Abstract
AIM
To assess the expression of nestin and glial fibrillary acidic protein (GFAP) in rat retina after optic nerve transection.
METHODS
Rats were randomly divided into normal control group, sham group and operation group, and used for establishing an animal model of optic nerve transection. Retinal specimen of each group was collected at 3, 48h, 7 and 14d postoperative. Nestin and GFAP expressions on sagittal sections were analyzed by immunohistochemical staining, and protein extraction was analyzed by Western blot.
RESULTS
Immunohistochemical analysis showed that nestin positive staining was rarely detected in normal control group and sham group, while sham group showed weak positive staining at 3h postoperative, the reaction gradually increased at 48h postoperative, and reached its maximum at 7d postoperative, and then decreased at 14d postoperative. Compared to the expression of GFAP, there was not statistically significant obvious difference among three groups (P>0.05). Result of Western blot method was consistent with that of immunohistochemical method.
CONCLUSION
The expression of nestin increased in a time dependent fashion in Müller cells of retina following optic nerve transection, which was statistically significant, but there was no obvious difference in GFAP expression. The results indicate that an increase in colloid synthesis in retina following optic nerve transection can improve the retinal neurons' environment.
Keywords: optic nerve transection, Müller cells, nestin, glial fibrillary acidic protein, rats
INTRODUCTION
Retinal degeneration is the most common disease caused by optic nerve injury in the ophthalmology. It can be the result of a trauma and pathological processes like optic neuritis, ischemic optic neuropathy, glaucoma, diabetes mellitus, retinal vessel occlusion, space-occupying lesions of visual pathway. All of these can lead to optic nerve injury and atrophy, vision loss or even blindness[1]. Due to the lack of timely and effective treatment measures for these diseases, half of patients end up losing their sight. Therefore, to explore the repair and regeneration methods of optic nerve injury, we must strive to focus on research strategy that can help optimize the optic nerve functions.
Central nervous system (CNS) in adult mammals includes two cell types: neurons and glial cells. A neuron is the basic unit of nerve signal generator having no regeneration capability after damage, but some special parts in it still maintain the neural stem and progenitor cell populations such as subependymal ventrical zone (SVZ) and subgranular zone (SGZ). They can self-renew and regenerate themselves[2]–[4]. Glial cells are known to play the role of support and protection in nervous system. Optic nerve is the part of CNS, and is composed of retinal ganglion cell axons and glial cells. Retina have two kinds of glial cells, one of them is the Müller cell. This cell forms the reticulate structure in the layers of the retina to control normal retinal homeostasis, vegetate and participate in the formation of blood-retinal barrier. It also has a close relationship with retinal ganglion cell and blood vessels wherein it plays a role in repairing the optic ganglion cells. The other one is astrocyte having a synergistic action with Müller cells.
A recent research showed that Müller cell has stem cells function, and was the main source of newly-born neurons, and was regarded as the cellular basis of retinal regeneration[5]. Müller cells contain massive amount of intermediate filament protein, the glial fibrillary acidic protein (GFAP) which is class IV intermediate filament protein and nestin, a class III intermediate filament protein. GFAP is a predominant skeleton protein and is expressed exclusively in astrocyte. However, the expression of GFAP in retina under pathological conditions has not been reported. Nestin, is mainly expressed in neural stem cells and neural precursor cells during the embryonic stage[6], and is down-regulated during neural stem cell differentiation into the neurons and glial cells, and then replaced by nonspecific fibroin GFAP[7]. Thus, nestin is widely used to identify the neural stem cell in basic and clinical research, and regarded as a biological marker of neural cell regeneration. Many experimental studies have found that nestin expression is up-regulated during retinal trauma[8] and stress state[9], while normal retinal has no expression.
In the current study expression levels of nestin and GFAP in Müller cell were investigated during trauma and stress state following retinal damage in an animal model of optic nerve transection to know the cellular differentiation process and related influencing factors in an order to find a new approach for the prevention and treatment of optic nerve associated injuries.
