Abstract
Mesenchymal stem cells (MSCs) isolated from various tissues have been well characterized for therapeutic application to clinical diseases. However, in contrast to MSCs from other animal species, the characteristics of feline MSCs have not been fully documented. In this study, we conducted extensive characterization of feline adipose tissue-derived MSCs (fAD-MSCs). Study fAD-MSCs were individually isolated from the intra-abdominal adipose tissues of six felines. The expression levels of cell surface markers and pluripotent markers were evaluated. Next, proliferation capacity was analyzed by performing cumulative population doubling level (CPDL) and doubling time (DT) calculation assays. Differentiation potentials of fAD-MSCs into mesodermal cell lineages were analyzed by examining specific staining and molecular markers. All fAD-MSCs positively expressed cell surface markers such as CD29, CD44, CD90, CD105, CD166, and MHC-I, while CD14, CD34, CD45, and CD73 were negatively expressed. The CPDL of the fAD-MSCs was maintained until passage 5 to 6 (P5 to P6), whereas DT increased after P3 to P4. Also, stem cell-specific pluripotent markers (Oct3/4, Nanog, and SSEA-4) were detected. Importantly, all fAD-MSCs demonstrated mesodermal differentiation capacity. These results suggest that fully characterized fAD-MSCs could be beneficial when considering the use of these cells in feline disease research.
Keywords: adipose tissue-derived mesenchymal stem cells, differentiation, feline, multipotent, proliferation
Introduction
Mesenchymal stem cells (MSCs) are undifferentiated cells that have the potential to regenerate and differentiate into multilineage cells, including adipogenic, chondrogenic, osteogenic, myogenic, and neurogenic cells [6,21,40]. MSCs can be isolated from various tissues such as bone marrow, adipose, and umbilical cord blood [8,10,13]. Compared to other tissues or organs, adipose tissue is a good source for MSCs due to its ease of isolation and plentiful availability with minimal risk to the donor organ [5,21]. Many different animals (porcine, canine, bovine, and equine) have been reported as donors of MSCs [4,7,18,24]. Feline species have been studied for potential isolation and characterization of MSCs. Feline bone marrow-derived MSCs (fBM-MSCs) and feline adipose tissue-derived MSCs (fAD-MSCs) were isolated and characterized by Martin et al. [20], Munoz et al. [23], and Quimby et al. [28]. They reported that fBM-MSCs and fAD-MSCs possess similar morphological features, such as cell surface antigens, and have the ability to differentiate in a manner similar to that of MSCs of other species, including mouse and human. Webb et al. [38] reported a comparative study of fBM-MSCs and fAD-MSCs. They reported that fBM-MSCs and fAD-MSCs have similar morphologies, but that fAD-MSCs have a higher proliferation rate than fBM-MSCs. However, they did not report on the potential of fBM-MSCs and fAD-MSCs to differentiate into multilineage cells. Kono et al. [16] reported a comparative study of feline mature adipocyte-derived dedifferentiated fat cells and fAD-MSCs. However, there is no comprehensive report on fAD-MSC characteristics including their proliferation ability during serial passages, pluripotent markers, molecular surface markers, and differentiation capability.
Oct3/4, Nanog, and Sox2 are specific markers for pluripotency and self-renewal of stem cells. In addition, SSEA-4, an early embryonic glycolipid antigen commonly used a marker for undifferentiated pluripotent embryonic stem cells, can identify adult mesenchymal stem cells [9,12]. Thus the need to examine the expression of Oct3/4, Nanog, Sox2, and SSEA-4 as pluripotent markers of fAD-MSCs. Clinical application of fAD-MSCs to several diseases such as chronic kidney disease [28,29,30], chronic allergic asthma [35], and chronic enteropathy [39] have been attempted. In this study, we carried out extensive characterization, including proliferation ability, pluripotent markers, molecular surface markers, and differentiation capability of fAD-MSCs. The results of this study may be critical in the preparation of fAD-MSCs for clinical applications.
Materials and Methods
Isolation of fAD-MSCs
Adipose tissue was individually collected from six adult female felines (age 0–1 years) during sterilization. Approximately 25 g of intra-abdominal adipose tissue was sampled from each feline. The tissue (n = 6) was washed with phosphate-buffered saline (PBS; Gibco, USA) to remove blood, and blood vessels were dissected out of the adipose tissue with sterile scissors and forceps. The fAD-MSCs were isolated according to a method described previously [17], with minor modifications. Briefly, the tissue was cut into pieces with scissors and incubated in PBS with 0.1% collagenase type I (Gibco) and 1% bovine serum albumin (BSA; Bioworld, USA) at 37℃ for 40 min with continuous shaking. The digested tissues were passed through a cell strainer to remove undigested tissue and then centrifuged at 300 × g for 5 min. The cell pellet obtained was resuspended in low glucose Dulbecco's Modified Eagle's Medium (Gibco), containing 10% heat-inactivated fetal bovine serum (FBS; Gibco), penicillin (100 unit/mL; Gibco), and streptomycin (100 µg/mL; Gibco). Subsequently, the cells were incubated in a tissue culture flask at 37℃ in a 5% CO2 incubator. Next day, the medium was discarded and fresh culture medium added to the cells. On reaching 80% to 90% confluency, the cells were passaged by trypsinization (every 4–5 days). The numbers of cells grown were counted at each passage.
