Abstract
Video-assisted thoracoscopic surgery (VATS) has been widely accepted as a minimally invasive surgery for treatment of early-stage lung cancer. However, various VATS approaches are available. In patients with lung cancer, VATS should achieve not only minimal invasiveness but also safety and oncological clearance. In this article, we introduce our method of VATS lobectomy.
Keywords: Video-assisted thoracoscopic surgery (VATS), minimally invasive surgery, confronting upside-down monitor setting
Introduction
Video-assisted thoracoscopic surgery (VATS) has acquired widespread favor with the rapid development of associated techniques. Lobectomy with systemic lymph node dissection (SND) is the standard treatment for patients with stage I or II non-small cell lung cancer. VATS lobectomy for treatment of primary lung cancer has become increasingly accepted as a minimally invasive surgery and is now widely performed (1-4). However, the monitor setting and port placement differ among hospitals. Two monitor settings are available: the look-up monitor setting and the confronting upside-down monitor setting. We consider both the safety and procedure radicality to be important in VATS lobectomy for lung cancer. As a result, we have adopted the confronting upside-down monitor setting and the performance of four incisions with which to assist operation of the surgical instruments.
Confronting upside-down monitor setting
The patient is placed in the lateral decubitus position under general anesthesia with double-lumen intubation. Two monitors are installed on the cranial side of the patient, and one of the monitors is placed upside-down (Figure 1). The surgeon stands on the right side of the patient regardless of the operation side (in a right-sided operation, dorsal side; in a left-sided operation, ventral side). The camera and second assistants stand on the left side of the patient. The surgeon can maintain a comfortable position and operate all instruments easily during surgery because he or she stands alone. We usually use a high-definition camera with a 30-degree lens. The camera assistant always keeps the camera in a horizontal position, and there are no mirror images of the surgical field. Each person obtains a correct view of the surgical field in the confronting monitor.
Figure 1.
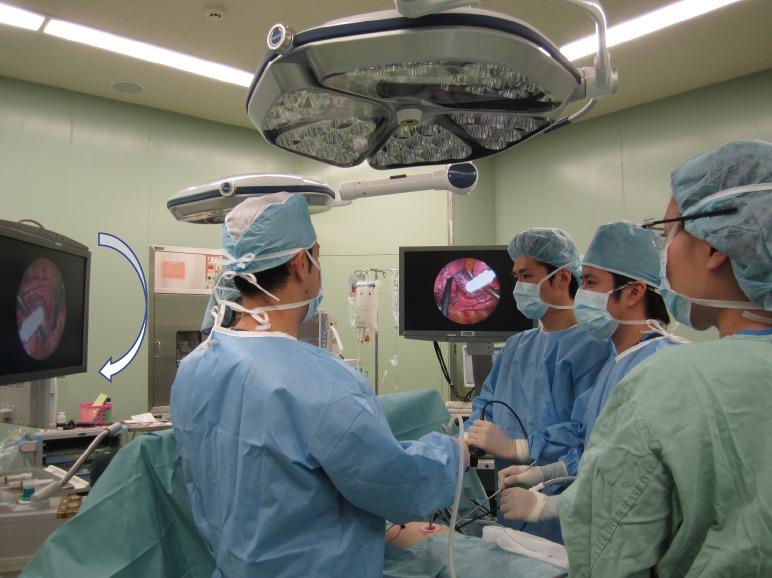
Confronting upside-down monitor setting. Two monitors are installed on the cranial side of the patient, and one of the monitors is placed upside down. The surgeon stands on the right side of the patient regardless of the operation side.
Port placement
During a right-sided operation, the surgical procedure is performed via a 2 to 3 cm utility incision on the posterior axillary line in the fifth intercostal space using endoscopic instruments and traditional instruments for sharp dissection. The latissimus dorsi is left intact, the serratus anterior is split, and the intercostal muscles are minimally divided about 5 cm to remove the resected specimens. A 15-mm incision is created on the anterior axillary line in the fourth intercostal space for the second assistant. Two 7-mm ports are placed in the center of the third intercostal space for the camera and posteriorly in the fourth intercostal space for the operator’s left hand. Two silicone rubber instruments (Lap ProtectorTM Minimini; Hakko Co., Ltd., Tokyo, Japan) are applied to maintain the two wounds in the open position (Figure 2). The operation is performed entirely via thoracoscopic visualization, and the role of each port is fixed. Achieving adequate retraction in the dissected area is sometimes more difficult during VATS than during open thoracotomy. Therefore, in our procedure, the second assistant performs retraction in the dissection area using two instruments. Thus, the role of the second assistant is necessary to perform sharp dissection. The camera port is placed in the third intercostal space during a right-sided operation and in the fifth intercostal space during a left-sided operation. Through this camera port, we can see all structures in the chest cavity using a 30-degree camera (Figure 3).
Figure 2.
