Abstract
The role of alpha-fetoprotein (AFP) in the specific setting of the diagnosis and prognosis of patients with hepatocellular cancer (HCC) waiting for liver transplantation (LT) is still controversial. Recently, a marked interest for this marker has been reported, mainly related to its ability to predict the outcome of HCC patients after LT. The growing number of papers in PubMed indicates that AFP has begun a “second life” in the particular context of LT. Looking at the most recent International Guidelines on HCC, it looks obvious that time is ripe to reevaluate the value of AFP in relation to its prognostic ability to identify HCC patients at high-risk for drop-out before and recurrence after LT. Many discrepancies exist worldwide regarding the use of biomarkers in HCC. In contrast to the Western world, AFP is widely used in Asian countries, the reason why being unclear. Indeed, in the (merely Western-dominated) HCC treatment algorithms, the role of AFP as a prognostic tumor marker is still considered to be “under investigation”. One should however realize that the underestimation of the value of AFP in the LT context will hamper further refinements of both the liver allograft allocation process and the selection of the best candidates for this procedure. Moreover, AFP has an important role to play in the monitoring of bridging and/or downstaging procedures bringing eventually the patient to transplantation. So, time has come to reconsider the role and value of AFP (dynamics) in the field of transplant oncology.
Keywords: Hepatocellular cancer (HCC), liver transplantation (LT), recurrence, alpha-fetoprotein (AFP), des-gamma-carboxy-prothrombin (DCP), liver allocation
Introduction
The role of alpha-fetoprotein (AFP) in the specific setting of the diagnosis and prognosis of patients with hepatocellular cancer (HCC) waiting for liver transplantation (LT) is still controversial, the main reason probably being the high rates of false positive and false negative results (1). Despite the fact that this marker was introduced in detecting HCC in the early 70s (2), its use in clinical practice widely varied since then, even leading to a (temporary) AFP obituary (3).
Initially, AFP was considered together with the radiological assessment of the tumor as a crucial tool to diagnose HCC; this combination became than incorporated on a worldwide basis into the HCC diagnostic flow-charts (4,5). Due to following analyses revealing that AFP had a limited added diagnostic value only to modern radiological assessment alone (6), AFP determination became less used in clinical practice (3).
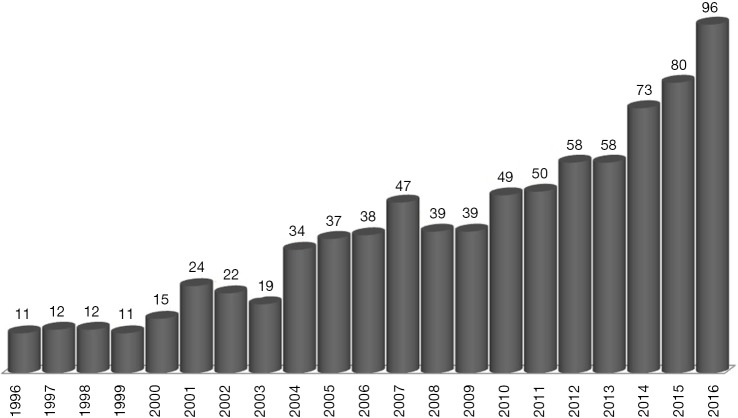
Indeed, about one-third of potential liver recipients present non-AFP-producing tumors (7). Despite this observation, there has been a rising interest for this tumor marker as a tool to predict the outcome of HCC patients after LT (8). The growing number of articles containing the terms: (alpha-fetoprotein) AND (liver transplant) AND (hepatocellular cancer) identified on PubMed is indicating that AFP started a “second life” (Figure 1).
Figure 1.
Search of Articles on PubMed when using the MESH terms: (alpha-fetoprotein) AND (liver transplant) AND (hepatocellular carcinoma). A total of 824 articles were available during the period 1996–2016.
The aim of the present review is to focus the attention on the specific role that AFP may play in the diagnostic as well as in the prognostic process of HCC patients undergoing LT. Particular attention has been given to identify the grades of evidence related to the inclusion of AFP in the most recent international and national HCC guidelines.
AFP and its diagnostic value
The recommendation statements originating from the international guidelines focusing on the diagnostic role of AFP in the detection of HCC are displayed in Table 1.