Flow cytometry (FACS) analysis
The fAD-MSCs at passage 2 (P2) were used for identification of cell surface markers. The cells (2.5 × 105 cells) were stained with the following antibodies: conjugated FITC and PE against CD14 (antibody clone TÜK4, MCA1568F; AbD Serotec, UK), CD34 (antibody clone 581, 555821; BD Bioscience, USA), CD44 (antibody clone IM7, ab19622; Abcam, UK), CD45 (antibody clone HI30, 555482; BD Bioscience), CD90 (antibody clone 5E10, 555596; BD Bioscience), and CD105 (antibody clone SN6, MCA1557F; AbD Serotec). The fAD-MSCs for non-specific binding control were stained with the following antibodies: FITC-conjugated mouse IgG1 κ (antibody clone MOPC-21, 555748; BD Bioscience), PE-conjugated mouse IgG1 (antibody clone A85-1, 550083; BD Bioscience), FITC-conjugated mouse IgG2a κ (antibody clone eBM2a, 11-4724-42; eBioscience, USA), and FITC-conjugated rat IgG2b κ (antibody clone A95-1, 553988; BD Bioscience). Cells were analyzed by using a BD FACSCalibur flow cytometer (BD Bioscience). CellQuest Pro software (BD Bioscience) was used for FACS data analysis.
Gene expression analysis with reverse transcriptase polymerase chain reaction (RT-PCR) and quantitative real-time RT-PCR (qRT-PCR)
Total RNA was extracted from fAD-MSCs at P2 by using the RNeasy Mini Kit (Qiagen, Germany). RNA concentration and quality was measured by using a Nano-drop 1000 spectrophotometer (Thermo Fisher, USA). The cDNA was generated by combining total RNA (1 µg), reverse primers (10 pmol), and superscript II reverse transcriptase (Invitrogen, USA). GoTaq DNA polymerase (Promega, USA) was used for RT-PCR. For RT-PCR analysis, target genes underwent predenaturation at 95℃ (5 min) and amplification in 35 cycles of 95℃ (45 sec), 58℃ (45 sec) or 60℃ (45 sec), and 72℃ (45 sec), followed by a final extension at 72℃ (5 min). For qRT-PCR analysis, a LightCycler 480 SYBR Green I Master kit (Roche Diagnostics, Switzerland) was used. The following qRT-PCR program was used: predenaturation at 95℃ for 10 min, amplification in 45 cycles of annealing at 58℃ or 60℃ for 10 sec, and elongation at 72℃ for 10 sec. Melting curve analysis was performed between 65℃ and 97℃. Each sample was analyzed in triplicate, and the qRT-PCR result was calculated by determining the threshold cycle (Ct) value. PCR products were separated on 2% agarose gel by electrophoresis, stained with Red Safe (iNtRon Biotechnology, Korea), and visualized under UV light. Each image was digitally captured using a CCD camera (Gel Doc, Bio-Rad Laboratories, USA). GAPDH was used in every PCR analysis for internal control and reference. We referred to a previous study [33] to determine the RT-PCR and qRT-PCR primers, except for the primer for Sox2 (NM_001173447.1).
Cumulative population doubling level (CPDL) and cell doubling time (DT) calculation
The fAD-MSCs were seeded at a density of 5 × 104 cells per well in a 6-well cell culture plate and maintained for 5 days until 80% to 90% confluence was reached. Cells were passaged every 5 days with trypsinization to the single cell for the CPDL. During continuous passages, the number of fAD-MSCs at both the seeding and harvesting times were determined to calculate the CPDL and DT. We calculated CPDL and DT using the formula:
| CPDL = ln(Nf/Ni)ln2 and DT = CT/CPDL, |
where, Ni is the initial cell number, Nf is the harvest cell number, In is the natural log, and CT is cell culture time for each passage [36].
Immuno-fluorescence staining (IFS)
The fAD-MSCs at P2 were fixed in 4% formaldehyde (Sigma-Aldrich, USA) in PBS for 15 min at room temperature (RT), washed twice with PBS, and permeabilized in 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 3 min at RT. Blocking was done by using 1% BSA in PBS for 60 min at RT. For multipluripotent marker analysis, the fixed fAD-MSCs were incubated for overnight at 4℃ with primary antibodies (1:100): Oct3/4 (antibody clone H-134; sc-9081, Santa Cruz Biotechnology, USA), Nanog (antibody clone RCK107, ab9220; Millipore, USA), Sox2 (antibody clone 245610, MAB2018; R&D Systems, USA), and SSEA-4 (antibody clone MC-813-70, MAB4304; R&D Systems). After washing with PBS, the fAD-MSCs were incubated with secondary antibodies (1:500): FITC-conjugated goat anti-mouse (A90-244F; Bethyl Laboratories, USA) and FITC-conjugated goat anti-rabbit (sc-2012; Santa Cruz Biotechnology), for 90 min at RT. After washing three times each, with 0.01% Triton X-100 in PBS and PBS, the fAD-MSCs were counterstained with DAPI (Santa Cruz Biotechnology). The stained fAD-MSCs were examined by using an Olympus FluoView confocal microscope (Olympus, Japan).