MATERIALS AND METHODS
Materials
Thirty-six SD rats, weighing 220±15 g, aged 2mo, half males and females, were randomly divided into 3 groups: normal control group (n=4), sham group (n=16) and operation group (n=16). SD rats of clean grade were obtained from Animal Experiment Center, Xi'an Jiaotong University (number of animal license SYXK 2005-002). The treatment and care of the animals were adherence to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and the animal research was approved by the Local Animal Care and Ethics Committee of Xi'an Jiaotong University (Shaanxi Province, China).
Main Reagents and Instruments
Nestin Mause McAb (No.66259-1-1g) and GFAP Mause McAb (No.6090-1-1g) as first antibody, AF488-conjugated Affinipure Goat Anti-Mouse IgG (H+L) (No. SA00006-1) as second antibody, were purchased from Proteintech Group, Inc. Electrophoresis apparatus (Tanon EPS300; Tanon Science & Technology Co., Ltd., Shanghai, China), chemiluminescent imaging systems (G:BOX 680X; Syngene Ltd., England), gel imaging analysis system (GelDoc 2000) were used. Analysis software was Quantity One Ver 4.62. IX5I fluorescence microscope (Olympus, Japan). Fluorescent intensity of immunohistochemical image was performed using Photoshop 7.0. HM500 O freezing microtome was obtained from Microm Co. Ltd. Germany.
Methods
To establish an animal model of optic nerve transection, anesthetized with 15% urethane (1 mL/kg) being injected into abdominal cavity in rats (75 mg/kg). Left margo supraorbitalis was performed on sagittal notch, then separated posterior pole and optic nerve terminal, cut optic never approximately 2 mm behind the globe by stratified suture to guarantee the central retinal vein system remained intact and avoid the optic traction. The surgical procedure of sham group was similar with model group, but sham group was not cut optic nerve. The animal had no treatment in normal control group, raising parallel as model group. At 3, 48h, 7 and 14d postoperative, 5 rats were randomly taken from three groups (4 eyes for sham group, 1 eye for model group, 2 eyes for normal group) underwent abdominal anesthesia, after internal fixation by 4% paraformaldehyde for 5min to completely remove eyeball, 2 eyes were analyzed using immuno histochemical, 2 eyes were analyzed using Western blot, each eyeball was analyzed from normal control group.
Immunohistochemical Analysis
The expression of nestin and GFAP on the radiographs were recorded and read under fluorescent Inverted Microscope according to the conventional method of fixation, frozen section and immunohistochemical staining. Western blot analysis: at 3, 48h, 7 and 14d postoperative, eyeball and cornea were completely removed, retina was took out, add 0.5 mL pH 7.2 phosphate buffer to EP tube for -80°C cryopreservation. Tissue lysate, protein extraction, protein concentration determination, SDS-PAGE (PolyAcrylamide Gel Electrophoresis), electric transfer and close were measured, then combined nestin and GFAP with first antibody and secondary antibody of actin to detect the moving object based on the chemiluminescence method, and image analyzer was used to measure the absorbance of each chromogenic strip, finally the absorbance of nestin and GFAP ratios were calculated to its relative content.
Statistical Analysis
All data are expressed in mean±standard deviation. Application of the GraphPad Prism 5.0 software (GraphPad, In. USA) was used in this study. Data were analyzed by one-way ANOVA, P values less than 0.05 was considered to be statistically significant.
