Abstract
AIM
To evaluate and compare the clinical outcomes with a diffractive bifocal and trifocal intraocular lens (IOL) during a 12-month follow-up.
METHODS
Prospective comparative study including 75 eyes of 38 patients (44-70y) undergoing uneventful cataract surgery. Each patient was randomly assigned to one type of IOL, bifocal (35 eyes) or trifocal (40 eyes). Visual, refractive, and contrast sensitivity changes were evaluated in a 12-month follow-up. The binocular defocus curve was also measured at 12mo postoperatively.
RESULTS
No statistically significant differences between groups were found in postoperative uncorrected and corrected distance visual acuities (P≥0.276). Postoperative corrected near visual acuity (33 cm) was significantly better in the trifocal group during all follow-up (P≤0.004) as well as 6-month uncorrected near (P=0.008) and distance-corrected near visual acuities (P=0.016) (33/40 cm). Significantly better uncorrected intermediate and distance corrected-intermediate visual acuity were found during all follow-up in the trifocal group (P<0.001), which was consistent with differences among groups in binocular defocus curve. Differences among groups in contrast sensitivity were minimal, being only significant at 6 months for some low to medium spatial frequencies (P≤0.006).
CONCLUSION
Bifocal and trifocal diffractive IOLs are able to provide an effective visual restoration which is maintained during a 12-month follow-up, with a clear benefit of the trifocal IOL for the intermediate vision.
Keywords: trifocal intraocular lens, bifocal intraocular lens, defocus curve, contrast sensitivity, cataract surgery
INTRODUCTION
Some previous research has demonstrated that trifocal diffractive intraocular lenses (IOLs) are an effective option to restore the distance, intermediate and near visual function after cataract surgery[1]–[11]. This type of IOLs has been shown to be superior to previous bifocal diffractive IOLs in terms of intermediate visual outcome in some clinical comparative studies[12]–[14] and optical bench experiences[15]–[16]. Similarly, trifocal IOLs have shown their benefit in intermediate vision compared to apodized refractive-diffractive IOLs[17]. Our research group evaluated and compared the clinical outcomes with a diffractive bifocal and trifocal IOL of the same material and haptic design during a 3-month follow-up[14]. We concluded that diffractive trifocality provided an improved intermediate vision compared to diffractive bifocality, but maintaining similar levels of visual and ocular optical quality postoperatively. However, to this date, there is no comparative study of the visual performance achieved with a bifocal and trifocal diffractive IOL in a medium-long term in order to confirm if the benefits of trifocality are maintained over time. The current study was aimed at evaluating and comparing the visual, refractive, and contrast sensitivity outcomes with a diffractive bifocal and trifocal IOL of the same material and with the same haptic design during a 12-month follow-up.
SUBJECTS AND METHODS
Patients
This prospective comparative study included 75 eyes of 38 patients with ages from 44 to 70y and undergoing uneventful cataract surgery with implantation of a bifocal or trifocal diffractive IOL. Each patient was randomly assigned to one type of implant, bifocal or trifocal. Accordingly, two groups were differentiated: bifocal group including 35 eyes of 18 patients implanted with the IOL AT LISA 801 (Carl Zeiss Meditec, Jena, Germany), with a bifocal diffractive optics, and trifocal group including 40 eyes of 20 patients implanted with the IOL AT LISA tri 839MP, with a trifocal diffractive optics. Inclusion criteria were visually significant cataract, presbyopic/pre-presbyopic patients demanding refractive, and corneal astigmatism below 1.25 D. Exclusion criteria were previous ocular surgery, antecedents of glaucoma, ocular inflammation or retinal detachment, active ocular disease, irregular corneal astigmatism, abnormal iris, macular degeneration or retinopathy, and neurophthalmic disease. All patients were properly informed about their inclusion and signed an informed consent form. The study complied with the principles of the Declaration of Helsinki, and was approved by the Clinic Ethics Committee.
Examination Protocol
A complete opthalmologic examination was performed preoperatively including refraction, keratometry, monocular uncorrected distance visual acuity (UDVA) and corrected visual acuity (CDVA), monocular uncorrected imtermediate visual acuity (UIVA) and corrected intermediate visual acuity (CIVA) measured at 66 cm and 80 cm, monocular uncorrected visual acuity (UNVA) and corrected near visual acuity (CNVA) measured at 33 cm and 40 cm, monocular distance-corrected near visual acuity (DCNVA) (33 and 40 cm) and intermediate visual acuity (DCIVA) (66 and 80 cm), Goldmann applanation tonometry, slit lamp examination, ocular aberrometry (OPD scan III, Nidek), corneal topography (OPD scan III, Nidek), biometry (IOL Master v.4.3, Carl Zeiss Meditec), and funduscopy. Visual acuities were measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts.
The following postoperative visits were scheduled: 1d, 1, 3, 6 and 12mo after surgery. The same tests as preoperatively were done at 6 and 12mo after surgery, with the additional inclusion of the evaluation of photopic contrast sensitivity measurements (CSV-1000). Likewise, defocus curves were also obtained binocularly at 12mo postoperatively to evaluate the range of functional function using a methodology previously described[3],[14], and the level of posterior capsular opacification (PCO) was evaluated in the central 4.3-mm zone using the EPCO 2000 software[18].