Table 1. Recommendations on the diagnostic role of alpha-fetoprotein as reported in the most recent international guidelines on hepatocellular cancer management.
| Group | References | Grade | Year | Ref. |
|---|---|---|---|---|
| APASL | AFP is not recommended as a confirmatory test in small HCC | 1B | 2017 | (9) |
| The cut-off value of AFP should be set at 200 ng/mL for surveillance programs when used in combination with US | 2B | |||
| The cut-off value of AFP can be set at lower value in a population with hepatitis virus suppression or eradication | 2B | |||
| AASLD | Surveillance using US, with or without AFP is suggested every 6 months | 2C | 2017 | (10) |
| EASL-EORTC | Accurate tumor biomarkers for early detection need to be developed. Data available with tested biomarkers (i.e., AFP, AFP-L3 and DCP) show that these tests are suboptimal for routine clinical practice | 2B | 2012 | (6) |
| APASL | Surveillance for HCC should be performed by US and AFP every 6 months | 2B | 2010 | (11) |
| a-Fetoprotein alone is not recommended for the diagnosis of HCC | 1A | |||
| Cutoff value of AFP should be set at 200 ng/mL for diagnosis | 1A | |||
| Simultaneous measurement of AFP and DCP provides higher sensitivity without decreasing specificity | 1A |
APASL, Asian Pacific Association for the Study of the Liver; AFP, alpha-fetoprotein; HCC, hepatocellular cancer; US, ultrasounds; AASLD, American Association For The Study Of Liver Diseases; EASL, European Association for the Study of the Liver; EORTC, European Organization for Research and Treatment of Cancer; DCP, des-gamma carboxy-prothrombin.
It is interesting to observe that differences exist between Eastern vs. Western guidelines. The 2010 (11) as well as the 2017 (9) Asian Pacific Association for the Study of the Liver (APASL) guidelines recommend to not use AFP alone to diagnose HCC (1A), mainly in case of small HCCs (1B). This last statement was suggested by a prospective study including 89 patients presenting at imaging a suspected small HCC (<2.0 cm), in which AFP levels were similar in the groups effectively having an HCC (n=60) vs. no HCC (n=39) (12). A systematic review further confirmed the limited role of AFP in the diagnosis of HCC, especially if the AFP values were beneath 200 ng/mL (13). The APASL Guidelines propose to use this value in combination with ultrasound in HCC surveillance programs (2B) (9). In case of HBV/HCV suppression or eradication, a lower cut-off value should be considered (2B) (9), based on the facts that viral infection leads to higher AFP baseline levels (14), and that complete and sustained response after anti-viral treatment consents to obtain lower AFP values (15).
APASL Guidelines also recommend to measure simultaneously AFP and des-gamma-carboxy-prothrombin (DCP), also known as protein induced by vitamin K absence/antagonist-II (PIVKA-II), in order to increase the overall sensitivity in the diagnosis of HCC without decreasing the specificity (1A) (11). Unfortunately, the clinical use of DCP has been almost exclusively done in Eastern countries. Conversely, its role in US and European patients has been poorly investigated. A meta-analysis of 40 studies confirmed that AFP alone has a lower diagnostic ability compared to the combination of AFP-DCP (areas under the curve: 0.835 vs. 0.874, respectively) (16). Conversely, the European guidelines put forward by the joined committees of the European Association for the Study of the Liver (EASL) and the European Organization for Research and Treatment of Cancer (EORTC) consider all HCC biomarkers (including AFP and PIVKA-II) as suboptimal tests for routine clinical practice (2B), and that more accurate tumor markers need to be developed in order to detect HCC in an earlier stadium (6).
A relative agreement has been observed between the Western and Eastern opinions in relation to the timing of HCC surveillance; APASL Guidelines suggest to perform testing including AFP and ultrasound on a semestrial basis (2B) (11). American Association for the Study of Liver Diseases (AASLD) Guidelines suggest to perform a 6-month surveillance using ultrasound, with or without AFP determination (2C). These recommendations are derived from a meta-analysis including 13 studies, in which an half-yearly surveillance using ultrasound revealed to have a higher diagnostic sensitivity (70%) than the yearly one (sensitivity dropping to 50%) (17).
AFP and its prognostic value in LT
The recommendations coming from international guidelines focusing on the prognostic role of AFP in HCC patients undergoing LT are displayed in Table 2.