RESULTS
Immunohistochemical Analysis
The expression of nestin
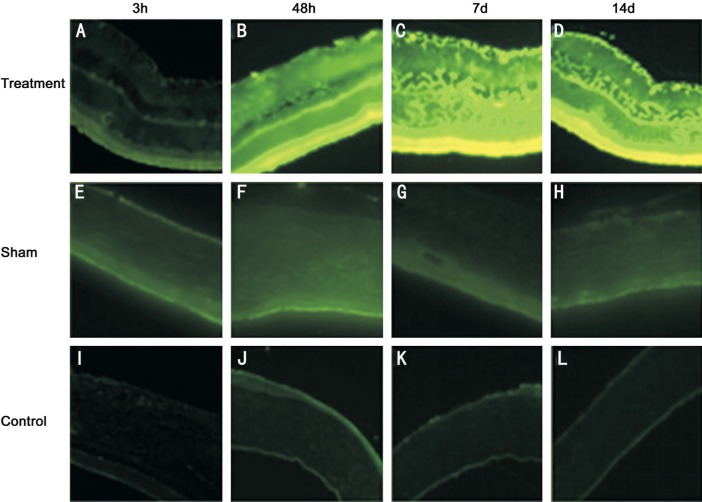
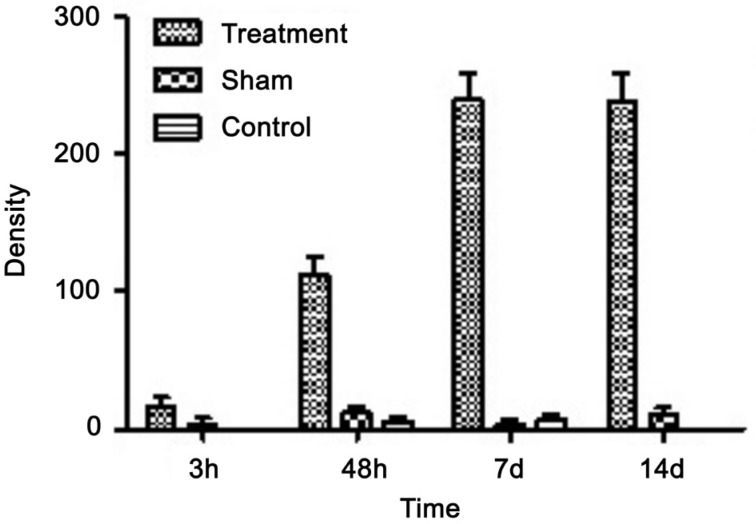
At 3, 48h, 7 and 14d after the optic nerve transection, nestin staining was negligible in normal control group and sham group (Figure 1E–1L), and there was no statistically significant difference between two groups. But operation group showed weak nestin-staining signal at 3h postoperative, mainly distributed in outer nuclera layer (ONL) and inner nuclear layer (INL) in the foot plate of Müller cells (Figure 1A). At 48h postoperative, nestin staining obviously increased in comparison with 3h postoperative, which was mainly distributed in ganglion cell layer (GCL) and inner plexiform layer (IPL) in internal limiting membrane of Müller cells. INL also had nestin staining, but it was very weak. At 7 and 14d postoperative, the distribution of nestin was obviously extending into ONL, INL, IPL, outer plexiform layer (OPL) and internal limiting membranes (ILM) (Figure 1C, 1D). The expression of nestin gradually increased along with the extension of time following the optic nerve transection, and reached its maximum at 7d postoperative (Figure 2).
Figure 1. The expression of nestin in rat retina after optic nerve transection.
Figure 2. The expression of retinal nestin after optic nerve transection in rat.
P<0.01, compared with control and sham groups.
