Abstract
AIM
To evaluate and compare structural optical coherence tomography (OCT)-based parameters, such as Bruch's membrane opening-minimum rim width (BMO-MRW), and retinal nerve fiber layer (RNFL) thickness in glaucoma patients with visual field (VF) defects, and to correlate both to mean deviation (MD) values of obtained standard achromatic perimetry (SAP) examinations.
METHODS
Patients with glaucoma and glaucomatous VF defects were enrolled in this prospective study and compared to age-matched healthy individuals. All study participants underwent a full ophthalmic examination and VF testing with SAP. Peripapillary RNFL thickness and BMO-MRW were acquired with SD-OCT. Correlation analyses between obtained global functional and global as well as sectorial structural parameters were calculated.
RESULTS
A consecutive series of 30 glaucomatous right eyes of 30 patients were included and compared to 36 healthy right eyes of 36 individuals in the control group. Global MD of values correlated significantly with global RNFL (Pearson corr. coeff: 0.632, P=0.001) and global BMO-MRW (Pearson corr. coeff: 0.746, P<0.001) values in the glaucoma group. Global MD and sectorial RNFL or BMO-MRW values correlated less significantly. In the control group, MD values did not correlate with RNFL or BMO-MRW measurements. A subgroup analysis of myopic patients (>4 diopters) within the glaucoma group (n=6) revealed a tendency for higher correlations between MD and BMO-MRW than MD and RNFL measurements.
CONCLUSION
In a clinical setting, RNFL thickness and BMO-MRW correlate similarly with global VF sensitivity in glaucoma patients with BMO-MRW showing higher correlations in myopic glaucoma patients.
Keywords: glaucoma, optical coherence tomography, Bruch's membrane opening-minimum rim width, retinal nerve fibre layer, myopia, standard automated perimetry, visual field defects
INTRODUCTION
Glaucoma is a leading cause of irreversible blindness worldwide. Thus, early diagnosis of this chronic disease is of crucial importance[1]. Additionally to visual field (VF) tests obtained with standard achromatic perimetry (SAP) as functional parameters, measurements of structural variables at and around the optic nerve head (ONH) have become more and more popular given the significant advances in technology. Especially non-invasively obtained, optical coherence tomography (OCT)-based structural parameters such as the retinal thickness of the posterior pole or the retinal nerve fiber layer (RNFL) thickness can be measured precisely with an accuracy in the range of a few microns. Another huge advantage is the possibility to compare point to point in follow up examinations[2]–[5].
Recently, the novel OCT-based parameter Bruch's membrane opening-minimum rim width (BMO-MRW) was introduced. This variable measures the shortest distance from Bruch's membrane opening (BMO) to the internal limiting membrane[6]–[8] using radial cross-sectional images through the ONH. The acquisition time for BMO-MRW examinations is longer than for circular RNFL measurements alone and require a better compliance of the examined patients than OCT-based RNFL measurements. BMO-MRW as a structural parameter, together with the established RNFL thickness, has been shown to successfully measure and correlate with the loss of neuro-retinal tissue, which happens in glaucoma patients as well as healthy elderly[9]. Yet, the significance or extent of novelty of this relatively new structural parameter BMO-MRW in daily clinical practice remains unclear.
The goal of this prospective study was to evaluate the structural OCT-based parameter BMO-MRW in glaucoma patients and compare the results with RNFL thickness values by correlating both parameters, BMO-MRW and RNFL, to global mean deviation (MD) values of SAP in a clinical routine setting.
SUBJECTS AND METHODS
Patients
This prospective cross-sectional study was conducted at the Glaucoma Department of a German University Eye Hospital (Department of Ophthalmology, Technical University of Munich). Inclusion criteria were patients with diagnosed primary open angle glaucoma (POAG) and documented glaucomatous VF defects in SAP who were compared to age-matched healthy persons without glaucoma and inconspicuous VF testing (control group). Exclusion criteria for the “glaucoma group” were any non-glaucomatous VF defects, corneal scars, history of any corneal surgery, nystagmus, or any pathology or suspected disease of the ONH or RNFL that may influence VF testing or structural ONH parameters.
All study participants underwent a full ophthalmic examination, including best-corrected visual acuity (BCVA) in decimals obtained with a Snellen projection chart, objective and subjective refraction, slit-lamp biomicroscopy, measurement of intraocular pressure (IOP) with Goldmann applanation tonometry (GAT), gonioscopy, and fundus examination by indirect ophthalmoscopy. The linear vertical cup disk ratio (CDR) was documented. VF testing with SAP was performed prior to dilation of pupils. The study adheres to the tenets of the Declaration of Helsinki (2008) and was approved by the Institutional Review Board.
