Abstract
AIM
To conduct a Meta-analysis for investigating the variations in intraocular pressure (IOP) and central corneal thickness (CCT) during normal pregnancy.
METHODS
We searched for clinical trials published up to November 2015 without language or region restrictions in PubMed, EMBASE, Web of Science, the Cochrane Central Register of Controlled Trials, Ovid, EBSCO, Elsevier, the Chinese Biomedicine Database, WanFang, CNKI, CQVIP and Google Scholar. Studies of the ocular changes observed in pregnant women were selected. The main outcomes were assessed by changes in IOP and CCT.
RESULTS
Fifteen studies were included. In subgroup analyses, IOP was significantly decreased during the second MD=-1.53, 95%CI (-2.19, -0.87); P<0.00001, and third MD=-2.91, 95%CI (-3.74, -2.08); P<0.00001 trimesters of pregnancy. CCT was increased during the second MD=10.12, 95%CI (2.01, 18.22); P=0.01, trimester of pregnancy; moreover, during the third trimester of pregnancy, the CCT displayed an increasing trend, but the difference was not significant MD=5.98, 95%CI (-1.11, 13.07); P=0.1.
CONCLUSION
A decrease in IOP is accompanied by an increase in CCT in the second and third trimesters of a normal pregnancy in women.
Keywords: intraocular pressure, central corneal thickness, pregnancy, Meta-analysis
INTRODUCTION
Intraocular pressure (IOP) is an important indicator used in the diagnosis and treatment of glaucoma[1]. In a functional assessment, “normal IOP” refers to the level that usually does not lead to glaucomatous damage to visual function, according to the statistical regularity of IOP. Normal IOP ranges from 10-21 mm Hg [Goldmann applanation tonometer (GAT)]; however, IOP values fluctuate cyclically throughout the day. Different local factors, such as trabecular outflow facility, corneal curvature, corneal thickness, sclera hardness, and intraocular inflammation, as well as systemic factors, such as age, gender, race, heredity, cardiovascular factors, movement or a change in posture, hormones, food, and drug factors, all affect IOP[2].
The most important treatment for glaucoma is to reduce the IOP. However, the IOP is affected by the central corneal thickness (CCT), and the CCT can induce variations in the IOP within a certain range. A CCT that is too thick or too thin will affect the corneal resistance. When the corneal thickness exceeds the average value (520 µm), the measured IOP will be greater than the actual value; in contrast, when the corneal thickness is less than the average value, the measured IOP will be less than its true value. Therefore, the CCT is an important indicator used in the diagnosis and treatment of glaucoma. The CCT has been reported to vary among different individuals and depends on ethnicity[3]. Age, corneal steepness, contact lens wear, mydriasis, hormones and diseases may also influence the CCT[4]–[5].
Pregnancy is the period when a woman carries a fetus in the uterus. Women undergo many changes during pregnancy, including ocular and systemic changes. Physiological changes in the cardiovascular, hematological, and immune systems, as well as in hormone levels and metabolism, occur during pregnancy[6]–[7]; however, hormonal changes are among the most significant systemic changes in pregnant women. Pregnancy and ocular changes are often associated, and the effect is occasionally permanent but is typically transient in nature[8]. Ocular effects during pregnancy are divided into pathological conditions, physiological changes or modifications of pre-existing conditions[9]. Physiological ocular changes, including the CCT, curvature, IOP, corneal sensitivity, outflow facility, contact lens intolerance and temporary refractive changes, were reported in previous studies[10]–[11].
Many studies have been conducted to determine the changes in IOP and CCT during pregnancy, but the data are limited and localized, and no Meta-analysis on this topic has been reported. In this study, we performed a systematic review and Meta-analysis with the aim of summarizing the variations in IOP and CCT during pregnancy.
MATERIALS AND METHODS
Two investigators (Wang C and Li AL) independently searched PubMed, EMBASE, Web of Science, the Cochrane Central Register of Controlled Trials, Ovid, EBSCO, Elsevier, the Chinese Biomedicine Database, WanFang, CNKI and CQVIP and Google Scholar for eligible articles with the search strategy (“Intraocular Pressure” OR “IOP” OR “Central Corneal Thickness” OR “CCT” AND “Pregnancy”). We performed the final search on November 1, 2015.