Table 2. Recommendations on the prognostic role of alpha-fetoprotein as reported in the most recent international guidelines on hepatocellular cancer management.
| Group | References | Grade | Year | Ref. |
|---|---|---|---|---|
| EASL | HCC is a particular MELD exception requiring extra points to get access to OLT. These points have to take into account size, number of nodules, AFP levels, recurrence after downstaging therapy | 2B | 2016 | |
| BTS-BASL | HCC patients out of current UK guidelines but who are within UCSFC and who also meet AFP <1,000 ng/mL, can be considered for LDLT | 1B | 2015 | |
| EASL-EORTC | Assessment of response in HCC should be based on the modification of the RECIST criteria (mRECIST). Use of changes in serum levels of biomarkers for assessment of response (i.e., AFP levels) is under investigation | 2B | 2012 | (6) |
| OLT for HCC Consensus Group | AFP concentrations add prognostic information in HCC patients and may be used for making decisions regarding OLT in combination with imaging criteria | 2B | 2012 | (18) |
| AFP concentrations before and after downstaging may add additional information | 4 | |||
| Periodic waiting-list monitoring should be performed by imaging and AFP measurements | 5 |
EASL, European Association for the Study of the Liver; HCC, hepatocellular cancer; MELD, model for end-stage liver disease; OLT, orthotopic liver transplantation; AFP, alpha-fetoprotein; BTS, the British Transplant Society; BASL, British Association for the Study of the Liver; UK, United Kingdom; UCSFC, University of California San Francisco Criteria; LDLT, living-donor liver transplantation; EORTC, European Organization for Research and Treatment of Cancer; RECIST, Response Evaluation Criteria in Solid Tumors.
The 2012 International Zurich HCC Consensus Conference, focusing on the role of LT in HCC patients, has been the first and, so far, the last opportunity to propose internationally accepted recommendations in this specific setting (18). Looking at the recommendations focusing on AFP, the Zurich Consensus states that AFP has an added prognostic value in HCC patients, and that this marker combined with imaging criteria may be useful to make decisions regarding the indication for LT (2B).
A vast amount of papers recently looked at the added value of AFP in the prediction of the post-LT outcome. The retrospective US Scientific Registry of Transplant Recipients analysis including 6,817 patients listed with the diagnosis of HCC showed that patients with down-staged AFP levels from > to ≤400 ng/mL had a better intent-to-treat survival when compared to patients which failed to reduce AFP after loco-regional treatments (81% vs. 48% at 3 years, P<0.001) (19). Another US study including 45,267 liver recipients reported a progressive increased risk for post-LT death when AFP increased [16–65 ng/mL: hazard ratio (HR) =1.38; 66–320 ng/mL: HR =1.65; >320 ng/mL: HR =2.37]. Milan Criteria (MC)-OUT patients with low AFP levels had excellent survivals; in contrast, MC-IN patients with high AFP levels had poor survivals (20).
A European study focusing on the combination of total tumor diameter <8 cm and AFP <400 ng/mL showed a very low five-year recurrence rate (4.9%) and high five-year disease-free survival rates (74.4%) when meeting the proposed criteria, with a contextual increase in the number of potentially transplantable patients compared to the conventional MC (+22.2% increase) (21). Another prospective study based on the combination of total tumor volume (TTV) exceeding 115 cm3 and AFP >400 ng/mL, showed that patients meeting TTV/AFP criteria had 4-year recurrence rates (9.4% vs. 4.5%; P=0.1) and post-LT survivals (74.6% vs. 78.7%; P=0.9) substantially similar to the ones observed in MC-IN cases (22).
The Zurich Consensus Conference also values the combination of AFP level and radiological assessment, in the context of downstaging procedures leading to a periodic tumor marker and imaging waiting-list monitoring (18). A large European multicenter study including 2,103 HCC patients identified four variables [namely, radiological response, AFP, model for end-stage liver disease (MELD) and MC status] as the best ones to reveal the highest intention-to-treat (ITT) benefit: cases with AFP >1,000 ng/mL, complete response or progression disease, low MELD and MC-IN status, had no benefit of LT compared to alternative (surgical and non-surgical) treatments. Conversely, patients without any risk factor (namely, AFP <1,000 ng/mL, partial response or stable disease, high MELD and MC-OUT status) had had benefit of 60 months when transplanted (23).