In vitro differentiation
Adipogenesis: For adipogenic differentiation, the fAD-MSCs at P2 were seeded at a density of 7.5 × 104 cells/well in 12-well tissue culture plates. The adipogenic medium was supplemented with 1 µM dexamethasone (Sigma-Aldrich), 200 µM indomethacin (Sigma-Aldrich), 500 µM isobutyl methyl xanthine (Sigma-Aldrich), and 20 µg/mL insulin (Sigma-Aldrich). Medium was changed every 3–4 days, and adipogenesis was induced for 21 days. The cells were stained with an Oil Red O stain kit (NovaUltra; IHC World, USA) for observation of red-colored lipid vacuoles in differentiated cells.
Chondrogenesis: For chondrogenic differentiation, 1 × 106 fAD-MSCs at P2 in 5 µL droplets of the growth medium were seeded in 4-well tissue culture plates; after 6 h, chondrogenic medium containing 100 nM dexamethasone, 25 µM ascorbic acid 2-phosphate (Sigma-Aldrich), and 1 ng/mL TGF-β (Lonza, USA) was added. Chondrogenesis was induced for 21 days, with changes to fresh medium every 3–4 days. To confirm chondrogenic differentiation, we used an Alcian Blue stain kit (NovaUltra; IHC World) and checked for blue color staining of proteoglycans.
Osteogenesis: For osteogenic differentiation, 7.5 × 104 cells/well at P2 were seeded on 12-well tissue culture plates, and grown in osteogenic differentiation medium (PT-4120; Lonza) for 21 days with a medium change every 3–4 days. We observed the orange-red colored calcium deposits by staining with an Alizarin Red stain kit (NovaUltra; IHC World).
Statistical analysis
Data from three independent experiments were analyzed by performing one-way ANOVA (analysis of variance) and data are presented as mean ± SD values. Differences among two groups were compared by using Student's t-test (JMP 6.0; SAS Institute, USA). Values of p < 0.05 were considered statistically significant.
Results
Isolation and proliferation ability of fAD-MSCs and expression of MSC surface markers
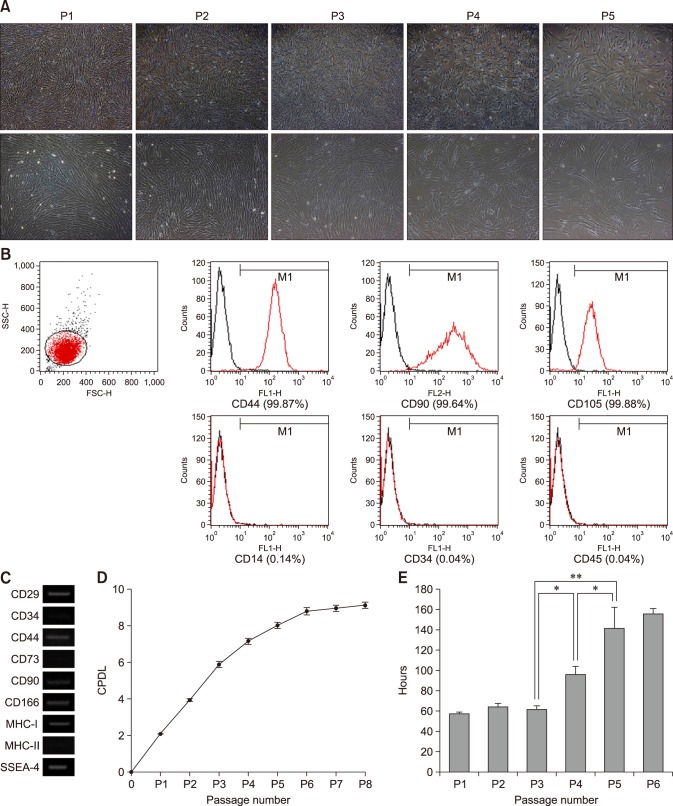
The fAD-MSCs were isolated from feline intra-abdominal adipose tissues (n = 6) and grown in plastic tissue culture flasks. The cells had a fibroblast-like spindle shape and formed a highly homogenous monolayer (panel A in Fig. 1). The fAD-MSCs were evaluated for the expression of cell surface markers such as CD29, CD34, CD44, CD73, CD90, CD166, MHC-I, and MHC-II by performing FACS and RT-PCR at P2. The FACS analysis revealed that the fAD-MSCs were strongly positive for CD44, CD90, and CD105, but negative for CD14, CD34, and CD45 at P2 (panel B in Fig. 1). The RT-PCR results showed that fAD-MSCs positively expressed CD29, CD44, CD90, CD166, and MHC-I, but negatively expressed CD34, CD73, and MHC-II at P2 (panel C in Fig. 1). During six consecutive passages, the CPDL of the fAD-MSCs steadily increased until P4 or P5. Individual passage CPDL results were: P1 (2.08 ± 0.02), P2 (3.95 ± 0.07), P3 (5.88 ± 0.15), P4 (7.14 ± 0.15), P5 (8.00 ± 0.16), and P6 (8.78 ± 0.19). There was little CPDL increase after P6 (panel D in Fig. 1). In accordance with the CPDL results, the DT values significantly increased to 95.7 ± 7.80 h (P4) and 141.3 ± 20.59 h (P5) from 62.1 ± 2.86 h (P3) (panel E in Fig. 1).