Intraocular Lenses
The AT LISA 801 (former Acri.LISA 376 D) is a diffractive IOL made of a foldable hydrophilic acrylate (water content 25%) with the following main characteristics: single-piece with C-loop shape, diameter of the biconvex optical zone of 6.0 mm, total diameter of 12.5 mm, company labelled A-constant of 118.0, posterior lens surface asphericity of -0.18, haptic angulation of 0°, hydrophobic lens surface properties, and refractive index of 1.46. The theoretical addition provided by this IOL at the IOL plane is +3.75 D[14].
The AT LISA tri 839MP is a diffractive trifocal preloaded IOL made of the same material and with the same main characteristics. The main differences with the bifocal design are the following: total diameter of 11.0 mm, four-haptic design, 360° square edge to prevent PCO, trifocal diffractive pattern within an IOL diameter of 4.3 mm and bifocal pattern between 4.3 and 6 mm of diameter, and company labelled A-constant of 118.6. A near addition of +3.33 D is theoretically provided by the IOL as well as an intermediate addition of +1.66 D, both calculated at the IOL plane[14].
Surgery
The same experienced surgeon (Mojzis P) performed all surgeries using sutureless micro-coaxial 2.2-mm phacoemulsification when the bifocal IOL was implanted and a technique of microincision (1.8-mm) when the implanted IOL was the trifocal design. Before surgery, topical anaesthesia and mydriatic drops were instilled in all cases. Once performed the incision and paracentesis, the capsulorhexis was created and the phacoemulsification procedure was performed. After this, IOLs were inserted through the main incisions into the capsular bag using the AT.Shooter A1-2000 injector (Carl Zeiss Meditec) and Viscoject 2.2 cartridge when the bifocal IOL was implanted, and the BLUEMIXS 180 injector (Carl Zeiss Meditec) for the trifocal IOL. A combination of topical antibiotic and steroid was prescribed to be applied four times daily during one week as postoperative topical treatment.
Statistical Analysis
The statistical analysis was performed using the SPSS statistics software package 15.0.1 for Windows (IBM, Armonk, NY, USA). First, the normality of all data distributions was examined with the Kolmogorov-Smirnov test. The Student's t-test for paired data was used to analyse all parameter comparisons between visits, and the Student's t-test for unpaired data for the comparison between groups when data were normally distributed. In contrast, when data were not normally distributed, the Wilcoxon Rank Sum test was used to assess the significance of differences between examinations, and the Mann-Whitney test for comparing groups. The same level of significance (P<0.05) was considered in all cases.
RESULTS
Table 1 displays a comparison between bifocal and trifocal groups of preoperative data. As shown, no significant differences between groups were found in manifest cylinder, corneal astigmatism, keratometry, and UDVA, UIVA and UNVA (P≥0.076). However, IOL power was found to be significantly higher in trifocal group compared to bifocal group (P=0.017).
Table 1. Comparative table showing the preoperative clinical data of eyes included in the two groups.
| Parameters | Bifocal group |
Trifocal group |
P | ||
| Mean (SD) | Median (Range) | Mean (SD) | Median (Range) | ||
| UDVA (logMAR) | 0.57 (0.36) | 0.50 (0.10 to 1.50) | 0.54 (0.36) | 0.40 (0.00 to 1.30) | 0.665 |
| CDVA (logMAR) | 0.20 (0.31) | 0.10 (-0.20 to 1.00) | -0.03 (0.10) | 0.00 (-0.20 to 0.20) | <0.001 |
| UNVA (logMAR, 33 cm) | 0.73 (0.32) | 0.70 (0.30 to 1.40) | 0.81 (0.22) | 0.90 (0.20 to 1.20) | 0.205 |
| CNVA (logMAR, 33 cm) | 0.24 (0.27) | 0.20 (-0.20 to 1.10) | 0.05 (0.10) | 0.00 (-0.20 to 0.20) | <0.001 |
| DCNVA (logMAR, 33 cm) | 0.63 (0.24) | 0.60 (0.10 to 1.40) | 0.55 (0.19) | 0.55 (0.20 to 0.90) | 0.065 |
| UNVA (logMAR, 40 cm) | 0.76 (0.34) | 0.70 (0.30 to 1.50) | 0.81 (0.24) | 0.85 (0.30 to 1.20) | 0.319 |