Another multicenter European study including 306 MC-IN and 116 MC-OUT patients similarly identified two risk factors for patient death and post-LT recurrence, namely the radiological response and the progression of AFP after loco-regional treatments. Interestingly, progressive disease and AFP slope >15 ng/mL/month were prognostic for poor survival rates, even in the very well selected population of MC-IN cases (24).
A study including 179 Belgian HCC patients (training set) and 110 Italian patients (validation set) proposed the Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) score, in which the AFP slope together with radiological response to loco-regional therapies were variables allowing to predict the risk for ITT death. In case of longer waiting times (>120 days), the score was better in predicting the risk for ITT death; in case of shorter waiting times, the score was better in identifying the risk for post-LT recurrence (25).
A Belgian study on 137 LT patients revealed that AFP level ≥400 ng/mL (HR =5.1; P<0.0001) was the unique risk factor for HCC recurrence after LT. Conversely, response to neo-adjuvant treatments was a useful prognostic tool for the risk of survival (26).
The 2012 EASL-EORTC (6) and the 2016 EASL Guidelines on LT (27) also value the role of combining variables such as AFP and response to neo-adjuvant approaches as predictors of efficacious treatment, mainly in the context of downstaging procedures.
The EASL-EORTC Guidelines propose to implement for the assessment of response to loco-regional treatments the modification of the RECIST criteria (mRECIST) and the use of the changes in serum levels of biomarkers (i.e., AFP levels) (2B) (6).
The EASL Guidelines on LT also state that HCC deserves a particular MELD exception status based on extra points in order to get access to LT, taking thereby into account size, number of tumor nodules, AFP levels as well as recurrence after downstaging therapy (2B) (27).
A US study showed that morphological and biological response to neo-adjuvant therapies allow to identify patients having a low-risk profile for both drop-out and/or death. A total of 398 T2 HCC listed for LT were analyzed: a subgroup of cases presenting one tumor of 2–3 cm, and both a complete response and AFP level ≤20 ng/mL after the first LRT had an incredibly low 2-year probability of drop-out (only 1.6%) vs. 26.5% in the other patients (P=0.004) (28).
The British Transplant Society (BTS) and Association for the Study of the Liver (BASL) Guidelines recommend that HCC patients out of current UK guidelines but within University of California San Francisco Criteria and who have an AFP level <1,000 ng/mL can be considered for living-donor LT (1B) (29).
This proposed cut-off level of 1,000 ng/mL has been validated in a metacentric French study including 537 HCC LT recipients. A prognostic score based on the combination of morphologic aspects and AFP >1,000 ng/mL was able to identify a subgroup of patients with a high risk of post-LT recurrence (30).
This cut-off was also investigated in a US population of 211 MC-IN patients; interestingly, this cut-off excluded only a 4.7% of patients for LT but contextually reduced HCC recurrence rate by 20% (31).
Curiously, none of the aforementioned Guidelines focused on other biomarkers, despite recent (merely Eastern) studies confirming the remarkable role of PIVKA-II in the prediction of post-LT recurrence (32). Also of note the complete absence of inflammatory markers as possible tools for identifying outcome of HCC patients (33).
AFP: time is ready to reconsider its value?
From many recent well conducted studies and meta-analyses, it becomes obvious that the role of AFP in the diagnostic as well as in the therapeutic process of HCC needs to be reevaluated. Especially, its role in the identification of HCC patients at high-risk for drop-out before and for recurrence after LT must be valued. Five years after the Zurich Guidelines of 2012, the opportunity time is there to fine-tune several recommendations.