Fig. 1. Expression of MSC surface markers and proliferation ability of fAD-MSCs. (A) Typically, cell morphology was fibroblast-like. (B and C) FACS and RT-PCR analysis: fAD-MSCs positively expressed CD29, CD44, CD90, CD105, CD166, and MHC-I, but CD14, CD34, CD45, CD73, and MHC-II were negatively expressed at P2. (C) GAPDH is shown as a control for RNA sample quality. (D) Cumulative population doubling level (CPDL) linearly increased until P4 or P5, and (E) doubling time (DT) did not increase until P3. CPDL and DT data from three independent experiments are expressed as mean ± SD values. 40× (upper panels in A), 100× (lower panels in A). *p < 0.05, **p < 0.001.
Expression of pluripotent stem cell markers
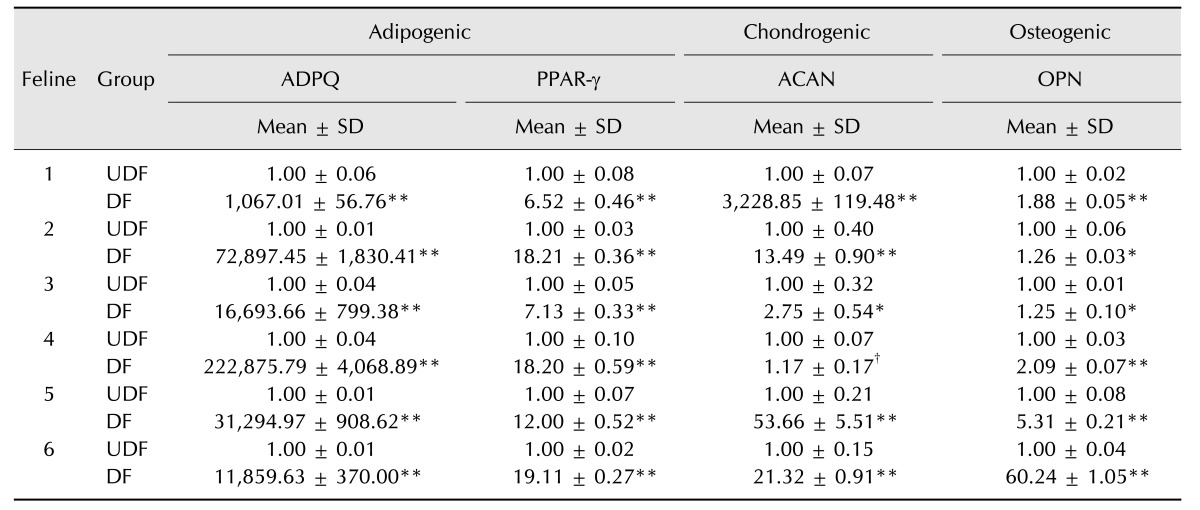
Expression of pluripotent stem cell markers in fAD-MSCs at P2 was observed by using RT-PCR (Oct3/4, Nanog, and Sox2; panel A in Fig. 2) and IFS (Oct3/4, Nanog, Sox2, and SSEA-4; panel B in Fig. 2). The fAD-MSCs positively expressed Oct3/4, Nanog, and SSEA-4, but negatively expressed Sox2. Similar results were obtained from both RT-PCR and IFS.
Fig. 2. Expression of pluripotent markers in fAD-MSCs at passage 2. (A and B) Images from RT-PCR and IFS analyses: fAD-MSCs positively expressed Oct3/4, Nanog, and SSEA-4, but Sox2 was negatively expressed. (A) GAPDH is shown as a control for RNA sample quality. 140×.
Multi-lineage differentiation ability
To determine their ability to differentiate, fAD-MSCs at P2 were induced to differentiate into adipocytes, chondrocytes, and osteocytes by using modified differentiation medium for adipocytes and chondrocytes and osteogenic medium for osteocytes.