Inclusion Criteria
1) Types of studies: cohort or cross-sectional studies of ocular changes in normal pregnant and non-pregnant women; 2) Type of exposure and population. The cohort study: pregnant women comprise the exposure group, and non-pregnant and postpartum women comprise the control group. None of the women in the study had ocular diseases, and all were tracked during follow up. The cross-sectional study: two groups, pregnant and non-pregnant, were compared without continuous observation; 3) Types of outcome measures: IOP of the eyes was measured with one of the three different methods: a Schiötz tonometer, a non-contact pneumotonometer (NCT), and a GAT. Readings were repeated 2--5 times and then averaged. The CCT of the eyes was measured with an ultrasonic pachymeter.
Exclusion Criteria
1) Meeting abstracts, case reports and review articles; 2) Articles with data that could not be extracted, were unreported, or were not clear; 3) Duplicate reports of data and unclear depictions of study characteristics.
The inclusion and exclusion of the studies were determined in an independent evaluation by 2 reviewers (Wang C and Pang Y). We assessed the methodological quality of the studies included in the Meta-analysis using the Newcastle-Ottawa Quality Assessment Scale (NOS)[12] for cohort studies and the Agency for Healthcare Research and Quality (AHRQ)[13] for cross-sectional studies. The NOS is a nine-point scale that assigns points based on the process of selecting the cohorts, cases and controls (0-4 points), the comparability of the cohorts with the cases and the controls (0-2 points), and the identification of the exposure and the outcomes of study participants (0-3 points). The overall reliability and validity of the research questions was graded as low (≤4), medium (4-7), or high (≥7). The NOS was developed to assess the quality of nonrandomized studies for the purpose of incorporating quality assessments in the interpretation of Meta-analytical results. This scale is recommended by the Cochrane Non-randomized Studies Methods Working Group. In the AHRQ for cross-sectional studies, the standard includes 11 questions. This approach, which is largely based on the approach developed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group, incorporates five domains: study limitations, consistency, directness, precision, and reporting bias. Grades (high, moderate, low, and insufficient) reflect our confidence in the evidence for a specific outcome based on the comparative benefits and harms of the interventions.
RevMan software (Review Manager, Version 5.3; The Cochrane Collaboration, 2014) was utilized for the Meta-analysis. Because some of the studies did not report on all outcomes of interest, we performed separate Meta-analyses for each comparison and outcome. Regarding the different clinical characteristics among study groups and the variation in sample sizes, we assumed that heterogeneity was present, even when statistical significance was not identified. Thus, we decided to combine data in a random-effects model to achieve more conservative estimates. The weighted mean difference (WMD) was used to analyze continuous variable outcomes, which were measured with standard test methods. The 95% confidence intervals (CI) were calculated, and P<0.05 was considered statistically significant. Heterogeneity among studies was assessed with the Q test and I2-statistics, P<0.10 and I2>50% indicated evidence of heterogeneity.
RESULTS
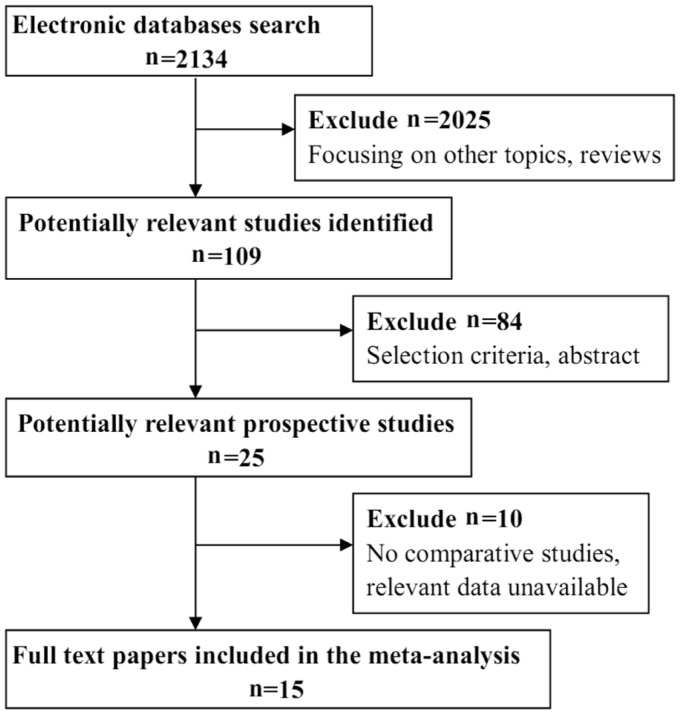
A total of 2134 records were identified through the database search. After browsing the titles and abstracts, 2109 unrelated and overlapping articles were removed. Twenty-five full-text articles were scrutinized for eligibility. Among these articles, 10 articles were excluded because they did not describe comparative studies or the relevant data were unavailable. Ultimately, 15 studies were included in this Meta-analysis. Figure 1 shows a flow diagram of the study selection process.