The expression of glial fibrillary acidic protein
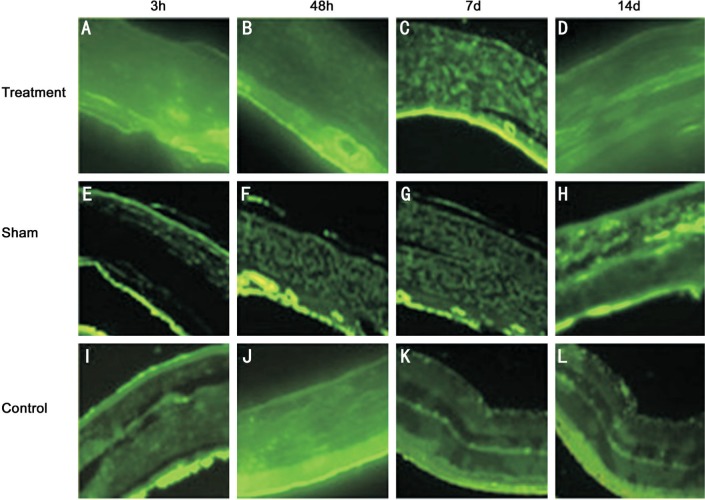
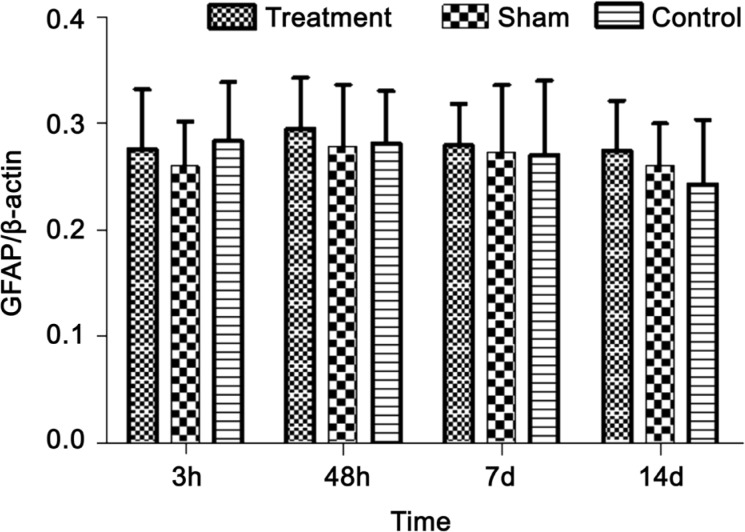
At 3, 48h, 7 and 14d postoperative, the fluorescence signal distribution of GFAP was diffused in control and sham groups, and the fluorescence intensity was not statistically significant between the two groups (Figures 3E–3L, 4). Similarly, the fluorescence signal of GFAP was not statistically significant in three groups (Figures 3A–3D, 4). These results showed that the expression level of GFAP was unchanged after optic nerve transection.
Figure 3. The expression of GFAP in rat retina after optic nerve transection.
Figure 4. The expression of retinal GFAP after optic nerve transection in rat.
Western Blot Analysis
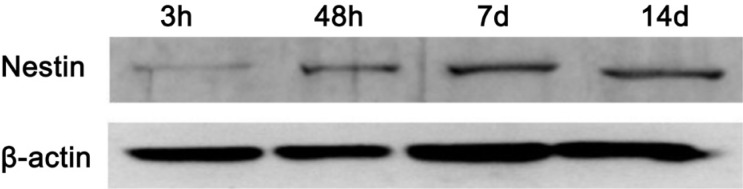
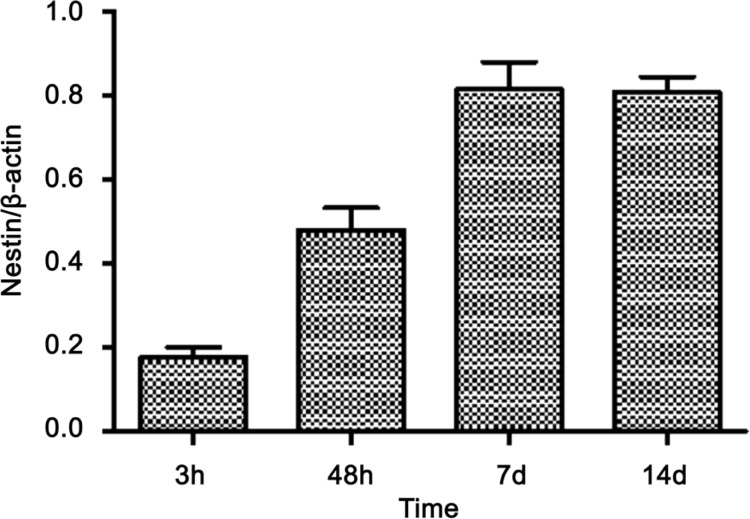
At 3, 48h, 7 and 14d postoperative, there was no expression of nestin in control and sham groups. However, at 3h postoperative, the expression of nestin was noticeable (Figure 5). At 48h postoperative, nestin expression obviously increased, and reached its maximum at 7d postoperative (Figures 5, 6), then decreased at 14d postoperative.