| CNVA (logMAR, 40 cm) | 0.27 (0.30) | 0.20 (0.00 to 1.50) | 0.07 (0.12) | 0.10 (-0.10 to 0.40) | <0.001 |
| DCNVA (logMAR, 40 cm) | 0.62 (0.28) | 0.60 (0.10 to 1.50) | 0.56 (0.18) | 0.60 (0.10 to 0.90) | 0.357 |
| UIVA (logMAR, 66 cm) | 0.61 (0.36) | 0.60 (0.00 to 1.50) | 0.70 (0.30) | 0.65 (0.20 to 1.30) | 0.318 |
| CIVA (logMAR, 66 cm) | 0.17 (0.28) | 0.10 (-0.20 to 1.10) | -0.02 (0.10) | 0.00 (-0.20 to 0.10) | 0.001 |
| DCIVA (logMAR, 66 cm) | 0.41 (0.27) | 0.30 (0.00 to 1.40) | 0.26 (0.20) | 0.30 (-0.10 to 0.80) | 0.007 |
| UIVA (logMAR, 80 cm) | 0.60 (0.33) | 0.60 (0.10 to 1.20) | 0.63 (0.27) | 0.60 (0.10 to 1.30) | 0.728 |
| CIVA (logMAR, 80 cm) | 0.18 (0.27) | 0.10 (-0.10 to 1.10) | 0.01 (0.09) | 0.00 (-0.20 to 0.20) | 0.001 |
| DCIVA (logMAR, 80 cm) | 0.39 (0.24) | 0.40 (0.00 to 1.10) | 0.20 (0.20) | 0.20 (-0.10 to 1.00) | <0.001 |
| Sphere (D) | 0.43 (3.15) | 0.75 (-7.25 to 9.00) | 0.91 (2.96) | 1.50 (-8.75 to 5.00) | 0.030 |
| Cylinder (D) | -0.54 (0.56) | -0.50 (-2.25 to 0.00) | -0.34 (0.35) | -0.25 (-1.25 to 0.00) | 0.076 |
| Spherical equivalent (D) | 0.20 (3.12) | 0.75 (-7.25 to 8.12) | 0.75 (3.01) | 1.38 (-8.75 to 4.88) | 0.038 |
| KM (D) | 43.63 (1.86) | 43.89 (40.59 to 47.64) | 43.12 (1.71) | 42.89 (39.71 to 46.88) | 0.174 |
| Corneal astigmatism (D) | 0.56 (0.28) | 0.53 (0.18 to 1.17) | 0.58 (0.22) | 0.58 (0.09 to 1.01) | 0.413 |
| AL (mm) | 23.35 (1.26) | 23.38 (19.23 to 25.31) | 23.32 (1.57) | 23.10 (21.05 to 28.09) | 0.181 |
| ACD (mm) | 3.32 (0.36) | 3.38 (2.48 to 3.85) | 3.18 (0.33) | 3.17 (2.55 to 4.05) | 0.023 |
| IOL power (D) | 20.79 (4.02) | 20.50 (12.50 to 33.00) | 22.18 (4.79) | 22.25 (11.50 to 29.50) | 0.017 |
SD: Standard deviation; D: Diopters; IOL: Intraocular lens; UDVA: Uncorrected distance visual acuity; CDVA: Corrected distance visual acuity; UNVA: Uncorrected near visual acuity; DCNVA: Distance-corrected near visual acuity; CNVA: Corrected near visual acuity; UIVA: Uncorrected intermediate visual acuity; CIVA: Corrected intermediate visual acuity; DCIVA: Distance-corrected intermediate visual acuity; KM: Mean keratometry; AL: Axial length; ACD: Anterior chamber depth.
Visual Acuity and Refractive Outcomes
Table 2 summarizes and compares the 6 and 12mo postoperative visual and refractive data in bifocal and trifocal groups. As shown, differences between groups in postoperative UDVA (6mo P=0.276; 12mo P=0.822) and CDVA (6mo P=0.334; 12mo P=0.487) were not statistically significant during the whole follow-up. In contrast, significant differences among groups were found in CNVA measured at 33 and 40 cm, with a significantly better visual outcome in the trifocal group (6mo P<0.001; 12mo P=0.004). Significant differences in UNVA (P=0.008) and DCNVA (P=0.016) measured at 33 cm among groups were found at 6mo after surgery but not at 12mo postoperatively (UNVA P=0.529, DCNVA P=0.627). Concerning near visual outcomes at 40 cm, significant differences were found among groups in CNVA during all follow-up, with the best outcome in the trifocal group (6mo P<0.001; 12mo P=0.004). Differences between groups in UNVA measured at 40 cm did not reach statistical significance (6mo P=0.053; 12mo P=0.857), whereas differences in DCNVA were only statistically significant at 6mo postoperatively (P=0.023). Concerning intermediate vision, significant differences were found in UIVA and DCIVA at 6 and 12mo after surgery (P<0.001), with the best visual outcome observed in the trifocal group. No significant differences between groups in CIVA were detected during the postoperative follow-up (P≥0.241). A trend to a more significant myopic residual sphere (6mo P=0.056; 12mo P=0.007) and spherical equivalent (6mo P=0.004; 12mo P=0.005) was found in the bifocal group compared to the trifocal. Cylinder was significantly higher in the bifocal group compared to the trifocal group at 6mo (P<0.001), but not at 12mo after surgery (P=0.538).