Figure 1. Flow diagram of the selection process.
Fifteen studies evaluated changes in IOP during pregnancy (Table 1)[10],[14]–[27]. The subjects in the control group were non-pregnant women, and subjects in the experimental group were pregnant women. Totally 1208 women were enrolled, including 701 in the pregnant group and 507 in the non-pregnant group. Pregnant women were divided into three subgroups based on the trimester of pregnancy. The pregnant women's IOP was measured in the first, second and third trimesters to assess objectives and outcomes. Four studies evaluated the changes in CCT during pregnancy (Table 1). Pregnant women's CCT was also measured in their first, second and third trimesters.
Table 1. Characteristics of the studies included in the review.
| References | Design | No. of patients1 | No. of eyes1 | Follow-up period (mo) | Age1 (a) | Primary outcome | Scoring tool | Scores |
| Green K, America, 1988[14] | Pro, Sel | 6, 6, 7/7 | 12, 12, 14/14 | 12 | 24-37/24-37 | IOP | NOS | 7 |
| Ziai N, America, 1994[15] | Pro, Sel | 15/15 | 15/15 | 10 | 24-32/24-32 | IOP, CCT | NOS | 6 |
| Efe YK, Turkey, 2012[10] | Pro, Sel | 25/25 | 25/25 | 12 | 21-35/21-35 | IOP, CCT | NOS | 9 |
| Ataş M, Turkey, 2014[25] | Pro, Sel | 54/54 | 54/54 | 12 | 18-38/18-38 | IOP, CCT | NOS | 9 |
| Centofanti M, Italy, 2002[21] | Pro | 27, 10/14 | 27, 10/14 | 5 | NC | IOP | NOS | 6 |
| Akar Y, Turkey, 2005[18] | Pro | 88/94 | 88/94 | 10 | 21-30/21-30 | IOP | NOS | 9 |
| Ebeigbe JA, Nigeria, 2011[16] | Pro | 100/100 | 100/100 | 11 | 20-35/20-35 | IOP | NOS | 7 |
| Saylik M, Turkey, 2014 [20] | Pro | 40/40 | 40/40 | 36 | 25-33/25-33 | IOP | NOS | 9 |
| Sen E, Turkey, 2014 [17] | Pro | 32/34 | 32/34 | 9 | 21-37/20-37 | IOP, CCT | NOS | 8 |
| Phillips CI, UK, 1985[22] | Cro | 20, 33/25 | 20, 33/25 | - | 20-35, 17-38/19-34 | IOP | AHRQ | High |
| Liu CD, China, 1990[24] | Cro | 60/30 | 60/30 | - | 22-29/22-29 | IOP | AHRQ | High |
| Zhang DX, China, 1991[23] | Cro | 60/30 | 60/30 | - | 23-28/23-28 | IOP | AHRQ | High |
| Qureshi IA, Pakistan, 1996[19] | Cro | 40/40 | 40/40 | - | 25-35/25-35 | IOP | AHRQ | High |
| Qureshi IA, Pakistan, 2000[26] | Cro | 40/40 | 40/40 | - | 21-30/21-30 | IOP | AHRQ | High |
| Goldich Y, Canada, 2014[27] | Cro | 60/60 | 60/60 | NC | NC | IOP | AHRQ | High |
Pro: Prospective nonrandomized; Cro: Cross-sectional; Sel: Self-controlled; NC: Not clear; NOS: The Newcastle-Ottawa Quality Assessment Scale; AHRQ: The Agency for Healthcare Research and Quality; IOP: Intraocular pressure; CCT: Central corneal thickness. 1Pregnant/non-pregnant.