Figure 5. The expression of nestin in rat retina after optic nerve transection.
Figure 6. The expression of retinal nestin after optic nerve transection in rat.
The expression of glial fibrillary acidic protein
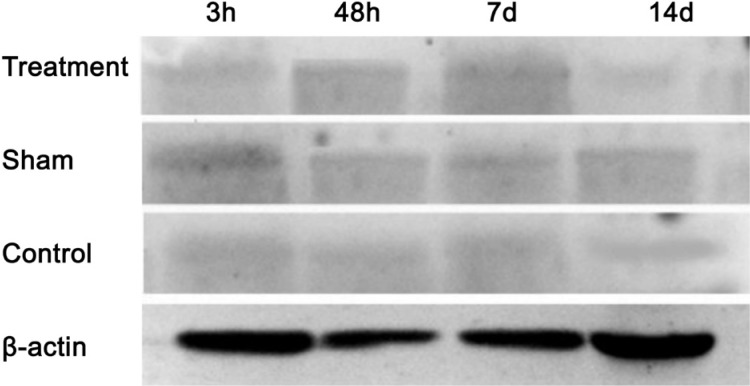
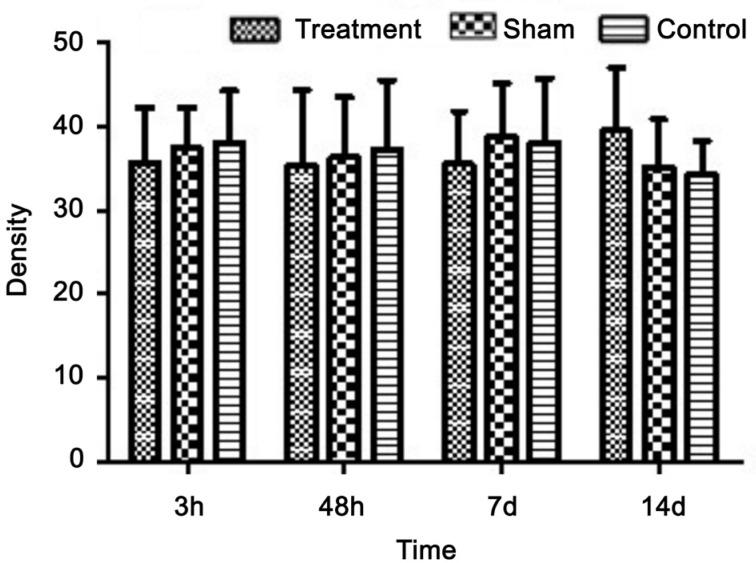
At 3, 48h, 7 and 14d postoperative, the expression of GFAP in rat retina was unchanged, and the results of sham and control groups were the same (Figure 7). Similarly, the operation group did not show any change at 3, 48h, 7 and 14d postoperative following the optic nerve transection (Figures 7, 8).
Figure 7. The expression of GFAP in rat retina after optic nerve transection.
Figure 8. The expression of retinal GFAP after optic nerve transection in rat.
DISCUSSION
Optic nerve is composed of retinal ganglion cell axons and support cells, which is considered to be part of the CNS. Like other central neurons, injury to the axon can be the effect on neuronal soma like disorder of axonal transportation, neurotrophic loss, neuronal degeneration, apoptosis and neuronal necrosis. Optic nerve and retina exhibit reaction to various form of injuries[10]–[12], but it is the Müller cells and astrocytes that tend to display the first reaction to the injury.
