF-α and iNOS mRNA expression levels, which suggest that gastrodin inhibited the production of proinflammatory factors through the regulation of their gene transcriptional level.
The p38 MAPK signaling pathway, one major neuroinflammation-related signaling pathway, can be activated by stresses and proinflammatory cytokines, has been implicated in many biological processes, including cell death, cell proliferation, cell migration and invasion[1]–[3]. Inhibition of the p38 pathway can block the production of TNF-α and other cytokines. Our previous finding has shown that phosphorylation p38 MAPK was much higher in the AOH retinas[8]. In an in vitro model of lipopolysaccharide (LPS)-stimulated microglia activation, gastrodin significantly attenuate the levels of proinflammatory cytokines by inhibition of the NF-κB signaling pathway and phosphorylation of MAPKs pathway[22]. In the rat model of posttraumatic stress disorder, Peng et al[23] found gastrodin could ameliorate anxiety-like behaviors and inhibit p38 MAPK phosphorylation of hippocampus. Taken together, we hypothesize that gastrodin may also influence the p38 MAPK phosphorylation in our AOH rat model.
Many reports have indicated that gastrodin can act upon multiple pathways, such as NF-κB, ERK1/2, and iNOS, among others, except on the p38 MAPK signaling pathway. Thus, gastrodin could possibly inhibit the microglia activation dependent on different pathways, including the p38 MAPK pathway. As expected, the phosphorylation level of p38 was significantly decreased as a result of continuous gastrodin treatment. Therefore, the p38 MAPK signaling pathway is the major signaling pathway through which gastrodin exerts its anti-inflammatory effects[23]. In addition to its anti-inflammatory effect, gastrodin can prevent the enhancement of extracellular glutamate level, which may provide extra insights to explain the neurochemical effects of gastrodin against RGCs damage induced by AOH[13]. Further investigations are needed to elucidate the direct effect of gastrodin on cultured primary RGCs.
In conclusion, we showed that gastrodin exerted its neuroprotective effects on RGCs in the AOH rat model. Gastrodin treatment significantly attenuated the production of microglia-mediated pro-inflammatory mediators, including TNF-α and iNOS. Furthermore, the level of phosphorylated p38 MAPK was significantly decreased by continuous gastrodin treatment. Taken together, these findings support our belief that the therapeutic potential of gastrodin in neuroprotective therapy for glaucoma and gastrodin can promote the survival of RGCs and may have a therapeutic potential in other degenerative optic neuropathies characterized by RGC apoptosis.
Acknowledgments
Foundation: Supported by the Natural Science Foundation of Shandong Province, China (No.ZR2017BH049).
Conflicts of Interest: Wang JW, None; Liu YM, None; Zhao XF, None; Zhang H, None.
REFERENCES
- 1.Seki M, Lipton SA. Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma. Prog Brain Res. 2008;173:495–510. doi: 10.1016/S0079-6123(08)01134-5. [DOI] [PubMed] [Google Scholar]
- 2.Katome T, Namekata K, Guo X, Semba K, Kittaka D, Kawamura K, Kimura A, Harada C, Ichijo H, Mitamura Y, Harada T. Inhibition of ASK1-p38 pathway prevents neural cell death following optic nerve injury. Cell Death Differ. 2013;20(2):270–280. doi: 10.1038/cdd.2012.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotamisligil GS, Davis RJ. Cell signaling and stress responses. Cold Spring Harb Perspect Biol. 2016;8(10):a006072. doi: 10.1101/cshperspect.a006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi W, Li F, Chen H, Wang Y, Zhu Y, Yang X, Zhu J, Wu F, Ouyang H, Ge J, Weinreb RN, Zhang K, Zhuo Yl. Caspase-8 promotes NLRP1/NLRP3 inflammasome activation and IL-1β production in acute glaucoma. Proc Natl Acad Sci U S A. 2014;111(30):11181–11186. doi: 10.1073/pnas.1402819111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31(2):152–181. doi: 10.1016/j.preteyeres.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Trost A, Motloch K, Bruckner D, Schroedl F, Bogner B, Kaser-Eichberger A, Runge C, Strohmaier C, Klein B, Aigner L, Reitsamer HA. Time-dependent retinal ganglion cell loss, microglial activation and blood-retina-barrier tightness in an acute model of ocular hypertension. Exp Eye Res. 2015;136:59–71. doi: 10.1016/j.exer.