As previously described, we added the following research content to the quality evaluation: for cohort studies, we used NOS, and for cross-sectional studies, we used AHRQ. Among the cohort studies, seven studies had a high grade, with a score ≥7, and two studies had a medium grade, with a score of 6. The studies lose points for their choices of the exposed and control groups and the follow-up period. According to the results from the cross-sectional studies, all studies have good descriptions in the items of the AHRQ, and they all had a high grade.
Data Analysis and Synthesis
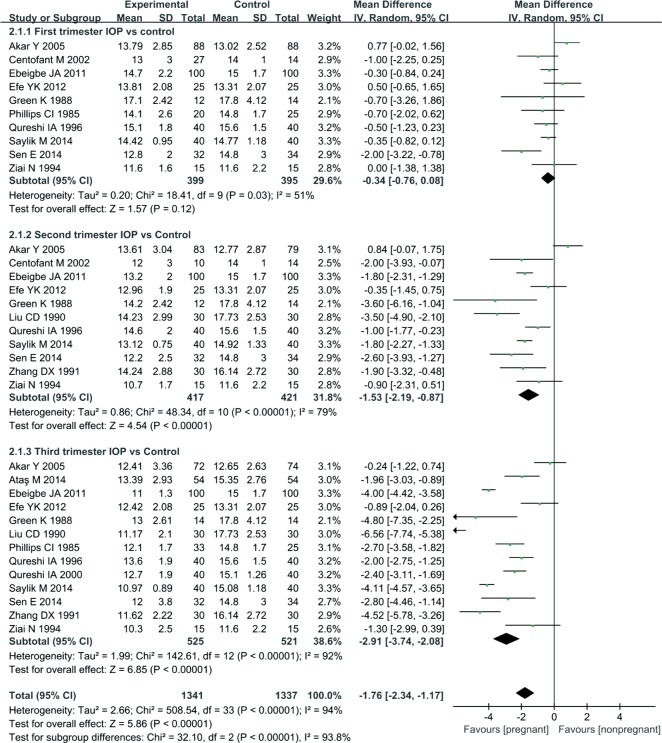
Fifteen studies reported a reduction in IOP at various time points: 10 reported a reduction in the first trimester, 11 reported a reduction in the second trimester, and 13 reported a reduction in the third trimester. According to the results of the Meta-analysis, a statistically significant difference between the first trimester of pregnancy and non-pregnancy was not observed, MD=-0.34, 95%CI (-0.76, 0.08), P=0.12. But, the IOP of the pregnant group was significantly reduced compared with that of the non-pregnant group, MD= -1.53, 95% CI (-2.19, -0.87), P<0.00001 during the second trimester; MD=-2.91, 95%CI (-3.74, -2.08), P<0.00001 during the third trimester (Figure 2). These significant differences indicate that the IOP is lower in pregnant women than in non-pregnant women.
Figure 2. Comparison of the percent reduction in IOP between pregnant and non-pregnant women at various time points.
I2: I2 heterogeneity statistic; Z: Z statistic.
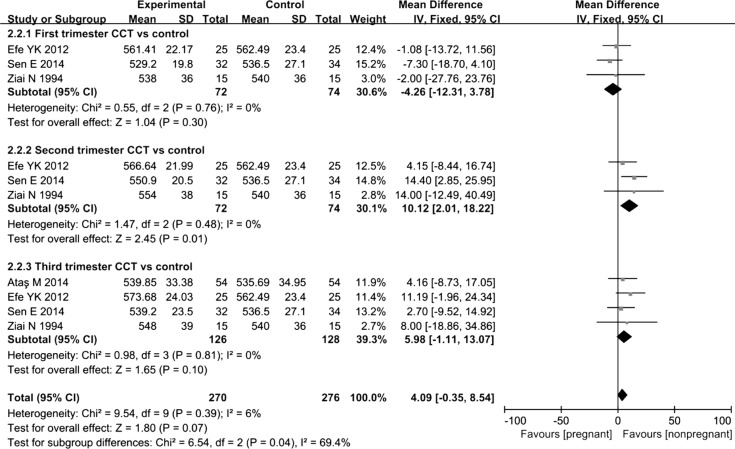
Four trials reported changes in CCT during pregnancy: 3 reported changes during the first trimester, 3 reported changes during the second trimester, and 4 reported changes during the third trimester. According to the results of the Meta-analysis, a statistically significant difference in CCT was not observed between women in the first and third trimesters of pregnancy and non-pregnant women, MD=-4.26, 95%CI (-12.31, 3.78), P=0.3 in the first trimester; MD=5.98, 95%CI (-1.11, 13.07), P=0.1 in the third trimester. However, during the third trimester of pregnancy, the CCT showed an obvious increasing trend. Moreover, the CCT of the pregnant group was significantly increased compared with that of the non-pregnant group, MD=10.12, 95%CI (2.01, 18.22), P=0.01 in the second trimester (Figure 3). Based on these results, the CCT was increased in pregnant women compared with that in non-pregnant women.