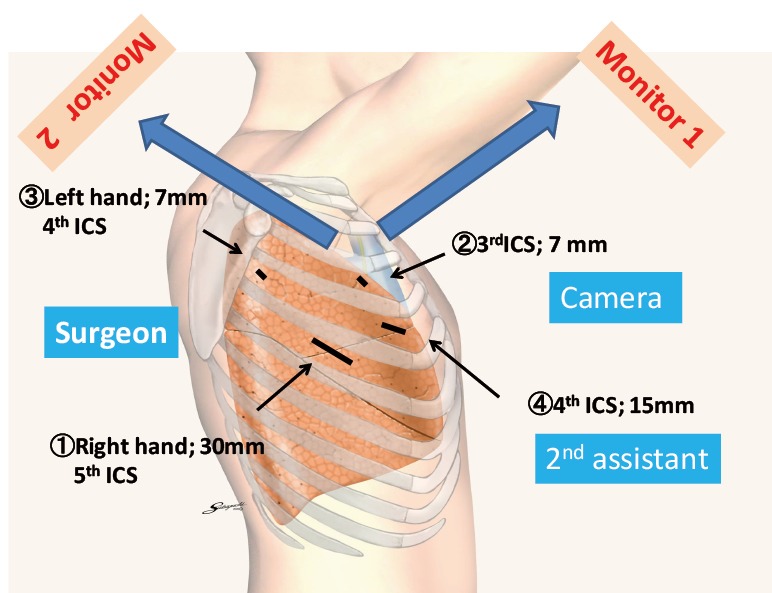
Port placement. Each person is able to view the confronting monitor. The surgeon looks at Monitor 1. In this monitor, the left side is the cranial side of the patient. The camera and second assistants look at Monitor 2. In this monitor, the right side is the cranial side of the patient. If the camera assistant keeps the camera in a horizontal position, mirror images and disorientation can be avoided.
Figure 3.
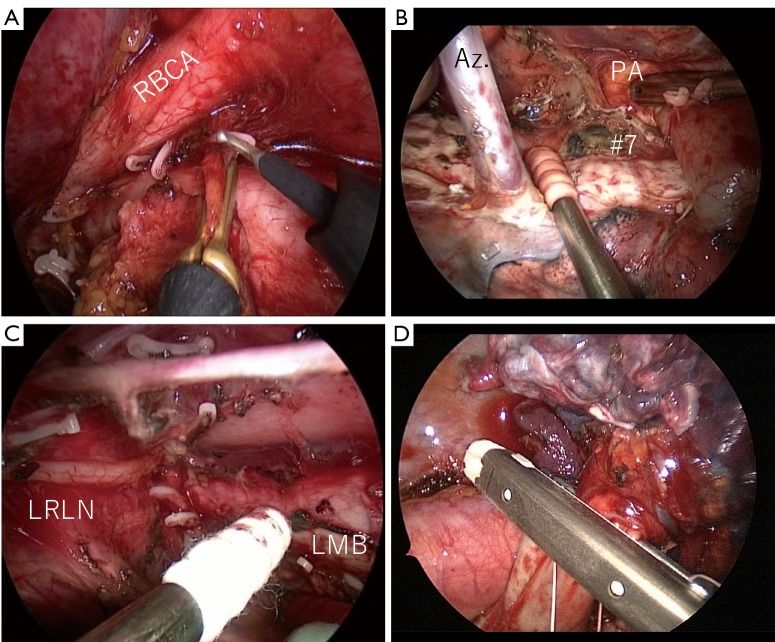
Surgical view of the confronting upside-down monitor setting. (A) Dissection of #2R; (B) final view after dissection of the right upper zone; (C) final view after dissection of station 4L; (D) division of A3 by an endoscopic stapler. Checking both the cartridge and anvil at the same time is important to ensure safe division of vessels. RBCA, right brachiocephalic artery; Az, azygos vein; PA, pulmonary artery; LRLN, left recurrent laryngeal nerve; LMB, left main bronchus.
Surgical procedure
After retraction of the lung by the second assistant, the pulmonary vessels are sharply dissected using a scissors. When the vessels are dissected, it is important to open the vessel sheath. The tissue behind the vessel is then bluntly dissected using a sponge stick. Pulmonary vessels of ≥7 mm in diameter are divided using a stapler, and those of <7 mm in diameter are divided after ligation by 2-0 silk. Creation of a tunnel between the entrance and exit is important before stapling an incomplete fissure (Figure 4). When the tunnel is made, dissection must be performed between the hilar lymph node and lung parenchyma to avoid pinching the lymph node while stapling the fissure.
Figure 4.

Video-assisted thoracoscopic surgery right upper lobectomy via confronting upside-down monitor setting (5). Under retraction by the second assistant, the pulmonary vessels are sharply exposed and the fissure is divided without crushing the hilar nodes. Available online: http://www.asvide.com/articles/1716
As a rule, hilar lymph node dissection is performed after dividing the pulmonary vessels and fissure. Hilar lymph node dissection is performed in conjunction with the lobe from an oncological viewpoint, and the bronchial artery is usually divided after ligation by 2-0 silk. We use this thread to create traction and countertraction in the dissected area. This allows us to avoid grasping the lymph nodes directly and helps to protect the capsule of the hilar lymph nodes. Protecting the capsule of the metastatic lymph node may prevent the spread of malignant cells into the chest cavity.