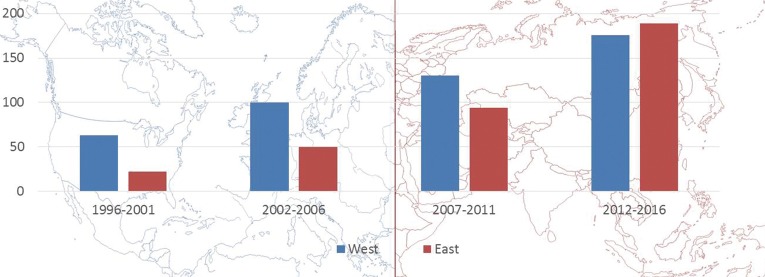
First of all, the discrepancy between Western and Eastern worlds concerning the use of HCC biomarkers should be taken away. As it can be taken out from Figure 2, the number of papers produced in Asia has nowadays clearly outgrown the number of studies originating from Occidental countries. There is now enough evidence, merely coming from Asiatic countries, in order to use both AFP and PIVKA-II in the diagnostic and therapeutic algorithm of HCC patients. The combination of AFP and PIVKA-II raises the sensitivity of detecting HCC, mainly in case of patients with normal AFP/increased DCP levels (16). It is still believed that PIVKA-II is not available in the Western world: however, a slightly growing but consistent number of articles coming from Europe and US have been recently reported, mainly thanks to the possibility to dose DCP also in Western countries (Wako Chemicals GmbH, Neuss, GERMANY) (32,34,35).
Figure 2.
Articles on PubMed focused on AFP, LT and HCC during the period 1996–2016: articles are categorized according to the period and the place of publication. AFP, alpha-fetoprotein; LT, liver transplantation; HCC, hepatocellular cancer.
Another recurring argument is that it is frequently said that Eastern and Western HCC patients are different. However, we were able to show that the main differences between Eastern and Western countries derive more from different approaches that from biological differences or different biological tumor behaviors (36).
If both the US and European Guidelines continue to completely rule out the prognostic role of AFP in the HCC algorithm, because they considered it to still be “under investigation”, the opportunity will be missed to refine markedly the liver allograft allocation and the patient LT selection processes. Moreover, one will stay far away from the todays “real” clinical practice, in which AFP fluctuations are commonly used to monitor patients inserted on the waiting list, and undergoing bridge or downstaging procedures. Time has come to routinely use the determination of AFP in both the diagnostic and therapeutic algorithm of HCC patients (37).
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Trevisani F, D’Intino PE, Morselli-Labate AM, et al. Serum α-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 2001;34:570-5. 10.1016/S0168-8278(00)00053-2 [DOI] [PubMed] [Google Scholar]
- 2.Sizaret P, Tuyns A, Martel N, et al. Alpha-Fetoprotein levels in normal males from seven ethnic groups with different hepatocellular carcinoma risks. Ann N Y Acad Sci 1975;259:136-55. 10.1111/j.1749-6632.1975.tb25410.x [DOI] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. α-fetoprotein for hepatocellular carcinoma diagnosis: the demise of a brilliant star. Gastroenterology 2009;137:26-9. 10.1053/j.gastro.2009.05.014 [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the barcelona-2000 EASL conference. J Hepatol 2001;35:421-30. 10.1016/S0168-8278(01)00130-1 [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 6.European Association For The Study Of The Liver. European Organisation For Research And Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Agopian VG, Harlander-Locke MP, Markovic D, et al. Evaluation of patients with hepatocellular carcinomas that do not produce α-fetoprotein. JAMA Surg 2017;152:55-64. 10.1001/jamasurg.2016.3310 [DOI] [PubMed] [Google Scholar]
- 8.Lai Q, Melandro F, Pinheiro RS, et al. Alpha-fetoprotein and novel tumor biomarkers as predictors of hepatocellular carcinoma recurrence after surgery: a brilliant star raises again. Int J Hepatol 2012;2012:893103. 10.1155/2012/893103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heimbach J, Kulik LM, Finn R, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2017. [Epub ahead of print]. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 11.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. 10.1007/s12072-010-9165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104. 10.1002/hep.21966 [DOI] [PubMed] [Google Scholar]
- 13.Tateishi R, Yoshida H, Matsuyama Y, et al. Diagnostic accuracy of tumor markers for hepatocellular carcinoma: a systematic review. Hepatol Int 2008;2:17-30. 10.1007/s12072-007-9038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KA, Lee JS, Jung ES, et al. Usefulness of serum alpha-fetoprotein (AFP) as a marker for hepatocellular carcinoma (HCC) in hepatitis C virus related cirrhosis: analysis of the factors influencing AFP elevation without HCC development. Korean J Gastroenterol 2006;48:321-6. [PubMed] [Google Scholar]