Standard Achromatic Perimetry
VF testing by SAP was obtained for each patient using Octopus 500 G1 (Haag-Streit Diagnostics, Köniz, Switzerland). A reliable VF test was defined as <25% of fixation losses, and <20% false positive and false negative answers. According to the definition of the European Glaucoma Society and previous works by Katz et al[10], a VF defect suspicious for glaucoma was defined as a cluster of at least three or more non-edge-contiguous points with significantly reduced sensitivity (P<0.05), out of these one with a significance of at least P<0.01 on the same side of the horizontal meridian in the pattern deviation plot. All of the included patients in the glaucoma group and none in the control group fulfilled these criteria.
Optical Coherence Tomography Measurements
All included patients were imaged with conventional circular peripapillary ONH cross sectional scans using the Heidelberg Spectralis OCT with an excitation wavelength of 870 nm and 40 000 A-scans per second. Global and six sectorial (supero-temporal, temporal, infero-temporal, infero-nasal, nasal, and supero-nasal) values for both, RNFL thickness and BMO-MRW (shortest distance from BMO to the internal limiting membrane) were obtained. The device-specific software-based classification scores for both parameters RNFL and BMO-MRW classified each obtained global as well as sectorial value into “within normal limits”, “borderline”, and “outside normal limits”.
Structure-function Correlations
For further analysis, BCVA and global MD values of the right eye of each patient of the “glaucoma group” and the “control group” were correlated with sectorial and global RNFL and BMO-MRW measurements.
Statistical Analysis
Data were collected and analyzed using SPSS Version 22.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov-Smirnov test was used for testing for normal distribution. Correlation analyses between obtained global functional (SAP) and global as well as sectorial structural (SD-OCT) parameters were calculated. Linear regression analyses were performed for comparison between metric and nominal/categorical data.
RESULTS
A consecutive series of 30 glaucomatous right eyes of 30 patients were included in this prospective study and compared to 36 right healthy eyes of 36 individuals in the control group. Patients' characteristics are given in Table 1. The glaucoma and the control group are age-matched and show similar BCVA and IOP values.
Table 1. Characteristics of glaucoma group and control group.
| Characteristics | Glaucoma group | Control group | P |
| n | 30 | 36 | |
| Refraction (D) | -1.51±2.97 | -0.27±3.37 | 0.149 |
| Age (a) | 66.73±10.53 | 65.03±14.88 | 0.589 |
| BCVA (decimal) | 0.615±0.309 | 0.679±0.278 | 0.449 |
| MD (db) | 8.65±7.02 | 2.18±0.56 | <0.001 |
| IOP (mm Hg) | 14.73±4.56 | 15.84±3.14 | 0.357 |
| CDR | 0.826±0.245 | 0.382±0.244 | <0.001 |
| RNFL global (µm) | 66.33±17.16 | 93.98±15.88 | <0.001 |
| RNFL (BMO-MRW) global (µm) | 66.47±19.54 | 95.22±15.67 | <0.001 |
| BMO-MRW global (µm) | 192.84±74.74 | 303.61±48.53 | <0.001 |
BCVA: Best corrected visual acuity; MD: Mean deviation of standard automated perimetry (SAP) testing; IOP: Intraocular pressure obtained with Goldmann applanation tonometry; CDR: Cup-to-disc ratio; RNFL global: Global retinal nerve fiber layer thickness obtained with SD-OCT; RNFL (BMO-MRW) global: Global retinal nerve fiber layer thickness obtained with SD-OCT during BMO-MRW measurements; BMO-MRW global: Shortest distance between bruch's membrane opening and internal limiting membrane.
Correlation Analyses
We did not find any correlations between BCVA or IOP and the structural OCT-based parameters RNFL or BMO-MRW measurements in the glaucoma group except for the temporal superior quadrant (BCVA and RNFL: Pearson corr. coeff.: 0.399, P=0.029; BCVA and BMO-MRW: Pearson corr. coeff.: 0.447, P=0.013).