Table 2. Comparative table showing the 3-month postoperative clinical data of eyes included in the two groups.
| Parameters | Postoperative 6mo |
Postoperative 12mo |
||||
| Bifocal group | Trifocal group | P | Bifocal group | Trifocal group | P | |
| UDVA | 0.00 (0.10)/0.00 (-0.10 to 0.40) | -0.03 (0.10)/0.00 (-0.20 to 0.20) | 0.276 | 0.01 (0.13)/0.00 (-0.10 to 0.40) | 0.02 (0.14)/0.00 (-0.20 to 0.40) | 0.822 |
| CDVA | -0.02 (0.10)/0.00 (-0.20 to 0.40) | -0.05 (0.08)/-0.05 (-0.20 to 0.10) | 0.334 | -0.01 (0.11)/0.00 (-0.20 to 0.40) | 0.01 (0.12)/0.00 (-0.20 to 0.40) | 0.487 |
| UNVA (33 cm) | 0.22 (0.10)/0.20 (0.10 to 0.50) | 0.15 (0.13)/0.10 (-0.10 to 0.40) | 0.008 | 0.19 (0.10)/0.20 (0.00 to 0.40) | 0.21 (0.15)/0.20 (0.00 to 0.60) | 0.529 |
| CNVA ( 33 cm) | 0.18 (0.08)/0.20 (0.10 to 0.40) | 0.07 (0.07)/0.10 (-0.10 to 0.20) | <0.001 | 0.19 (0.10)/0.20 (0.00 to 0.40) | 0.12 (0.08)/0.10 (0.00 to 0.40) | 0.004 |
| DCNVA (33 cm) | 0.21 (0.11)/0.20 (0.10 to 0.50) | 0.15 (0.13)/0.10 (-0.10 to 0.40) | 0.016 | 0.19 (0.11)/0.20 (0.00 to 0.40) | 0.21 (0.15)/0.20 (0.00 to 0.60) | 0.627 |
| UNVA (40 cm) | 0.27 (0.18)/0.30 (0.00 to 0.70) | 0.20 (0.10)/0.20 (0.00 to 0.40) | 0.053 | 0.25 (0.13)/0.30 (0.00 to 0.60) | 0.25 (0.14)/0.20 (0.00 to 0.60) | 0.857 |
| CNVA (40 cm) | 0.25 (0.16)/0.30 (0.00 to 0.60) | 0.14 (0.08)/0.10 (0.00 to 0.30) | <0.001 | 0.23 (0.12)/0.20 (0.00 to 0.50) | 0.16 (0.08)/0.20 (0.00 to 0.40) | 0.004 |
| DCNVA (40 cm) | 0.29 (0.18)/0.30 (0.00 to 0.70) | 0.20 (0.10)/0.20 (0.00 to 0.40) | 0.023 | 0.25 (0.13)/0.30 (0.00 to 0.60) | 0.24 (0.14)/0.20 (0.00 to 0.50) | 0.811 |
| UIVA (66 cm) | 0.27 (0.18)/0.30 (-0.10 to 0.60) | 0.08 (0.09)/0.10 (-0.10 to 0.30) | <0.001 | 0.26 (0.17)/0.30 (-0.10 to 0.70) | 0.09 (0.11)/0.10 (-0.10 to 0.30) | <0.001 |
| CIVA (66 cm) | 0.06 (0.10)/0.00 (-0.10 to 0.40) | 0.06 (0.07)/0.10 (-0.10 to 0.20) | 0.751 | 0.10 (0.12)/0.10 (-0.10 to 0.40) | 0.07 (0.09)/0.10 (-0.10 to 0.30) | 0.241 |
| DCIVA (66 cm) | 0.28 (0.16)/0.30 (-0.10 to 0.60) | 0.08 (0.09)/0.10 (-0.10 to 0.30) | <0.001 | 0.27 (0.17)/0.30 (-0.10 to 0.70) | 0.08 (0.10)/0.10 (-0.10 to 0.30) | <0.001 |
| UIVA (80 cm) | 0.23 (0.17)/0.20 (0.00 to 0.60) | 0.07 (0.08)/0.10 (-0.20 to 0.20) | <0.001 | 0.25 (0.18)/0.30 (-0.10 to 0.60) | 0.08 (0.13)/0.05 (-0.10 to 0.40) | <0.001 |
| CIVA (80 cm) | 0.06 (0.11)/0.00 (-0.10 to 0.40) | 0.05 (0.08)/0.10 (-0.20 to 0.20) | 0.749 | 0.05 (0.11)/0.00 (-0.10 to 0.40) | 0.05 (0.10)/0.00 (-0.10 to 0.40) | 0.906 |
| DCIVA (80 cm) | 0.24 (0.17)/0.30 (-0.10 to 0.60) | 0.07 (0.08)/0.10 (-0.20 to 0.20) | <0.001 | 0.26 (0.18)/0.30 (-0.10 to 0.60) | 0.08 (0.13)/0.05 (-0.10 to 0.40) | <0.001 |
| Sphere (D) | -0.16 (0.43)/0.00 (-1.00 to 1.00) | 0.04 (0.36)/0.00 (-0.50 to 0.75) | 0.056 | -0.18 (0.47)/-0.25 (-1.25 to 1.25) | 0.09 (0.39)/0.25 (-0.50 to 0.75) | 0.007 |
| Cylinder (D) | -0.41 (0.26)/-0.50 (-1.00 to 0.00) | -0.20 (0.19)/-0.25 (-0.50 to 0.00) | <0.001 | -0.37 (0.34)/-0.25 (-1.25 to 0.00) | -0.31 (0.29)/-0.25 (-1.00 to 0.00) | 0.538 |
| Spherical equivalent (D) | -0.36 (0.42)/-0.25 (-1.25 to 0.50) | -0.06 (0.34)/-0.13 (-0.62 to 0.62) | 0.004 | -0.36 (0.46)/-0.38 (-1.38 to 0.75) | -0.07 (0.35)/0.00 (-0.62 to 0.62) | 0.005 |
| Corneal astigmatism (D) | 0.63 (0.38)/0.59 (0.05 to 1.42) | 0.55 (0.22)/0.55 (0.11 to 1.08) | 0.610 | 0.59 (0.35)/0.53 (0.10 to 1.37) | 0.59 (0.23)/0.59 (0.18 to 1.11) | 0.599 |
SD: Standard deviation; D: Diopters; IOL: Intraocular lens; UDVA: Uncorrected distance visual acuity (logMAR); CDVA: Corrected distance visual acuity (logMAR); UNVA: Uncorrected near visual acuity (logMAR); DCNVA: Distance-corrected near visual acuity (logMAR); CNVA: Corrected near visual acuity (logMAR); UIVA: Uncorrected intermediate visual acuity (logMAR); CIVA: Corrected intermediate visual acuity (logMAR); DCIVA: Distance-corrected intermediate visual acuity (logMAR).