Müller cell is the most common type of glial cell found in the retina as it spans across the entire thickness of the neural retina from vitreous surface to the retinal pigment epithelium (RPE). Cell body is localized in the inner nuclear compartment. Astrocytes mainly exist in the nerve fiber layer, which is composed of CNS and retinal ganglion cell axons. Glial cell maintains the basic structure and function of the retina. The foot plate of Müller cell expresses GFAP and glutamate synthase during the development process, and it reveals a differentiation phenotype of retinal progenitor cells (RPCs)[13]–[15]. During retinal injury, Müller cell can change the expression of nestin as it does during the process of differentiation and proliferation[10],[16]–[17]. The retinal neurons can regenerate at the lesion site of neuronal toxicity without any obvious difference in the expression of GFAP. The expression of nestin is up-regulated in hereditary retinal degeneration as seen in a rat model[18].
This study was designed to observe the expression of nestin and GFAP by immunohistochemical and Western blot analyses after optic nerve transection. The results showed no obvious differences in the expression of GFAP in normal control and operation groups (Figures 1–8), and the ocular tissue damage had no effect on GFAP expression. Our study found that operation group exhibited nestin expression at 3h postoperative, and reached its maximum at 7d postoperative, then decreased at 14d postoperative. Nestin was mainly distributed in ONL and INL in the foot plate of Müller cells (Figure 1A), extending from GCL to RPE, and then spanning across the entire thickness of the retina. It also indicated that the expression of nestin increased with time after the optic nerve transection, further confirming that the Müller cells had the corresponding reaction after optic nerve transection. In CNS, the neuronal soma influences the axonal transportation when nerve synapses are damaged. It can lead to neuronal degeneration, apoptosis and necrosis. Retina as a part of CNS behaves the same way. After optic nerve transection, the function of axoplasmic transport was lost, retinal ganglion cells had no nutrition and stimulation, which caused degeneration and death. Meanwhile, neural stem cells/retinal progenitor cells of Müller cell may show the activation through differentiation and transdifferentiation processes and might compensate for the loss of retinal ganglion cells in an order to reconstruct the retinal structure. The results in this study did reveal the intermediate filament protein synthesis along with the increased expression of nestin.
Our results also found that the expression of GFAP in rat retina had no obvious differences in three groups at each time points after the optic nerve transection. GFAP is an intermediate filament protein expressed primarily in astrocytes. Nawashiro et al[19] reported cerebral hemorrhage in 12 of 15 GFAP−/− mice after percussive head injury by mechanical stress, while there was no cerebral hemorrhage in all of the 14 wild-type mice studied. These results indicated that intermediate filament protein played an important role in protecting the CNS during injury. Pekny and Lane[20] also reported that GFAP−/−-Vim−/− mice retina was spared from endometrial tissues after the mechanical damage, however, Vim−/− mice didn't get the same protective advantage post injury[20]. These results strongly suggest that GFAP play a vital role in protecting the retinal architecture. Astrocytes are a type of glial cells in mammalian retina, and they perform many functions including material exchange, signal transduction, retinal metabolism, maintenance of extracellular ion balance and the blood-brain barrier. They also in the repair process of the brain and spinal cord, mostly via their active participation in the retinal microvascular remodeling related events.
In short, these experimental results proved that Müller cell was active following optic nerve transection, and the phenomenon of activation-induced cell repair mechanisms appears to be the hallmarks of many diseases such as glaucoma, macular degeneration, diabetic retinopathy, and retinitis pigmentosa. Furthermore, our results also suggested that the changes in nestin composition seems to improve the overall retinal neurons environment towards protecting the structural integrity of the retina, and retinal ganglion cells through the process of transdifferentiation. Therefore, Müller cell-induced activation combined with subretinal injection of RPCs might be helpful in the repair and treatment of various forms of retinopathy.
Acknowledgments
Foundation: Supported by Special Scientific Research Program of Shaanxi Provincial Education Department (No.16JK1665).
Conflicts of Interest: Wang L, None; Li P, None.