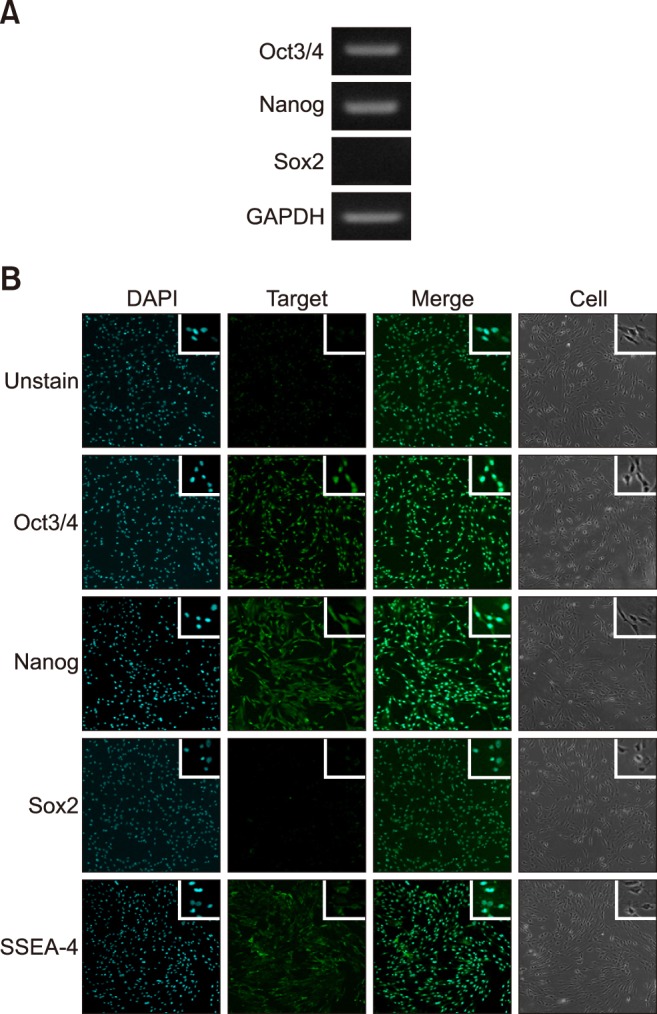
Adipogenesis: After 21 days of culture, the cells were stained with Oil Red O and examined for the presence of intracellular lipid accumulation. The differentiated cells showed many stained lipid droplets, but the non-induced control cells did not stain positively (panel A in Fig. 3). Based on the qRT-PCR analysis, mRNA expression levels of adipogenic markers such as adiponectin (ADPQ) and peroxisome proliferator-activated receptor γ (PPAR-γ) were significantly high in the differentiated cells (Table 1).
Fig. 3. Multilineage differentiation potential of fAD-MSCs at P2. (A) fAD-MSCs, after being induced in adipogenic differentiation medium, formed lipid droplets. (B) MSCs formed proteoglycans when incubated in chondrogenic differentiation medium. (C) Calcium mineralization was formed after incubation in osteogenic differentiation medium. Oil Red O (A; red), Alcian Blue (B; blue), and Alizarin Red (C; orange red). 200× (A and B), 100× (C).
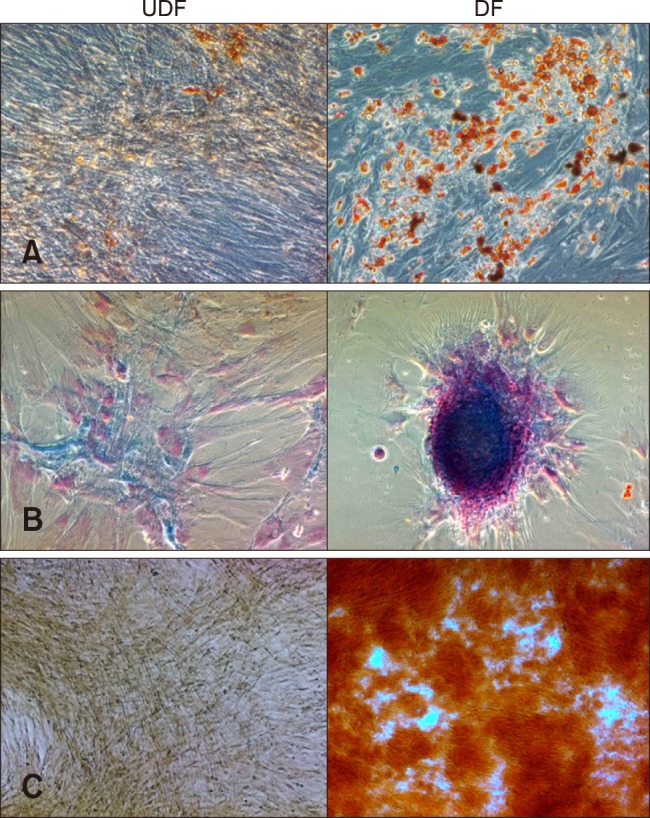
Table 1. Relative expression levels of each differentiation marker.
Expression levels were normalized to the level of GAPDH mRNA. The levels of expression from three independent experiments were evaluated and are expressed as mean ± SD values. ADPQ, adiponectin; PPAR-γ, peroxisome proliferator-activated receptor γ; ACAN, aggrecan; OPN, osteopontin; UDF, undifferentiation; DF, differentiation. UDF vs. DF (*p < 0.05, **p < 0.001, †p > 0.05).
Chondrogenesis: After 21 days of culture, the cells were stained with Alcian Blue and examined for the presence of proteoglycans. The proteoglycans were stained blue in the differentiated cells, but positive staining was not detected in the non-induced control cells (panel B in Fig. 3). The qRT-PCR results showed that the mRNA expression level of the chondrogenic marker aggrecan (ACAN) was highly elevated in the differentiated cells (Table 1).