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Crowston JG, Kong YX, Trounce IA, Dang TM, Fahy ET, Bui BV, Morrison JC, Chrysostomou V. An acute intraocular pressure challenge to assess retinal ganglion cell injury and recovery in the mouse. Exp Eye Res. 2015;141:3–8. doi: 10.1016/j.exer.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Valiente-Soriano FJ, Nadal-Nicolás FM, Rovere G, Chen S, Huang W, Agudo-Barriuso M, Jonas JB, Vidal-Sanz M, Zhang X. MicroRNA regulation in an animal model of acute ocular hypertension. Acta Ophthalmol. 2017;95(1):e10–e21. doi: 10.1111/aos.13227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: focus on natural products. Molecules. 2011;16(2):1021–1043. doi: 10.3390/molecules16021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu Y, Li C, Shen W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer's disease. Neuropathology. 2014;34(4):370–377. doi: 10.1111/neup.12115. [DOI] [PubMed] [Google Scholar]
- 11.Wong SB, Hung WC, Min MY. The role of gastrodin on hippocampal neurons after N-Methyl-D-Aspartate excitotoxicity and experimental temporal lobe seizures. Chin J Physiol. 2016;59(3):156–164. doi: 10.4077/CJP.2016.BAE385. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X, Zou Y, Xu H, Fan L, Guo H, Li X, Li G, Zhang X, Dong M. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012;1482:13–21. doi: 10.1016/j.brainres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Xu X, Lu Y, Bie X. Protective effects of gastrodin on hypoxia-induced toxicity in primary cultures of rat cortical neurons. Planta Med. 2007;73(7):650–654. doi: 10.1055/s-2007-981523. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Chen S, Zhang X, Huang W, Jonas JB. Intravitreal triamcinolone acetonide, retinal microglia and retinal ganglion cell apoptosis in the optic nerve crush model. Acta Ophthalmol. 2016;94(5):e305–e311. doi: 10.1111/aos.12698. [DOI] [PubMed] [Google Scholar]
- 15.Kumar H, Kim IS, More SV, Kim BW, Bahk YY, Choi DK. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson's disease model. Evid Based Complement Alternat Med. 2013;2013:514095. doi: 10.1155/2013/514095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng Z, Wang S, Chen G, Cai M, Liu R, Deng J, Liu J, Zhang T, Tan Q, Hai C. Gastrodin alleviates cerebral ischemic damage in mice by improving anti-oxidant and anti-inflammation activities and inhibiting apoptosis pathway. Neurochem Res. 2015;40(4):661–673. doi: 10.1007/s11064-015-1513-5. [DOI] [PubMed] [Google Scholar]
- 17.Xiao MM, Zhang YQ, Wang WT, Han WJ, Lin Z, Xie RG, Cao Z, Lu N, Hu SJ, Wu SX, Dong H, Luo C. Gastrodin protects against chronic inflammatory pain by inhibiting spinal synaptic potentiation. Sci Rep. 2016;6:37251. doi: 10.1038/srep37251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallego BI, Salazar JJ, de Hoz R, Rojas B, Ramírez AI, Salinas-Navarro M, Ortín-Martínez A, Valiente-Soriano FJ, Avilés-Trigueros M, Villegas-Perez MP, Vidal-Sanz M, Triviño A, Ramírez JM. IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J Neuroinflammation. 2012;9:92. doi: 10.1186/1742-2094-9-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan L, Neufeld AH. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64(5):523–532. doi: 10.1002/jnr.1104. [DOI] [PubMed] [Google Scholar]
- 20.Sivakumar V, Foulds WS, Luu CD, Ling EA, Kaur C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J Pathol. 2011;224(2):245–260. doi: 10.1002/path.2858. [DOI] [PubMed] [Google Scholar]
- 21.Dheen ST, Jun Y, Yan Z, Tay SS, Ling EA. Retinoic acid inhibits expression of TNF-alpha and iNOS in activated rat microglia. Glia. 2005;50(1):21–31. doi: 10.1002/glia.20153. [DOI] [PubMed] [Google Scholar]
- 22.Dai JN, Zong Y, Zhong LM, Li YM, Zhang W, Bian LG, Ai QL, Liu YD, Sun J, Lu D. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS One. 2011;6(7):e21891. doi: 10.1371/journal.pone.0021891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng Z, Wang H, Zhang R, Chen Y, Xue F, Nie H, Chen Y, Wu D, Wang Y, Wang H, Tan Q. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol Res. 2013;62(5):537–545. doi: 10.33549/physiolres.932507. [DOI] [PubMed] [Google Scholar]