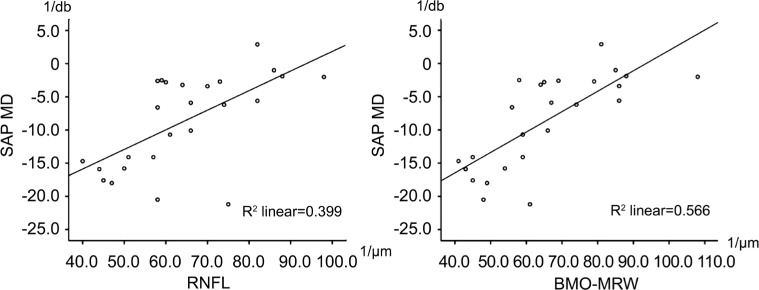
Global MD values correlated significantly with global RNFL and global BMO-MRW values in the glaucoma group (Table 2). Correlation analyses between global MD and sectorial RNFL or BMO-MRW values correlated less significantly (Tables 3, 4). Correlation between MD and BMO-MRW was slightly better than between MD and RNFL, as can be seen in Figure 1. In the control group, global MD values did not correlate with global or sectorial RNFL or BMO-MRW measurements.
Table 2. Correlation (Pearson correlation coefficient) between global MD and global OCT-based RNFL and BMO-MRW measurements.
| Correlations | Correlation coefficient (Pearson) | P |
| Global MD and RNFL | 0.632 | 0.001a |
| Global MD and BMO-MRW | 0.746 | <0.001a |
aP<0.05 are significant correlations.
Table 3. Correlations (Pearson correlation coefficient) between global MD and sectorial OCT-based RNFL measurements.
| Global MD and sectorial RNFL | Correlation coefficient (Pearson) | P |
| Temp | 0.471 | 0.017a |
| Temp sup | 0.733 | <0.001a |
| Temp inf | 0.579 | 0.002a |
| Nas | 0.133 | 0.527 |
| Nas sup | 0.436 | 0.029a |
| Nas inf | 0.394 | 0.051 |
Temp: Temporal sector; Temp sup: Temporal superior sector; Temp inf: Temporal inferior sector; Nas: Nasal sector; Nas sup: Nasal superior sector; Nas inf: Nasal inferior sector. aP<0.05 are significant correlations.
Table 4. Correlations (Pearson correlation coefficient) between global MD and sectorial OCT based BMO-MRW measurements.
| Global MD and sectorial BMO-MRW | Correlation coefficient (Pearson) | P |
| Temp | 0.591 | 0.002a |
| Temp sup | 0.671 | <0.001a |
| Temp inf | 0.629 | 0.001a |
| Nas | 0.413 | 0.040a |
| Nas sup | 0.569 | 0.003a |
| Nas inf | 0.555 | 0.004a |
Temp: Temporal sector; Temp sup: Temporal superior sector; Temp inf: Temporal inferior sector; Nas: Nasal sector; Nas sup: Nasal superior sector; Nas inf: Nasal inferior sector. aP<0.05 are significant correlations.
Figure 1. Scatter plots between MD (y-axis) and RNFL (x-axis) as well as BMO-MRW (x-axis) values revealing a higher R2 for the correlations between MD and BMO-MRW.
Subgroup Analysis for Myopia
We analyzed the myopic patients (>4 diopters) within the glaucoma group (n=6). Here we found a tendency for better correlation between MD and BMO-MRW than between MD and RNFL measurements (Table 5).
Table 5. Correlation (Pearson correlation coefficient) between global MD and global OCT-based RNFL and BMO-MRW measurements of myopic patients (>4 diopters, n=6).
| Correlations | Correlation coefficient (Pearson) | P |
| Global MD and RNFL | 0.623 | 0.186a |
| Global MD and BMO-MRW | 0.874 | 0.023a |
aP<0.05 are significant correlations.
DISCUSSION
A clear understanding of the structure-function relationship in glaucomatous eyes remains a challenge and a subject of current and future research. The vast majority of patients with POAG reveal structural changes before functional impairments occur, while in some patients VF changes can either appear simultaneously with RNFL thinning or even precede the observed structural changes[2],[11]–[12]. Despite the attempt to relate function to structure with various models[13]–[15], it remains of high importance to monitor functional as well as structural parameters in patients with known glaucoma[16].
The recently introduced novel structural parameter BMO-MRW measures the shortest distance from BMO to the internal limiting membrane, and is regarded to be a sensitive and stable structural parameter in diagnosis and follow up of glaucoma patients[6],[17].
When MD values of VF tests from patients with manifest glaucoma were correlated with both structural OCT-based parameters BMO-MRW and RNFL, we observed that sectorial structure-function analyses were inferior to global correlation analyses. Our observation of a moderate to good structure-function relationship between global RNFL as well as BMO-MRW and VF sensitivity in the glaucoma group are in agreement with our understanding of glaucoma being a general neurodegenerative disease[18]–[19].
The only exception were the temporal superior and temporal inferior sectors showing a higher structure-function relationship than the other sectors confirming our current understanding that those areas seem to be more sensitive to glaucomatous changes.