Osteogenesis: After 21 days of culture, the cells were stained with Alizarin Red and analyzed for the presence of calcium mineralization. Calcium staining was evident within the differentiated cells, but the control cells did not show any calcium mineralization (panel C in Fig. 3). Analysis of the qRT-PCR results revealed that the mRNA expression level of the osteogenic marker osteopontin (OPN) was significantly higher in the differentiated cells than in non-induced control cells (Table 1).
Discussion
The therapeutic potentials of adipose tissue-derived MSCs (AD-MSCs) have been investigated in many animal studies. For example, canine AD-MSCs were shown to be clinically effective for orthopedic condition and hip dysplasia treatments [3,11,21,22,37]. In addition, equine AD-MSCs have shown beneficial effects on the clinical signs of tendonitis, endometriosis, and bone spavin [19,21,25,31]. Clinical trials using fAD-MSCs have also been reported. A case of chronic enteropathy was significantly improved after fAD-MSCs treatment with no side effects observed [39]. Other studies have reported no significant effects in chronic kidney disease and chronic allergic asthma after stem cell therapy [21,28,29,30,35]. However, regardless of the active clinical trials, fAD-MSCs have not been fully characterized in terms of their proliferation activity, surface markers, pluripotent markers, and quantitative differentiation capacity; characteristics that are highly important in clinical applications of multipotent MSCs [6,21,24,36]. Therefore, in this study, we undertook extensive characterization of MSCs isolated from feline intra-abdominal adipose tissue.
In this study, we successfully isolated MSCs from intra-abdominal adipose tissues of six felines. The fAD-MSCs appeared to have the typical fibroblast-like shape associated with MSCs. During serial passages, the fAD-MSCs lost their proliferation potency after P4 or P5. In comparison with AD-MSCs of other species, fAD-MSCs demonstrate relatively low proliferation activity. In canine and miniature pig, AD-MSCs maintain their proliferative activity until P10 [14] and P14 [17], respectively. Therefore, for therapeutic application, fAD-MSCs may have to be prepared at early passage.
The results of our RT-PCR and FACS analyses confirmed the presence of MSC surface markers (CD29+/CD44+/CD90+/CD105+/CD34−) and major histocompatibility complex (MHC) molecules (MHC-I+/MHC-II−), which were also observed in previous studies [16,20,23,28,38]. The lack of MHC-II expression may confer upon fAD-MSCs a beneficial effect in allogeneic cell therapies due to their low immunogenicity [33]. A notable new result in our study was our observation of no expression of CD73 on fAD-MSCs; CD73 has been previously reported to be a MSC marker [6,27]. In addition, we detected expression of CD166 on fAD-MSCs. CD166, referred to as an activated leukocyte-cell adhesion molecule, is considered to be a marker of MSCs, to define MSCs when coexpressed with CD105, and to have a role in osteogenic differentiation potential [1,2].
The expressions of pluripotent genes Oct3/4, Nanog, Sox2, and SSEA-4 were examined in fAD-MSCs in our study. Oct3/4 and Nanog were expressed in fAD-MSCs, but Sox2 expression was not detected. Munoz et al. [23] showed that Oct3/4 and Sox2 are expressed in undifferentiated fBM-MSCs. In addition, Han et al. [12] and Park et al. [26] showed that Sox2 has an important role in the enhanced proliferation of MSCs. Therefore, the lack of expression of Sox2 in fAD-MSCs could be one reason for the low proliferation activity of fAD-MSCs. In addition, MSCs have tissue-specific characteristics. Riekstina et al. [32] reported that human BM-MSCs did not express Sox2, but other tissues, AD, dermis, and heart-derived MSCs express Sox2. In canine, Takemitsu et al. [34] reported qRT-PCR results showing that AD-MSCs and BM-MSCs had different gene expression levels of Nanog. As MSCs have tissue-specific characteristics [32], the lack of expression of Sox2 in our fAD-MSCs suggests the presence of tissue-specific expression in feline intra-abdominal adipose tissue MSCs.
SSEA-4 is a marker of human embryonic stem cells (ESCs) and is expressed in very early cleavage to blastocyst stage embryos. SSEA-4 is a surface marker associated with ESC pluripotency and differentiation. Gang et al. [9] reported that mouse bone marrow-derived MSCs expressed SSEA-4. MSCs in canine adipose and bone marrow did not express SSEA-4 [34]. However, equine bone marrow, amniotic fluid, and umbilical cord matrix-derived and porcine amniotic fluid-derived MSCs have been shown to express SSEA-4 [7,18]. Kokkinaki et al. [15] used SSEA-4 as an indicator marker for the isolation of stem cells from human testes. In addition, they showed that a SSEA-4-positive subpopulation highly expressed spermatogonial stem cell markers, and that the subpopulation was maintained over the long term. In our study, we observed the expression of SSEA-4 in fAD-MSCs.