Lobe-specific SND is usually performed in VATS lobectomy. In mediastinal lymph node dissection, we remove the tissue from adjacent organs and skeletonize the anatomic structures. Retraction by the second assistant is also necessary to perform SND (Figures 5,6).
Figure 5.

Hilar node dissection and right upper zone systemic lymph node dissection (6). In this particular patient, we performed en bloc lymph node dissection. The nodes were dissected in conjunction with the right upper lobe. Available online: http://www.asvide.com/articles/1717
Figure 6.

Mediastinal lymph node dissection of 4L (7). The upper lobe branch of the vagus nerve is retracted by 2-0 silk. The station 4L lymph nodes are then dissected from the surrounding tissue, including the left recurrent laryngeal nerve. The station 10 to 4L lymph nodes are dissected en bloc. Available online: http://www.asvide.com/articles/1718
Results
From April 2008 to December 2016, 913 consecutive patients with primary lung cancer underwent VATS lobectomy at our institution. The mean operation time was 198 min (range, 45–488 min). The mean blood loss was 48 g (range, 0–700 g). Two procedures were converted to open thoracotomy (conversion rate, 0.2%; pulmonary artery bleeding, n=1; silicotic nodes, n=1). The median chest drainage duration was 1 day (range, 1–6 days), and the median postoperative hospital stay was 7 days (range, 3–45 days). There was no 30-day mortality.
Discussion
Although open thoracotomy has long been considered the standard approach to lung cancer surgery, many reports have described the effectiveness of VATS for early-stage lung cancer (1-4). Two monitor settings are available for VATS lobectomy. One is the look-up monitor setting. In this monitor setting, the camera port is usually placed in the seventh or eighth intercostal space, and one monitor is placed on the cranial side of the patient. Therefore, the upside in the monitor is the cranial side of the patient. The surgeon and the assistant can watch the same monitor. In our opinion, however, detection of the tip of the instruments is sometimes difficult from the seventh or eighth intercostal space. In addition, the scope interferes with the other instruments in areas far from the camera port.
Several recent reports have described uniportal VATS lobectomy (8-11). However, uniportal VATS has the same drawbacks as does the look-up monitor setting. Therefore, we perform VATS lobectomy with lobe-specific SND via the confronting upside-down monitor setting. Sufficient retraction by the second assistant is essential to perform sharp dissection of any structures, as in open thoracotomy. In addition, if bleeding occurs, it is important to clear the operation field for safety. Under retraction by the second assistant, the surgeon can operate any instruments using both hands. To ensure the safety of the procedure, it is important to check the tip of instruments because vessel injury at blind spots may be fatal. The instrument tips can be easily checked using this monitor setting and port position of the camera.
In conclusion, the most important issues of VATS lobectomy with SND for primary lung cancer are safety and curability. We must choose the surgical approach that can optimize the procedure being performed.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lewis RJ, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgical non-rib spreading simultaneously stapled lobectomy: a more patient-friendly oncologic resection. Chest 1999;116:1119-24. 10.1378/chest.116.4.1119 [DOI] [PubMed] [Google Scholar]
- 2.McKenna RJ, Jr, Wolf RK, Brenner M, et al. Is lobectomy by video-assisted thoracic surgery an adequate cancer operation? Ann Thorac Surg 1998;66:1903-8. 10.1016/S0003-4975(98)01166-7 [DOI] [PubMed] [Google Scholar]
- 3.Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644-6. 10.1016/S0003-4975(00)01909-3 [DOI] [PubMed] [Google Scholar]
- 4.Ichinose J, Kohno T, Fujimori S, et al. Locoregional control of thoracoscopic lobectomy with selective lymphadenectomy for lung cancer. Ann Thorac Surg 2010;90:235-9. 10.1016/j.athoracsur.2010.03.049 [DOI] [PubMed] [Google Scholar]
- 5.Mun M, Ichinose J, Matsuura Y, et al. Video-assisted thoracoscopic surgery right upper lobectomy via the confronting upside-down monitor setting. Asvide 2017;4:402. Available online: http://www.asvide.com/articles/1716 [DOI] [PMC free article] [PubMed]
- 6.Mun M, Ichinose J, Matsuura Y, et al. Hilar node dissection and right upper zone systemic lymph node dissection. Asvide 2017;4:403. Available online: http://www.asvide.com/articles/1717
- 7.Mun M, Ichinose J, Matsuura Y, et al. Mediastinal lymph node dissection of 4L. Asvide 2017;4:404. Available online: http://www.asvide.com/articles/1718
- 8.Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc 1992;2:244-7. [PubMed] [Google Scholar]
- 9.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. 10.1016/j.athoracsur.2012.10.070 [DOI] [PubMed] [Google Scholar]
- 10.Sihoe AD. The evolution of minimally invasive thoracic surgery: implications for the practice of uniportal thoracoscopic surgery. J Thorac Dis 2014;6:S604-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]