When comparing OCT-based RNFL to BMO-MRW, we observed that BMO-MRW showed slightly higher correlations to functional MD values compared to RNFL but interpret those findings only as tendencies and not significant differences.
Our subgroup analysis of myopic patients (>4 diopters, n=6) revealed better correlations between functional VF sensitivity and BMO-MRW compared to RNFL measurements. The temporal superior sector was the only sector to show a constant significant sectorial structure-function relationship, thus again supporting the notion of the temporal sectors of the ONH and peripapillary region to reveal a better structure-function relationship than other sectors in glaucoma patients[20]–[21].
The varying structure-function correlations between both OCT-based RNFL and BMO-MRW parameters, especially in myopic patients, can be explained with the fact, that for RNFL measurements, the peripapillary circular scan is placed manually around the ONH and does not take into account altered anatomical papillary or peripapillary structures since the predefined diameter and circular shape of the scan remain the same. In contrast, BMO-MRW measurements are based on the actual anatomical opening of Bruch's membrane of the examined eye and therefore automatically respect structural variations of the evaluated ONHs and their peripapillary structures.
Recent publications have shown a decline of BMO-MRW and RNFL thickness as a natural degenerative process in healthy subjects over time. Interestingly, those changes of BMO-MRW measurements did not highly correlate with the observed changes of RNFL measurements[7]. This observed difference also might point to the above mentioned difference of the two obtained structural OCT-based parameters with BMO-MRW being less dependent on structural variations of the ONHs. Future longitudinal studies will have to compare both parameters RNFL and BMO-MRW over time in glaucoma patients with an expected necessity to adjust for age[9] and eventually also for sectors.
A limitation of our one-center study is the relatively small number of patients and its cross-sectional character which does not allow for interpretation regarding possible developments over time. Another limitation is the small number of myopic patients in our subgroup analysis which does not allow to draw strong conclusions independent of the results, but provides a useful hint for a possible better suitability of BMO-MRW compared to RNFL measurements in myopic patients. This careful observation has to be evaluated in larger groups of myopic patients over time.
In summary, we were able to correlate the structural OCT-based parameters RNFL and BMO-MRW to functional VF sensitivity in glaucoma patients. Both parameters RNFL and BMO-MRW seem to be similarly suitable for diagnosis in a clinical setting with BMO-MRW showing higher correlations than RNFL in myopic glaucoma patients.
Acknowledgments
Conflicts of Interest: Reznicek L, None; Burzer S, None; Laubichler A, None; Nasseri A, None; Lohmann CP, None; Feucht N, None; Ulbig M, None; Maier M, None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miglior S, Zeyen T, Pfeiffer N, Cunha-Vaz J, Torri V, Adamsons I, European Glaucoma Prevention Study (EGPS) Group Results of the European glaucoma prevention study. Ophthalmology. 2005;112(3):366–375. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Airaksinen PJ, Tuulonen A, Alanko HI. Rate and pattern of neuroretinal rim area decrease in ocular hypertension and glaucoma. Arch Ophthalmol. 1992;110(2):206–210. doi: 10.1001/archopht.1992.01080140062028. [DOI] [PubMed] [Google Scholar]
- 4.Zangwill LM, Williams J, Berry CC, Knauer S, Weinreb RN. A comparison of optical coherence tomography and retinal nerve fiber layer photography for detection of nerve fiber layer damage in glaucoma. Ophthalmology. 2000;107(7):1309–1315. doi: 10.1016/s0161-6420(00)00168-8. [DOI] [PubMed] [Google Scholar]