- 15.Males S, Gad RR, Esmat G, et al. Serum alpha-foetoprotein level predicts treatment outcome in chronic hepatitis C. Antivir Ther 2007;12:797-803. [PubMed] [Google Scholar]
- 16.Hu B, Tian X, Sun J, et al. Evaluation of individual and combined applications of serum biomarkers for diagnosis of hepatocellular carcinoma: a meta-analysis. Int J Mol Sci 2013;14:23559-80. 10.3390/ijms141223559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009;30:37-47. 10.1111/j.1365-2036.2009.04014.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clavien PA, Lesurtel M, Bossuyt PM, et al. OLT for HCC Consensus Group Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol 2012;13:e11-22. 10.1016/S1470-2045(11)70175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merani S, Majno P, Kneteman NM, et al. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol 2011;55:814-9. 10.1016/j.jhep.2010.12.040 [DOI] [PubMed] [Google Scholar]
- 20.Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl 2013;19:634-45. 10.1002/lt.23652 [DOI] [PubMed] [Google Scholar]
- 21.Lai Q, Avolio AW, Manzia TM, et al. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant 2012;26:E125-31. 10.1111/j.1399-0012.2011.01572.x [DOI] [PubMed] [Google Scholar]
- 22.Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015;62:158-65. 10.1002/hep.27787 [DOI] [PubMed] [Google Scholar]
- 23.Lai Q, Vitale A, Iesari S, et al. Intention-to-treat survival benefit of liver transplantation in patients with hepatocellular cancer. Hepatology 2017. [Epub ahead of print]. 10.1002/hep.29342 [DOI] [PubMed] [Google Scholar]
- 24.Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl 2013;19:1108-18. [DOI] [PubMed] [Google Scholar]
- 25.Lai Q, Nicolini D, Inostroza Nunez M, et al. A novel prognostic index in patients with hepatocellular cancer waiting for liver transplantation: Time-Radiological-response-Alpha-fetoprotein-INflammation (TRAIN) score. Ann Surg 2016;264:787-96. 10.1097/SLA.0000000000001881 [DOI] [PubMed] [Google Scholar]
- 26.Ciccarelli O, Lai Q, Goffette P, et al. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int 2012;25:867-75. 10.1111/j.1432-2277.2012.01512.x [DOI] [PubMed] [Google Scholar]
- 27.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol 2016;64:433-85. 10.1016/j.jhep.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 28.Mehta N, Dodge JL, Goel A, et al. Identification of liver transplant candidates with hepatocellular carcinoma and a very low dropout risk: implications for the current organ allocation policy. Liver Transpl 2013;19:1343-53. 10.1002/lt.23753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manas D, Burnapp L, Andrews PA. Summary of the British Transplantation Society UK Guidelines for Living Donor Liver Transplantation. Transplantation 2016;100:1184-90. 10.1097/TP.0000000000001128 [DOI] [PubMed] [Google Scholar]
- 30.Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3. 10.1053/j.gastro.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 31.Hameed B, Mehta N, Sapisochin G, et al. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl 2014;20:945-51. 10.1002/lt.23904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lai Q, Iesari S, Levi Sandri GB, et al. Des-gamma-carboxy prothrombin in hepatocellular cancer patients waiting for liver transplant: a systematic review and meta-analysis. Int J Biol Markers 2017. [Epub ahead of print]. 10.5301/ijbm.5000276 [DOI] [PubMed] [Google Scholar]
- 33.Lai Q, Castro Santa E, Rico Juri JM, et al. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl Int 2014;27:32-41. 10.1111/tri.12191 [DOI] [PubMed] [Google Scholar]
- 34.Chaiteerakij R, Zhang X, Addissie BD, et al. Combinations of biomarkers and Milan criteria for predicting hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl 2015;21:599-606. 10.1002/lt.24117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poté N, Cauchy F, Albuquerque M, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol 2015;62:848-54. 10.1016/j.jhep.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 36.Lai Q, Avolio AW, Lerut J, et al. Recurrence of hepatocellular cancer after liver transplantation: the role of primary resection and salvage transplantation in East and West. J Hepatol 2012;57:974-9. 10.1016/j.jhep.2012.06.033 [DOI] [PubMed] [Google Scholar]
- 37.Lai Q, Avolio AW, Manzia TM, et al. Role of alpha-fetoprotein in selection of patients with hepatocellular carcinoma waiting for liver transplantation: must we reconsider it? Int J Biol Markers 2011;26:153-9. 10.5301/JBM.2011.8557 [DOI] [PubMed] [Google Scholar]