Mean (SD)/median (range)
The improvement at 6mo in UDVA, CDVA, UNVA (33 and 40 cm), DCNVA (33 and 40 cm), UIVA (66 and 80 cm), DCIVA (66 and 80 cm), and CIVA (80 cm) was statistically significant in the bifocal group (P≤0.046). In this same group, significant changes between 6 and 12mo after surgery were only detected in CIVA (66 cm) (P=0.010). In the trifocal group, a significant improvement at 6mo was detected in UNVA, UNVA (33 and 40 cm), DCNVA (33 and 40 cm), CNVA (40 cm), and UIVA, CIVA and DCIVA measured at 66 and 80 cm (P≤0.007). Between 6 and 12mo after surgery, significant changes in CDVA, and in UNVA, CNVA and DCNVA measured at 33 cm were only detected in eyes implanted with the trifocal IOL.
Defocus Curve
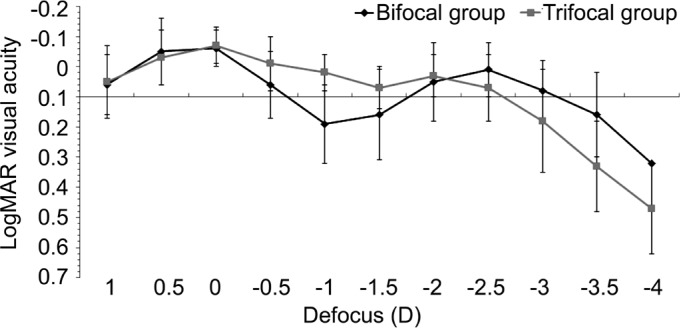
Mean defocus curves obtained in bifocal and trifocal groups at 12mo after surgery are displayed in Figure 1. Significantly better visual acuity was found in the trifocal group compared to the bifocal group for the defocus levels of -1.00 (P<0.001) and -1.50 D (P=0.030). In contrast, the visual acuity for the defocus of -3.00 (P=0.045), -3.50 (P=0.002) and -4.00 D (P=0.005) was significantly better in the bifocal group (Figure 1).
Figure 1. Mean defocus curve in bifocal and trifocal groups.
Contrast Sensitivity Outcomes
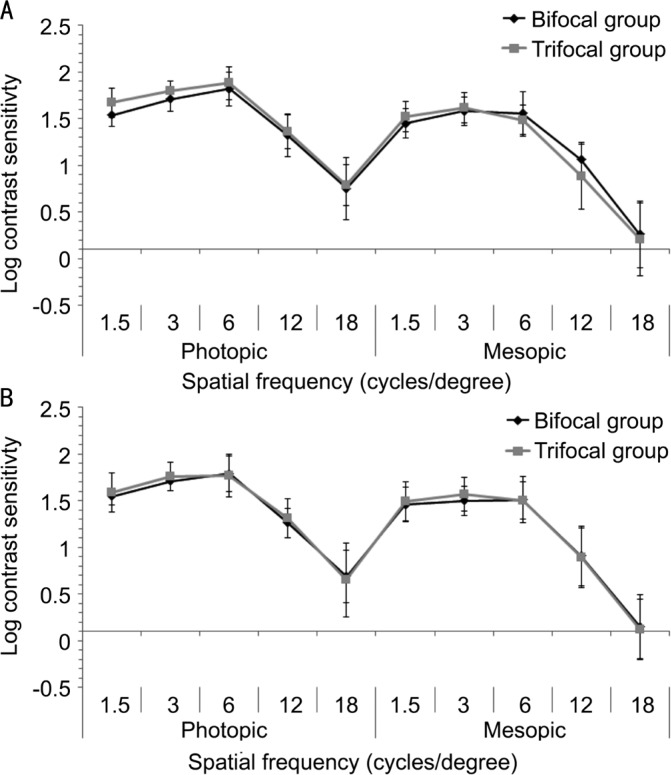
Mean contrast sensitivity function in bifocal and trifocal groups of eyes at 6 and 12mo postoperatively are displayed in Figure 2. Only statistically significant differences among groups were found in 6-month photopic contrast sensitivity values for the spatial frequency of 3 cycles/degree (P=0.001) and 6-month mesopic contrast sensitivity values for the spatial frequencies of 6 (P=0.006) and 12 cycles/degree (P=0.014). In the bifocal group, only significant changes in contrast sensitivity were found for the values measured under mesopic conditions for the spatial frequencies of 3 (P=0.015) and 12 cycles/degree (P=0.006). In the trifocal group, the significant changes in contrast sensitivity occurred among 6 and 12mo postoperatively for the photopic values corresponding to the spatial frequencies of 1.5 (P=0.018), 3 (P=0.041), 6 (P=0.011) and 18 cycles/degree (P=0.022) as well as for the mesopic values corresponding to the spatial frequency of 3 cycles/degree (P=0.044).