Importantly, we also assessed fAD-MSC differentiation capability. The fAD-MSCs were shown to successfully differentiate into multilineage cells (adipogenic, chondrogenic, and osteogenic cell line differentiation). Rutigliano et al. [33] reported that differentiated feline progenitor-like amniotic epithelial cells did not express ADPQ after induction into adipocytes. We examined the expression levels of the mesodermal lineage markers ADPQ, PPAR-γ, ACAN, and OPN by performing qRT-PCR. Although there were differences in the expression levels of these markers among the fAD-MSCs obtained from six felines, their expression levels in differentiated cells were significantly higher than those in undifferentiated cells.
MSCs are attractive vehicles for developing cell-based therapies and for the study of diseases. In the present study, we successfully isolated and extensively characterized MSCs obtained from the intra-abdominal adipose tissues of six felines. We confirmed that the features and potentials of fAD-MSCs are similar to those in various other animal MSCs. We suggest that the results of this study can be used as a reference for further improvement of isolation and characterization of fAD-MSCs, which can be useful in subsequent implementation of various therapeutic applications.
Acknowledgments
This work was funded by the Animal and Plant Quarantine Agency (N-1541780-2013-22-02), Korea.
Footnotes
Conflict of Interest: The authors declare no conflict of interests.
References
- 1.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–1532. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 2.Arai F, Ohneda O, Miyamoto T, Zhang XQ, Suda T. Mesenchymal stem cells in perichondrium express activated leukocyte cell adhesion molecule and participate in bone marrow formation. J Exp Med. 2002;195:1549–1563. doi: 10.1084/jem.20011700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black LL, Gaynor J, Adams C, Dhupa S, Sams AE, Taylor R, Harman S, Gingerich DA, Harman R. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther. 2008;9:192–200. [PubMed] [Google Scholar]
- 4.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Isolation and multilineage differentiation of bovine bone marrow mesenchymal stem cells. Cell Tissue Res. 2005;319:243–253. doi: 10.1007/s00441-004-1012-5. [DOI] [PubMed] [Google Scholar]
- 5.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Lu Z, Cheng D, Peng S, Wang H. Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells. PLoS One. 2011;6:e19964. doi: 10.1371/journal.pone.0019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 9.Gang EJ, Bosnakovski D, Figueiredo CA, Visser JW, Perlingeiro RCR. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109:1743–1751. doi: 10.1182/blood-2005-11-010504. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S, Franklin DM, Leddy HA, Robey PG, Storms RW, Gimble JM. Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 11.Guercio A, Di Marco P, Casella S, Cannella V, Russotto L, Purpari G, Di Bella S, Piccione G. Production of canine mesenchymal stem cells from adipose tissue and their application in dogs with chronic osteoarthritis of the humeroradial joints. Cell Biol Int. 2012;36:189–194. doi: 10.1042/CBI20110304. [DOI] [PubMed] [Google Scholar]
- 12.Han SM, Han SH, Coh YR, Jang G, Chan Ra J, Kang SK, Lee HW, Youn HY. Enhanced proliferation and differentiation of Oct4- and Sox2-overexpressing human adipose tissue mesenchymal stem cells. Exp Mol Med. 2014;46:e101. doi: 10.1038/emm.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 14.Kang BJ, Ryu HH, Park SS, Koyama Y, Kikuchi M, Woo HM, Kim WH, Kweon OK. Comparing the osteogenic potential of canine mesenchymal stem cells derived from adipose tissues, bone marrow, umbilical cord blood, and Wharton's jelly for treating bone defects. J Vet Sci. 2012;13:299–310. doi: 10.4142/jvs.2012.13.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokkinaki M, Djourabtchi A, Golestaneh N. Long-term culture of human SSEA-4 positive spermatogonial stem cells (SSCs) J Stem Cell Res Ther. 2011;2:pii: 2488. doi: 10.4172/2157-7633.S2-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kono S, Kazama T, Kano K, Harada K, Uechi M, Matsumoto T. Phenotypic and functional properties of feline dedifferentiated fat cells and adipose-derived stem cells. Vet J. 2014;199:88–96. doi: 10.1016/j.tvjl.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Lee AY, Lee J, Kim CL, Lee KS, Lee SH, Gu NY, Kim JM, Lee BC, Koo OJ, Song JY, Cha SH. Comparative studies on proliferation, molecular markers and differentiation potential of mesenchymal stem cells from various tissues (adipose, bone marrow, ear skin, abdominal skin, and lung) and maintenance of multipotency during serial passages in miniature pig. Res Vet Sci. 2015;100:115–124. doi: 10.1016/j.rvsc.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Lovati AB, Corradetti B, Lange Consiglio A, Recordati C, Bonacina E, Bizzaro D, Cremonesi F. Comparison of equine bone marrow-, umbilical cord matrix and amniotic fluidderived progenitor cells. Vet Res Commun. 2011;35:103–121. doi: 10.1007/s11259-010-9457-3. [DOI] [PubMed] [Google Scholar]