Figure 3. Comparison of the percent increase in CCT between pregnant and non-pregnant women at various time points.
I2: I2 heterogeneity statistic; Z: Z statistic.
DISCUSSION
To the best of our knowledge, this Meta-analysis is the first to examine the changes in IOP and CCT during pregnancy, and 15 studies were included. Based on the results of this Meta-analysis, the IOP was decreased during the second and third trimesters of pregnancy, and CCT was increased during the second and third trimesters of pregnancy.
The results showing a decrease in IOP in the second and third trimesters of pregnancy were consistent with the findings of previous studies[15]–[20],[23]–[25]. Moreover, according to Akar et al[18], IOP readings obtained using GAT and Schiötz tonometers are significantly lower than readings obtained using NCT, and the measurements obtained using GAT and Schiötz tonometers were consistent and did not change significantly with advancing pregnancy. Based on our results, the CCT increased during the second and third trimesters of pregnancy, consistent with the results of the studies by Ziai et al[15], Nebbioso et al[5] and Efe et al[10]. Concurrently, Sen et al[17] reported that the mean CCT value is higher in pregnant women than in non-pregnant women, but the difference was not statistically significant.
According to current research, changes in IOP and CCT result from a number of mechanisms[22],[28]–[31]. The majority of proposed mechanisms underlying the decrease in IOP and the increase in CCT during pregnancy indicate an association between female hormones and increased outflow. The increased levels of estrogen, progesterone, relaxin and β-human chorionic gonadotropin (β-HCG) that occur during pregnancy cause the changes of IOP and CCT. A woman's progesterone level begins to increase at approximately 20wk of gestation and continues to increase until the end of the third trimester[15]. In addition, estrogen levels first increase at 9wk and peak at 31-35wk of gestation[32]. In our Meta-analysis, significant reductions in IOP and increases in CCT were observed in the second and the third trimesters of pregnancy, but the change in CCT in the third trimester of pregnancy was not significant. Changes in IOP are negatively correlated with progesterone, estrogen and relaxin levels, and changes in the CCT are positively correlated with the levels of female hormones; hence, we believe that the three hormones may induce a decrease in the IOP and an increase in the CCT.
However, what is the mechanism of action? When present at sufficient levels, progesterone, a glucocorticoid receptor antagonist, has been reported to increase aqueous outflow, ultimately reducing the IOP[28]. Glucocorticoid receptors are expressed in the outflow apparatus; thus, their inhibition decreases the IOP. The increased levels of progesterone and estrogen that occur in pregnancy have been reported to dilate vessels in the circulatory system, leading to decreased arterial pressure and reduced aqueous humor production and a subsequent reduction in the IOP[29]. Furthermore, during pregnancy, relaxin release relaxes the mother's pelvic ligaments, and the pubis symphysis becomes elastic and the sacroiliac joints become relatively limber. These changes allow the fetus to more easily pass through the birth canal[25]. According to Philips and Gore[22], the softening of ligaments in late pregnancy may extend to the ligament of the corneoscleral envelope to produce reduced corneoscleral rigidity and a subsequent decrease in the IOP. Relaxin has also been shown to increase the passage of liquid between collagen in soft tissues and promotes aqueous humor outflow, causing a decrease in the IOP[33]. Additionally, β-HCG can reduce the IOP. The mechanism by which β-HCG decreases the IOP is through the activation of adenylate cyclase[30]. However, the highest β-HCG concentrations are observed during the first trimester, and, in our study, decreased IOP occurred in the second and the third trimesters. Therefore, the outflow facility and the β-HCG concentrations must be measured to clarify this association. An association between corneal thickness and estrogen levels has been reported[34]. Hormones have been suggested to have direct or secondary effects on the cornea, such as systemic water retention due to the estrogen-induced upregulation of the renin-aldosterone system[34].