Figure 2. Mean contrast sensitivity function in bifocal and trifocal groups at 6 (A) and 12mo (B) after surgery.
Complications
Mean EPCO scores at 12mo postoperatively were 0.49 (SD 0.67, median 0.21, range 0.00 to 3.00) and 0.33 (SD 0.47, median 0.15, range: 0.00 to 2.02) in the bifocal and trifocal groups, respectively (P=0.506). A total of 4 (11.4%) and 3 eyes (7.5%) developed significant PCO in the bifocal and trifocal groups, respectively, and a YAG capsulotomy was required (P=0.560).
DISCUSSION
Our research group reported in a previous study a comparative analysis of the outcomes obtained with the AT LISA bifocal and trifocal IOLs at 3mo after surgery[14]. As in the current study, we obtained no significant differences with the two types of diffractive IOLs in UDVA and CDVA[14]. Therefore, the two IOLs evaluated provide a good and comparable distance visual outcome. These results are also consistent with those obtained in previous studies evaluating each type of IOL[1]–[4],[6],[8],[12]–[14]. Concerning near vision, we have found significant differences among the bifocal and trifocal implant, as in our previous comparative study[14]. Specifically, significantly better CNVA was obtained with the trifocal IOL compared to the bifocal IOL at 6 and 12mo after surgery. Likewise, UNVA measured at 33 cm and DCNVA measured at 33 and 40 cm were significantly better in the trifocal group at 6mo, but not at 12mo. In our previous comparative study at 3mo postoperatively[14], better UNVA, CNVA and DCNVA were obtained with the trifocal IOL compared to the bifocal IOL. Therefore, it seems that the initial UNVA and DCNVA outcomes are better with the trifocal IOL, but with time become comparable to those obtained with the bifocal IOL. The residual refraction seems to play a major role on this finding. As in our previous study[14], the residual cylinder was significantly higher in the group of eyes implanted with the bifocal IOL and it has been shown that small amounts of residual astigmatism in eyes implanted with diffractive IOLs can induce significant levels of degradation of the visual quality[19]. The use of a larger incision (2.2 mm) for the insertion of the bifocal IOL may be the reason for the larger manifest cylinder found in the bifocal group. At 6mo postoperatively, postoperative corneal astigmatism was slightly higher in the bifocal group, but the difference with the trifocal group did not reach statistical significance. Previous studies have a shown a trend to higher corneal cylinder in the initial postoperative period when 2.2 mm corneal incision is used in cataract surgery compared to the use of 1.8-mm microincision[20]–[21]. Regarding the visual outcomes obtained in our sample, better distance-corrected visual acuities were obtained under monocular conditions (shown in Tables) compared to those obtained binocularly (shown in the defocus curve), which is consistent with previous studies reporting this beneficial contribution of both eyes to the binocular visual performance when implanted with diffractive multifocal IOLs[22].
In agreement with the outcomes obtained in optical bench simulation studies[15]–[16], the trifocal IOL provided significantly better intermediate visual outcomes which confirms that a third effective focus is provided by this optical design. Specifically, UIVA and DCIVA were significantly better at 6 and 12mo after surgery with the trifocal IOL compared to the bifocal. In previous comparative studies[13]–[14], significantly better UIVA and DCIVA values have been reported in the initial postoperative period after the implantation of the same trifocal IOL compared to the bifocal. The superiority of the trifocal over the bifocal IOL in terms of visual outcome was also found in our series in the analysis of the 12-month postoperative binocular defocus curve. Specifically, significantly better visual acuity in the trifocal group compared to the bifocal group was found for the defocus levels of -1.00 and -1.50 D that correspond to the vergence demands of intermediate vision. Similarly, other authors have found in the initial postoperative period better visual outcome in the intermediate range of defocus for the trifocal IOL compared to the bifocal[12]–[14]. In our series, the 12-month distance-corrected visual acuity for the defocus of -3.00, -3.50 and -4.00 D was significantly better in the bifocal group, suggesting that the bifocal IOL provides a near focus closer to the eye than the trifocal IOL. It should be considered that although the two IOLs evaluated were diffractive, the light distribution provided by the diffractive platform is different.