- 19.Mambelli LI, Mattos RC, Winter GHZ, Madeiro DS, Morais BP, Malschitzky E, Miglino MA, Kerkis A, Kerkis I. Changes in expression pattern of selected endometrial proteins following mesenchymal stem cells infusion in mares with endometrosis. PLoS One. 2014;9:e97889. doi: 10.1371/journal.pone.0097889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–886. doi: 10.1016/s0301-472x(02)00864-0. [DOI] [PubMed] [Google Scholar]
- 21.Marx C, Silveira MD, Nardi NB. Adipose-derived stem cells in veterinary medicine: characterization and therapeutic applications. Stem Cells Dev. 2015;24:803–813. doi: 10.1089/scd.2014.0407. [DOI] [PubMed] [Google Scholar]
- 22.Marx C, Silveira MD, Selbach I, da Silva AS, de Macedo Braga LMG, Camassola M, Nardi NB. Acupoint injection of autologous stromal vascular fraction and allogeneic adipose-derived stem cells to treat hip dysplasia in dogs. Stem Cells Int. 2014;2014:391274. doi: 10.1155/2014/391274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz JL, Greco SJ, Patel SA, Sherman LS, Bhatt S, Bhatt RS, Shrensel JA, Guan YZ, Xie G, Ye JH, Rameshwar P, Siegel A. Feline bone marrow-derived mesenchymal stromal cells (MSCs) show similar phenotype and functions with regards to neuronal differentiation as human MSCs. Differentiation. 2012;84:214–222. doi: 10.1016/j.diff.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neupane M, Chang CC, Kiupel M, Yuzbasiyan-Gurkan V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng Part A. 2008;14:1007–1015. doi: 10.1089/ten.tea.2007.0207. [DOI] [PubMed] [Google Scholar]
- 25.Nicpoń J, Marycz K, Grzesiak J. Therapeutic effect of adipose-derived mesenchymal stem cell injection in horses suffering from bone spavin. Pol J Vet Sci. 2013;16:753–754. doi: 10.2478/pjvs-2013-0107. [DOI] [PubMed] [Google Scholar]
- 26.Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK, Kang KS. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012;19:534–545. doi: 10.1038/cdd.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 28.Quimby JM, Webb TL, Gibbons DS, Dow SW. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: a pilot study. J Feline Med Surg. 2011;13:418–426. doi: 10.1016/j.jfms.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quimby JM, Webb TL, Habenicht LM, Dow SW. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: results of three sequential pilot studies. Stem Cell Res Ther. 2013;4:48. doi: 10.1186/scrt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quimby JM, Webb TL, Randall E, Marolf A, Valdes-Martinez A, Dow SW. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: a randomized, placebo-controlled clinical trial in eight cats. J Feline Med Surg. 2016;18:165–171. doi: 10.1177/1098612X15576980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricco S, Renzi S, Del Bue M, Conti V, Merli E, Ramoni R, Lucarelli E, Gnudi G, Ferrari M, Grolli S. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol. 2013;26(1 Suppl):61–68. doi: 10.1177/03946320130260s108. [DOI] [PubMed] [Google Scholar]
- 32.Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G, Muiznieks I, Muceniece R, Ancans J. Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev. 2009;5:378–386. doi: 10.1007/s12015-009-9094-9. [DOI] [PubMed] [Google Scholar]
- 33.Rutigliano L, Corradetti B, Valentini L, Bizzaro D, Meucci A, Cremonesi F, Lange-Consiglio A. Molecular characterization and in vitro differentiation of feline progenitor-like amniotic epithelial cells. Stem Cell Res Ther. 2013;4:133. doi: 10.1186/scrt344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemitsu H, Zhao D, Yamamoto I, Harada Y, Michishita M, Arai T. Comparison of bone marrow and adipose tissue-derived canine mesenchymal stem cells. BMC Vet Res. 2012;8:150. doi: 10.1186/1746-6148-8-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trzil JE, Masseau I, Webb TL, Chang CH, Dodam JR, Cohn LA, Liu H, Quimby JM, Dow SW, Reinero CR. Long-term evaluation of mesenchymal stem cell therapy in a feline model of chronic allergic asthma. Clin Exp Allergy. 2014;44:1546–1557. doi: 10.1111/cea.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vidal MA, Kilroy GE, Johnson JR, Lopez MJ, Moore RM, Gimble JM. Cell growth characteristics and differentiation frequency of adherent equine bone marrow-derived mesenchymal stromal cells: adipogenic and osteogenic capacity. Vet Surg. 2006;35:601–610. doi: 10.1111/j.1532-950X.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 37.Vilar JM, Batista M, Morales M, Santana A, Cuervo B, Rubio M, Cugat R, Sopena J, Carrillo JM. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Vet Res. 2014;10:143. doi: 10.1186/1746-6148-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb TL, Quimby JM, Dow SW. In vitro comparison of feline bone marrow-derived and adipose tissue-derived mesenchymal stem cells. J Feline Med Surg. 2012;14:165–168. doi: 10.1177/1098612X11429224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webb TL, Webb CB. Stem cell therapy in cats with chronic enteropathy: a proof-of-concept study. J Feline Med Surg. 2015;17:901–908. doi: 10.1177/1098612X14561105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin. 2013;34:747–754. doi: 10.1038/aps.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]