Overall, hormones have a major influence on the changes in IOP during pregnancy. Two enzyme systems are involved in aqueous humor secretion: carbonic anhydrase and Na/K-ATPase[35]. Antagonists of these enzyme systems reduce the formation of the aqueous humor and reduce the IOP. Changes in the levels of hormones and metabolites produced during pregnancy may antagonize these enzyme systems[35]. Additionally, estrogen, progesterone, and androgen receptors have been identified in the nuclei of stromal and endothelial cells of the human cornea[36]. Giuffrè et al[37] also reported variations in corneal biomechanical properties during the menstrual cycle. These changes probably occur due to the expression of these receptors in the cornea. Therefore, the increased estrogen and progesterone levels observed during pregnancy may lead to ocular changes, including changes in the IOP and corneal thickness[17]. However, additional experimental studies are required to define the changes in CCT during pregnancy.
A number of limitations of this systematic review and Meta-analysis should be noted. First, although we collected all published clinical evidence, the pooled sample size of this Meta-analysis was still relatively small, particularly for the CCT measurements. This limitation weakened the statistical power. Second, the ethnicity of participants in most of the available studies included in the Meta-analysis was Asian; therefore, our findings may not be applicable to other populations. Third, all studies included in the systematic review were published in English or Chinese; however, relevant studies may have been published in other languages. Finally, the heterogeneity between trials in our Meta-analysis was important for some comparisons and endpoints. The heterogeneity may be due to the characteristics of the studies, such as the different outcome measures of IOP and CCT, different countries, age, and gravidity. A Meta-analysis is more reliable if the trials being analyzed are highly similar. We therefore used a random effects model that addresses heterogeneity and provides conservative estimates.
Acknowledgments
Foundation: Supported by the National Nature Science Foundation of China (No.81570841).
Conflicts of Interest: Wang C, None; Li AL, None; Pang Y, None; Lei YQ, None; Yu L, None.
REFERENCES
- 1.Scheetz TE, Faga B, Ortega L, Roos BR, Gordon MO, Kass MA, Wang K, Fingert JH. Glaucoma risk alleles in the ocular hypertension treatment study. Ophthalmology. 2016;123(12):2527–2536. doi: 10.1016/j.ophtha.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao F, Miller JP, Miglior S, Beiser JA, Torri V, Kass MA, Gordon MO. The effect of changes in intraocular pressure on the risk of primary open-angle glaucoma in patients with ocular hypertension: an application of latent class analysis. BMC Med Res Methodol. 2012;12:151. doi: 10.1186/1471-2288-12-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aghaian E, Choe JE, Lin S, Stamper RL. Central corneal thickness of Caucasians, Chinese, Hispanics, Filipinos, African Americans, and Japanese in a glaucoma clinic. Ophthalmology. 2004;111(12):2211–2219. doi: 10.1016/j.ophtha.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Saeedi O, Pillar A, Jefferys J, Arora K, Friedman D, Quigley H. Change in choroidal thickness and axial length with change in intraocular pressure after trabeculectomy. Br J Ophthalmol. 2014;98(7):976–979. doi: 10.1136/bjophthalmol-2013-304433. [DOI] [PubMed] [Google Scholar]