To evaluate the potential impact of trifocality on visual and ocular optical quality, contrast sensitivity and ocular higher order aberrations were also measured in the current series. Some differences of small magnitude but statistically significant were found for 6-month postoperative photopic contrast sensitivity values for the spatial frequency of 3 cycles/degree and mesopic contrast sensitivity values for the spatial frequencies of 6 and 12 cycles/degree. However, no significant differences in contrast sensitivity among groups were detected at the end of the follow-up, as in other studies comparing both IOLs but in a shorter term[12],[14]. This is consistent with the absence of significant differences in CDVA during the follow-up. The presence of some differences between IOLs for low and medium spatial frequencies at 6mo may be attributable to several factors such as inherent intra-individual variability or the potential impact of the development of some level of PCO during the follow-up in some cases. In our series, mean EPCO scores at 12mo postoperatively were 0.49±0.67 and 0.33±0.47 in the bifocal and trifocal groups, respectively. These values are similar to those reported in previous series evaluating the same type of IOL[3]. A total of 11.4% and 7.5% of eyes developed significant PCO in the bifocal and trifocal IOL groups, respectively, being necessary a YAG capsulotomy. When our contrast sensitivity outcomes are compared to those reported in other studies using the same method of measurement, they are better than those obtained with refractive multifocal IOLs and similar to those obtained with other diffractive bifocal IOLs[23]–[24]. However, as expected, our contrast sensitivity outcomes are somewhat more limited than those obtained with monofocal IOLs[23]–[24]. This is coherent as diffractive IOLs distribute light to different foci, reducing the level of contrast that is present in each of them[25].
This paper has some limitations that should be acknowledged. First, an intra-individual design may have been a better option for our study, but unfortunately this design was not possible in our center when the study was conducted. Likewise, the relevance of the comparison may have been increased if the bifocal plate haptic IOL AT.LISA with the same IOL body design and the same injection protocol would have been used. Third, pupil size was not measured in this study and therefore we cannot characterize accurately the real impact of this factor on the visual outcomes obtained. These limitations must be considered for improving and optimizing future comparative studies of bifocal and trifocal IOLs.
In conclusion, an effective distance, intermediate and near visual rehabilitation can be achieved after cataract surgery with implantation of a trifocal diffractive IOL, with the advantage of maintaining levels of visual quality comparable to those obtained with diffractive bifocality. An improved intermediate visual outcome is obtained with the trifocal IOL compared to the bifocal IOL during the first 12mo after its implantation.
Acknowledgments
Conflicts of Interest: Mojzis P, None; Kukuckova L, None; Majerova K, None; Ziak P, None; Piñero DP, None.
REFERENCES
- 1.Kohnen T, Titke C, Böhm M. Trifocal intraocular lens implantation to treat visual demands in various distances following lens removal. Am J Ophthalmol. 2016;161:71–77.e1. doi: 10.1016/j.ajo.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 2.Kretz FT, Breyer D, Diakonis VF, Klabe K, Henke F, Auffarth GU, Kaymak H. Clinical outcomes after binocular implantation of a new trifocal diffractive intraocular lens. J Ophthalmol. 2015;2015:962891. doi: 10.1155/2015/962891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mojzis P, Majerova K, Hrckova L, Piñero DP. Implantation of a diffractive trifocal intraocular lens: one-year follow-up. J Cataract Refract Surg. 2015;41(8):1623–1630. doi: 10.1016/j.jcrs.2014.11.050. [DOI] [PubMed] [Google Scholar]
- 4.Carballo-Alvarez J, Vazquez-Molini JM, Sanz-Fernandez JC, Garcia-Bella J, Polo V, García-Feijoo J, Martinez-de-la-Casa JM. Visual outcomes after bilateral trifocal diffractive intraocular lens implantation. BMC Ophthalmol. 2015;15:26. doi: 10.1186/s12886-015-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marques JP, Rosa AM, Quendera B, Silva F, Mira J, Lobo C, Castelo-Branco M, Murta JN. Quantitative evaluation of visual function 12 months after bilateral implantation of a diffractive trifocal IOL. Eur J Ophthalmol. 2015;25(6):516–524. doi: 10.5301/ejo.5000638. [DOI] [PubMed] [Google Scholar]
- 6.Law EM, Aggarwal RK, Kasaby H. Clinical outcomes with a new trifocal intraocular lens. Eur J Ophthalmol. 2014;24(4):501–508. doi: 10.5301/ejo.5000407. [DOI] [PubMed] [Google Scholar]
- 7.Cochener B, Vryghem J, Rozot P, Lesieur G, Chevalier JP, Henry JM, David T, Lesueur L, Gatinel D, Ganem C, Blanckaert J, Van Acker E, Heireman S, Ghekiere S. Clinical outcomes with a trifocal intraocular lens: a multicenter study. J Refract Surg. 2014;30(11):762–768. doi: 10.3928/1081597X-20141021-08. [DOI] [PubMed] [Google Scholar]