- 5.Silverman AL, Hammel N, Khachatryan N, Sharpsten L, Medeiros FA, Girkin CA, Liebmann JM, Weinreb RN, Zangwill LM. Diagnostic accuracy of the spectralis and cirrus reference databases in differentiating between healthy and early glaucoma eyes. Ophthalmology. 2016;123(2):408–414. doi: 10.1016/j.ophtha.2015.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis AS, O'Leary N, Yang H, Sharpe GP, Nicolela MT, Burgoyne CF, Chauhan BC. Influence of clinically invisible, but optical coherence tomography detected, optic disc margin anatomy on neuroretinal rim evaluation. Invest Ophthalmol Vis Sci. 2012;53(4):1852–1860. doi: 10.1167/iovs.11-9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan BC, Danthurebandara VM, Sharpe GP, Demirel S, Girkin CA, Mardin CY, Scheuerle AF, Burgoyne CF. Bruch's membrane opening minimum rim width and retinal nerve fiber layer thickness in a normal white population: a multicenter study. Ophthalmology. 2015;122(9):1786–1794. doi: 10.1016/j.ophtha.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardiner SK, Ren R, Yang H, Fortune B, Burgoyne CF, Demirel S. A method to estimate the amount of neuroretinal rim tissue in glaucoma: comparison with current methods for measuring rim area. Am J Ophthalmol. 2014;157(3):540–549.e1-e2. doi: 10.1016/j.ajo.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vianna JR, Danthurebandara VM, Sharpe GP, Hutchison DM, Belliveau AC, Shuba LM, Nicolela MT, Chauhan BC. Importance of normal aging in estimating the rate of glaucomatous neuroretinal rim and retinal nerve fiber layer loss. Ophthalmology. 2015;122(12):2392–2398. doi: 10.1016/j.ophtha.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Katz J, Sommer A, Gaasterland DE, Anderson DR. Comparison of analytic algorithms for detecting glaucomatous visual field loss. Arch Ophthalmol. 1991;109(12):1684–1689. doi: 10.1001/archopht.1991.01080120068028. [DOI] [PubMed] [Google Scholar]
- 11.Keltner JL, Johnson CA, Anderson DR, Levine RA, Fan J, Cello KE, Quigley HA, Budenz DL, Parrish RK, Kass MA, Gordon MO, Ocular Hypertension Treatment Study Group The association between glaucomatous visual fields and optic nerve head features in the ocular hypertension treatment study. Ophthalmology. 2006;113(9):1603–1612. doi: 10.1016/j.ophtha.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 12.Hirooka K, Manabe S, Tenkumo K, Nitta E, Sato S, Tsujikawa A. Use of the structure-function relationship in detecting glaucoma progression in early glaucoma. BMC Ophthalmol. 2014;14:118. doi: 10.1186/1471-2415-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson WH, Horner DG. Assessing assumptions of a combined structure-function index. Ophthalmic Physiol Opt. 2015;35(2):186–193. doi: 10.1111/opo.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danthurebandara VM, Sharpe GP, Hutchison DM, Denniss J, Nicolela MT, McKendrick AM, Turpin A, Chauhan BC. Enhanced structure-function relationship in glaucoma with an anatomically and geometrically accurate neuroretinal rim measurement. Invest Ophthalmol Vis Sci. 2014;56(1):98–105. doi: 10.1167/iovs.14-15375. [DOI] [PubMed] [Google Scholar]
- 15.Hu R, Marin-Franch I, Racette L. Prediction accuracy of a novel dynamic structure-function model for glaucoma progression. Invest Ophthalmol Vis Sci. 2014;55(12):8086–8094. doi: 10.1167/iovs.14-14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik R, Swanson WH, Garway-Heath DF. Structure-function relationship in glaucoma: past thinking and current concepts. Clin Exp Ophthalmol. 2012;40(4):369–380. doi: 10.1111/j.1442-9071.2012.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hua R, Gangwani R, Guo L, McGhee S, Ma X, Li J, Yao K. Detection of preperimetric glaucoma using Bruch membrane opening, neural canal and posterior pole asymmetry analysis of optical coherence tomography. Sci Rep. 2016;6:21743. doi: 10.1038/srep21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeer KA, van der Schoot J, Lemij HG, de Boer JF. RPE-normalized RNFL attenuation coefficient maps derived from volumetric OCT imaging for glaucoma assessment. Invest Ophthalmol Vis Sci. 2012;53(10):6102–6108. doi: 10.1167/iovs.12-9933. [DOI] [PubMed] [Google Scholar]
- 19.Leaney J, Healey PR, Lee M, Graham SL. Correlation of structural retinal nerve fibre layer parameters and functional measures using Heidelberg Retinal Tomography and Spectralis spectral domain optical coherence tomography at different levels of glaucoma severity. Clin Exp Ophthalmol. 2012;40(8):802–812. doi: 10.1111/j.1442-9071.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- 20.Reznicek L, Seidensticker F, Mann T, Hubert I, Buerger A, Haritoglou C, Neubauer AS, Kampik A, Hirneiss C, Kernt M. Correlation between peripapillary retinal nerve fiber layer thickness and fundus autofluorescence in primary open-angle glaucoma. Clin Ophthalmol. 2013;7:1883–1888. doi: 10.2147/OPTH.S49112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reznicek L, Muth D, Vogel M, Hirneiss C. Structure-function relationship between flicker-defined form perimetry and spectral-domain optical coherence tomography in glaucoma suspects. Curr Eye Res. 2017;42(3):418–423. doi: 10.1080/02713683.2016.1190848. [DOI] [PubMed] [Google Scholar]