- 5.Nebbioso M, Fazio S, Di Blasio D, Pescosolido N. Hypobaric hypoxia: effects on intraocular pressure and corneal thickness. Scientific World Journal. 2014;2014:585218. doi: 10.1155/2014/585218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boardman H, Ormerod O, Leeson P. Haemodynamic changes in pregnancy: what can we learn from combined datasets? Heart. 2016;102(7):490–491. doi: 10.1136/heartjnl-2015-309166. [DOI] [PubMed] [Google Scholar]
- 7.Yenerel NM, Küçümen RB. Pregnancy and the eye. Turk J Ophthalmol. 2015;45(5):213–219. doi: 10.4274/tjo.43815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiteri Cornish K, Hrabovsky M, Scott NW, Myerscough E, Reddy AR. The short- and long-term effects on the visual system of children following exposure to maternal substance misuse in pregnancy. Am J Ophthalmol. 2013;156(1):190–194. doi: 10.1016/j.ajo.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Sheth BP, Mieler WF. Ocular complications of pregnancy. Curr Opin Ophthalmol. 2001;12(6):455–463. doi: 10.1097/00055735-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Efe YK, Ugurbas SC, Alpay A, Ugurbas SH. The course of corneal and intraocular pressure changes during pregnancy. Can J Ophthalmol. 2012;47(2):150–154. doi: 10.1016/j.jcjo.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Weinreb RN, Lu A, Beeson C. Maternal corneal thickness during pregnancy. Am J Ophthalmol. 1988;105(3):258–260. doi: 10.1016/0002-9394(88)90006-2. [DOI] [PubMed] [Google Scholar]
- 12.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. 2011. Available at: www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 13.Helfand M, Balshem H. Principles in developing and applying guidance. Methods Guide for Effectiveness and Comparative Effectiveness Reviews [Internet] Rockville (MD): Agency for Healthcare Research and Quality (US); 2008-. AHRQ Methods for Effective Health Care 2009 Aug 08. [PubMed]
- 14.Green K, Phillips CI, Cheeks L, Slagle T. Aqueous humor flow rate and intraocular pressure during and after pregnancy. Ophthalmic Res. 1988;20(6):353–357. doi: 10.1159/000266751. [DOI] [PubMed] [Google Scholar]
- 15.Ziai N, Ory SJ, Khan AR, Brubaker RF. β-Human chorionic gonadotropin, progesterone, and aqueous dynamics during pregnancy. Arch Ophthalmol. 1994;112(6):801–806. doi: 10.1001/archopht.1994.01090180099043. [DOI] [PubMed] [Google Scholar]
- 16.Ebeigbe JA, Ebeigbe PN, Ighoroje AD. Intraocular pressure in pregnant and non-pregnant Nigerian women. Afr J Reprod Health. 2011;15(4):20–23. [PubMed] [Google Scholar]
- 17.Sen E, Onaran Y, Nalcacioglu-Yuksekkaya P, Elgin U, Ozturk F. Corneal biomechanical parameters during pregnancy. Eur J Ophthalmol. 2014;24(3):314–319. doi: 10.5301/ejo.5000378. [DOI] [PubMed] [Google Scholar]
- 18.Akar Y, Yucel I, Akar ME, Zorlu G, Ari ES. Effect of pregnancy on intraobserver and intertechnique agreement in intraocular pressure measurements. Ophthalmologica. 2005;219(1):36–42. doi: 10.1159/000081781. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi IA, Xi XR, Wu XD. Intraocular pressure trends in pregnancy and in the thrid trimester hypertensive patients. Acta Obstet Gynecol Scand. 1996;75(9):816–819. doi: 10.3109/00016349609054709. [DOI] [PubMed] [Google Scholar]
- 20.Saylik M, Saylik SA. Not only pregnancy but also the number of fetuses in the uterus affects intraocular pressure. Indian J Ophthalmol. 2014;62(6):680–682. doi: 10.4103/0301-4738.120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centofanti M, Migliardi R, Bonini S, Manni G, Bucci MG, Pesavento CB, Amin CS, Harris A. Pulsatile ocular blood flow during pregnancy. Eur J Ophthalmol. 2002;12(4):276–280. doi: 10.1177/112067210201200404. [DOI] [PubMed] [Google Scholar]