- 8.Mojzis P, Peña-García P, Liehneova I, Ziak P, Alió JL. Outcomes of a new diffractive trifocal intraocular lens. J Cataract Refract Surg. 2014;40(1):60–69. doi: 10.1016/j.jcrs.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 9.Alió JL, Montalbán R, Peña-García P, Soria FA, Vega-Estrada A. Visual outcomes of a trifocal aspheric diffractive intraocular lens with microincision cataract surgery. J Refract Surg. 2013;29(11):756–761. doi: 10.3928/1081597X-20131021-05. [DOI] [PubMed] [Google Scholar]
- 10.Sheppard AL, Shah S, Bhatt U, Bhogal G, Wolffsohn JS. Visual outcomes and subjective experience after bilateral implantation of a new diffractive trifocal intraocular lens. J Cataract Refract Surg. 2013;39(3):343–349. doi: 10.1016/j.jcrs.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Voskresenskaya A, Pozdeyeva N, Pashtaev N, Batkov Y, Treushnicov V, Cherednik V. Initial results of trifocal diffractive IOL implantation. Graefes Arch Clin Exp Ophthalmol. 2010;248(9):1299–1306. doi: 10.1007/s00417-010-1424-8. [DOI] [PubMed] [Google Scholar]
- 12.Cochener B. Prospective clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses. J Refract Surg. 2016;32(3):146–151. doi: 10.3928/1081597X-20160114-01. [DOI] [PubMed] [Google Scholar]
- 13.Postolache C, Postolache O. Comparation of refractive results with bifocal implants AT LISA 809 and trifocal AT LISA tri839. Rom J Ophthalmol. 2015;59(2):100–102. [PMC free article] [PubMed] [Google Scholar]
- 14.Mojzis P, Kukuckova L, Majerova K, Liehneova K, Piñero DP. Comparative analysis of the visual performance after cataract surgery with implantation of a bifocal or trifocal diffractive IOL. J Refract Surg. 2014;30(10):666–672. doi: 10.3928/1081597X-20140903-06. [DOI] [PubMed] [Google Scholar]
- 15.Madrid-Costa D, Ruiz-Alcocer J, Ferrer-Blasco T, García-Lázaro S, Montés-Micó R. Optical quality differences between three multifocal intraocular lenses: bifocal low add, bifocal moderate add, and trifocal. J Refract Surg. 2013;29(11):749–754. doi: 10.3928/1081597X-20131021-04. [DOI] [PubMed] [Google Scholar]
- 16.Gatinel D, Houbrechts Y. Comparison of bifocal and trifocal diffractive and refractive intraocular lenses using an optical bench. J Cataract Refract Surg. 2013;39(7):1093–1099. doi: 10.1016/j.jcrs.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 17.Gundersen KG, Potvin R. Comparison of visual outcomes after implantation of diffractive trifocal toric intraocular lens and a diffractive apodized bifocal toric intraocular lens. Clin Ophthalmol. 2016;10:455–461. doi: 10.2147/OPTH.S103375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findl O, Buehl W, Menapace R, Georgopoulos M, Rainer G, Siegl H, Kaider A, Pinz A. Comparison of 4 methods for quantifying posterior capsule opacification. J Cataract Refract Surg. 2003;29(1):106–111. doi: 10.1016/s0886-3350(02)01509-2. [DOI] [PubMed] [Google Scholar]
- 19.Zheleznyak L, Kim MJ, MacRae S, Yoon G. Impact of corneal aberrations on through-focus image quality of presbyopia-correcting intraocular lenses using an adaptive optics bench system. J Cataract Refract Surg. 2012;38(10):1724–1733. doi: 10.1016/j.jcrs.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Can I, Takmaz T, Yildiz Y, Bayhan HA, Soyugelen G, Bostanci B. Coaxial, microcoaxial, and biaxial microincision cataract surgery: prospective comparative study. J Cataract Refract Surg. 2010;36(5):740–746. doi: 10.1016/j.jcrs.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Elkady B, Piñero D, Alió JL. Corneal incision quality: microincision cataract surgery versus microcoaxial phacoemulsification. J Cataract Refract Surg. 2009;35(3):466–474. doi: 10.1016/j.jcrs.2008.11.047. [DOI] [PubMed] [Google Scholar]
- 22.Kretz FT, Müller M, Gerl M, Gerl RH, Auffarth GU. Binocular function to increase visual outcome in patients implanted with a diffractive trifocal intraocular lens. BMC Ophthalmol. 2015;15:110. doi: 10.1186/s12886-015-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil MA, Varón C, Cardona G, Vega F, Buil JA. Comparison of far and near contrast sensitivity in patients symmetrically implanted with multifocal and monofocal IOLs. Eur J Ophthalmol. 2014;24(1):44–52. doi: 10.5301/ejo.5000335. [DOI] [PubMed] [Google Scholar]
- 24.Schmitz S, Dick HB, Krummenauer F, Schwenn O, Krist R. Contrast sensitivity and glare disability by halogen light after monofocal and multifocal lens implantation. Br J Ophthalmol. 2000;84(10):1109–1112. doi: 10.1136/bjo.84.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brito P, Salgado-Borges J, Neves H, Gonzalez-Meijome J, Monteiro M. Light-distortion analysis as a possible indicator of visual quality after refractive lens exchange with diffractive multifocal intraocular lenses. J Cataract Refract Surg. 2015;41(3):613–622. doi: 10.1016/j.jcrs.2014.07.033. [DOI] [PubMed] [Google Scholar]