REFERENCES
- 1.Yin DP, Liu L. Recent advances on the empirical study of the optic nerves' reparation and regeneration following injury. Guoji Yanke Zazhi (Int Eye Sci) 2013;13(6):1152–1156. [Google Scholar]
- 2.Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299(1):283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331(1):179–191. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- 4.Monnin J, Morand-Villeneuve N, Michel G, Hicks D, Versaux-Botteri C. Production of neurospheres from mammalian Muller cells in culture. Neurosci Lett. 2007;421(1):22–26. doi: 10.1016/j.neulet.2007.04.073. [DOI] [PubMed] [Google Scholar]
- 5.Chohan A, Singh U, Kumar A, Kaur J. Müller stem cell dependent retinal regeneration. Clinica Chimica Acta. 2017;464:160–164. doi: 10.1016/j.cca.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 6.Lendahl U, Zimmerman LB, Mckay RDG. CNS system cells express a new class of intermedite filament protein. Cell. 1990;60(4):585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 7.Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8(4):1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma TP, McDowell CM, Liu Y, Wagner AH, Thole D, Faga BP, Wordinger RJ, Braun TA, Clark AF. Optic nerve crush induces spatial and temporal gene expression patterns in retina and optic nerve of BALB/cJmice. Mol Neurodegener. 2014;9(1):14. doi: 10.1186/1750-1326-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wohl SG, Schmeer CW, Kretz A, Witte OW, Isenmann S. Optic nerve lesion increases cell proliferation and Nestin expression in the adult mouse eye in vivo. Exp Neurol. 2009;219(1):175–186. doi: 10.1016/j.expneurol.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1–40. doi: 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Dyer MA, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3(9):873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- 12.Xue LP, Lu J, Cao Q, Hu S, Ding P, Ling EA. Müller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience. 2006;139(2):723–732. doi: 10.1016/j.neuroscience.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 13.Walcott JC, Provis JM. Müller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin Exp Ophthalmol. 2003;31(3):246–249. doi: 10.1046/j.1442-9071.2003.00638.x. [DOI] [PubMed] [Google Scholar]
- 14.Wilding C, Bell K, Funke S, Beck S, Pfeiffer N, Grus Fh. GFAP antibodies show protective effect on oxidatively stressed neuroretinal cells via interaction with ERP57. Pharmacol Sci. 2015;127(3):298–304. doi: 10.1016/j.jphs.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Akagi T, Haruta M, Akita J, Nishida A, Honda Y, Takahashi M. Different characteristics of rat retinal progenitor cells from different culture periods. Neurosci Lett. 2003;341(2):213–216. doi: 10.1016/s0304-3940(03)00177-0. [DOI] [PubMed] [Google Scholar]
- 16.Kohno H, Sakai T, Kitahara K. Induction of nestin, Ki-67, and cyclin D1 expression in Müller cells after laser injury in adult rat retina. Graefes Arch Clin Exp Ophthalmol. 2006;244(1):90–95. doi: 10.1007/s00417-005-0030-7. [DOI] [PubMed] [Google Scholar]
- 17.Cuenca N, Fernández-Sánchez L, Campello L, Maneu V, De la Villa P, Lax P, Pinilla I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Valamanesh F, Monnin J, Morand-Villeneuve N, Michel G, Zaher M, Miloudi S, Chemouni D, Jeanny JC, Versaux-Botteri C. Nestin expression in the retina of rats with inherited retinal degeneration. Exp Eye Res. 2013;110(5):26–34. doi: 10.1016/j.exer.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Nawashiro H, Messing A, Azzam N, Brenner M. Mice lacking GFAP are hypersensitive to traumatic cerebrospinal injury. Neuroreport. 1998;9(8):1691–1696. doi: 10.1097/00001756-199806010-00004. [DOI] [PubMed] [Google Scholar]
- 20.Pekny M, Lane EB. Intermediate filaments and stress. Exp Cell Res. 2007;313(10):2244–2254. doi: 10.1016/j.yexcr.2007.04.023. [DOI] [PubMed] [Google Scholar]