- 22.Phillips CI, Gore SM. Ocular hypotensive effect of late pregnancy with and without high blood pressure. Br J Ophthalmol. 1985;69(2):117–119. doi: 10.1136/bjo.69.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang DX, Cui SX, Hao YM. Preliminary observation intraocular pressure change of pregnant women. Journal of Xi'an Jiaotong University (Medical Sciences) 1991;12:184–185. [Google Scholar]
- 24.Liu CD. The intraocular pressure of pregnancy women. Chinese Journal of Practical Ophthalmology. 1990;8:30. [Google Scholar]
- 25.Ataş M, Duru N, Ulusoy DM, Altınkaynak H, Duru Z, Açmaz G, Ataş FK, Zararsız G. Evaluation of anterior segment parameters during and after pregnancy. Cont Lens Anterior Eye. 2014;37(6):447–450. doi: 10.1016/j.clae.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi IA, Xi XR, Yaqob T. The ocular hypotensive effect of late pregnancy is higher in multigravidae than in primigravidae. Graefes Arch Clin Exp Ophthalmol. 2000;238(1):64–67. doi: 10.1007/s004170050011. [DOI] [PubMed] [Google Scholar]
- 27.Goldich Y, Cooper M, Barkana Y, Tovbin J, Lee Ovadia K, Avni I, Zadok D. Ocular anterior segment changes in pregnancy. J Cataract Refract Surg. 2014;40(11):1868–1871. doi: 10.1016/j.jcrs.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 28.Perogamvros I, Ray DW, Trainer PJ. Regulation of cortisol bioavailability-effects on hormone measurement and action. Nat Rev Endocrinol. 2012;8(12):717–727. doi: 10.1038/nrendo.2012.134. [DOI] [PubMed] [Google Scholar]
- 29.Pilas-Pomykalska M, Luczak M, Czajkowski J, Woźniak P, Oszukowski P. Changes in intraocular pressure during pregnancy. Klin Oczna. 2004;106(1-2 Suppl):238–239. [PubMed] [Google Scholar]
- 30.Elman J, Caprioli J, Sears M, Mead A, Rubin P. Chorionic gonadotropin decreases intraocular pressure and aqueous humor flow in rabbit eyes. Invest Ophthalmol Vis Sci. 1987;28(1):197–200. [PubMed] [Google Scholar]
- 31.Wilke K. Episcleral venous pressure and pregnancy. Acta Ophthalmol Suppl. 1975;125(125):40–41. doi: 10.1111/j.1755-3768.1975.tb01228.x. [DOI] [PubMed] [Google Scholar]
- 32.Yaron Y, Cherry M, Kramer RL, O'Brien JE, Hallak M, Johnson MP, Evans MI. Second-trimester maternal serum marker screening: maternal serum alphafetoprotein, beta-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. 1999;181(4):968–974. doi: 10.1016/s0002-9378(99)70334-0. [DOI] [PubMed] [Google Scholar]
- 33.Kass MA, Sears ML. Hormonal regulation of intraocular pressure. Surv Ophthalmol. 1977;22(3):153–176. doi: 10.1016/0039-6257(77)90053-4. [DOI] [PubMed] [Google Scholar]
- 34.Goldich Y, Barkana Y, Pras E, Fish A, Mandel Y, Hirsh A, Tsur N, Morad Y, Avni I, Zadok D. Variations in corneal biomechanical parameters and central corneal thickness during the menstrual cycle. J Cataract Refract Surg. 2011;37(8):1507–1511. doi: 10.1016/j.jcrs.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 35.Huang Q, Rui EY, Cobbs M, Dinh DM, Gukasyan HJ, Lafontaine JA, Mehta S, Patterson BD, Rewolinski DA, Richardson PF, Edwards MP. Design, synthesis, and evaluation of NO-donor containing carbonic anhydrase inhibitors to lower intraocular pressure. J Med Chem. 2015;58(6):2821–2833. doi: 10.1021/acs.jmedchem.5b00043. [DOI] [PubMed] [Google Scholar]
- 36.Allam RS, Khalil NM. Evaluation of sex differences in corneal hysteresis. Eur J Ophthalmol. 2015;25(5):391–395. doi: 10.5301/ejo.5000572. [DOI] [PubMed] [Google Scholar]
- 37.Giuffrè G, Di Rosa L, Fiorino F, Bubella DM, Lodato G. Variations in central corneal thickness during the menstrual cycle in women. Cornea. 2007;26(2):144–146. doi: 10.1097/01.ico.0000244873.08127.3c. [DOI] [PubMed] [Google Scholar]