Abstract
Small-breed dogs (n = 168; weight < 15 kg) diagnosed with myxomatous mitral valve degeneration based on a routine clinical examination, radiology, electrocardiography, and echocardiography at the Seoul National University Veterinary Medical Teaching Hospital were included in this study. Survival periods were determined, and there were significant differences in survival rates among the three International Small Animal Cardiac Health Council classes. The mean follow-up period was 14.3 ± 12.1 months. Univariate analysis revealed that dyspnea, pulmonary edema, and vertebral heart score were significantly associated with survival time (p < 0.05). Additionally, age, left atrial-to-aortic root ratio, ejection fraction, and left ventricular end diastolic volume were associated with an increased risk of death (p < 0.1), while body weight, body condition score, systolic blood pressure, arrhythmia, syncope, fractional shortening, and end systolic volume were not associated with an increased risk of death. These results suggest that among the assessed variables dyspnea, pulmonary edema, and vertebral heart score could be useful prognostic factors for providing patient information to owners.
Keywords: congestive heart failure, mitral valve disease, prognosis, survival rate, vertebral heart score
Introduction
Atrioventricular valve disease is the most common cardiovascular disease in small-breed dogs, accounting for more than 70% of reported heart disease cases [10]. The mitral valve is more susceptible than the tricuspid valve to disease and readily undergoes myxomatous degeneration, resulting in myxomatous mitral valve degeneration (MMVD) [4]. It has been reported that Cavalier King Charles Spaniels, Miniature Poodles, Miniature Schnauzers, Chihuahuas, Pomeranians, Fox Terriers, Cocker Spaniels, and Pekingese breeds tend to be particularly prone to MMVD [10]. The progression of MMVD leads to congestive heart failure and finally might induce dyspnea, syncope, cyanosis, and even death. In addition, other changes, such as hypertension, pulmonary edema (PE), and arrhythmia can occur [10]. Diagnosis of MMVD requires a thorough physical examination including auscultation, and, for confirmation, echocardiography should be carried out [13]. During echocardiography, mitral regurgitation can be imaged by using color-flow Doppler imaging [1]. Although many medications (e.g., diuretics, pimobendan, and ACE inhibitors) are available to relieve the symptoms of MMVD [6,15,17], they are only effective for symptom relief. Thus, expectation of the affected animal's lifespan based on the clinical symptoms during treatment is important in order when providing prognostic information about the dog to its owners. However, many symptoms are possible, and which parameter is an important factor in patient prognosis is unclear.
MMVD is particularly evident in the smallest dog breeds [11]; however, few studies have evaluated factors affecting the survival time of dogs with MMVD [2]. The aim of this retrospective study was to describe the signalment and clinical symptoms of 168 small-breed dogs diagnosed with MMVD based on a routine examination and echocardiographic results and to retrospectively review the prognostic variables for and survival time of small-breed dogs with MMVD.
Materials and Methods
Study population
The medical records of small-breed dogs (< 15 kg) examined at the Seoul National University Veterinary Medical Teaching Hospital (SNU VMTH) from 2010 to 2013 were reviewed. One hundred and sixty-eight dogs diagnosed with MMVD during that four-year period were eligible for inclusion in the study. All of the dogs underwent a general clinical examination and a cardiology consultation. During the routine clinical examination, cardiac auscultation and general physical examination were performed to investigate whether the clinical symptoms (i.e., cough and dyspnea) were caused by a cardiovascular disorder, and echocardiography was used to confirm the diagnosis.
Inclusion criteria
Small-breed dogs, weighing less than 15 kg, were included in this study. Physical, chest radiograph, and echocardiographic examinations were performed for all dogs. Some dogs with concurrent disease such as chronic kidney disease, liver failure or hormonal disease (e.g., Cushing's disease, diabetes mellitus, or hypothyroidism) were included only if the condition was well managed with medicinal therapy.
The numbers of dogs showing clinical signs such as cough, cyanosis, dyspnea, exercise intolerance, and syncope were determined based on the chief complaints of owners and the results of physical examination at first visit. If that resting respiratory rate was ≥ 60 breaths/min and was accompanied by shortness of breath, the dog categorized as having dyspnea. One or a combination of echocardiographic features, such as color Doppler identification of any degree of mitral valve regurgitation, two-dimensional (2D) detection of mitral valve prolapse, any degree of mitral valve leaflet thickening or both, were required for inclusion in the study. Dogs were classified as International Small Animal Cardiac Health Council (ISACHC) class I if they did not have present or past clinical signs of congestive heart failure or radiographic evidence of cardiac enlargement. Dogs were classified as ISACHC class II if they had mild to moderate clinical signs at rest or with mild exercise and heart enlargement on thoracic radiographs. Dogs with obvious clinical signs were classified as ISACHC class III.
Exclusion criteria
Dogs with congenital heart disease, bacterial endocarditis, dilated cardiomyopathy, or heartworm infections were excluded because these conditions may have a direct or indirect influence on mitral valve function. Animals with other medical problems that could significantly affect their survival were also excluded (e.g., dogs that had sepsis, neoplasia or immune-mediated hemolytic anemia). Any dogs with clinical evidence of respiratory disease such as the presence of moderate to marked bronchial, interstitial, or alveolar pulmonary patterns were excluded.
Study design
All data, such as signalments (sex, breed, age, body weight, and body condition score [BCS]), clinical symptoms at presentation, progression of symptoms after initial diagnosis, general physical examination (auscultation, arrhythmia, and systolic blood pressure [SBP]), electrocardiogram results, concurrent heart problems, vertebral heart score (VHS), echocardiographic results, and medications being administered, in addition to information on the survival of each dog, were collected retrospectively. Signalments, physical examination results, clinical symptoms, and treatment history were obtained from each case record. The BCS was assessed by using a nine-point body condition scoring system. Heart murmur was graded into 4 scales. The SBP was measured by performing Doppler sphygmomanometry at the first visit to the SNU VMTH. An electrocardiogram was obtained with the animal in a right lateral recumbent position, and the result was interpreted and confirmed by veterinary interns and residents in the Department of Veterinary Internal Medicine. Concurrent cardiovascular diseases, such as tricuspid valve insufficiency (TVI) and PE, were diagnosed via radiography. When tracheal collapse (TC) was suspected, its presence was confirmed by using fluoroscopy.
As part of the cardiac examination, thoracic radiographs were taken left-laterally and ventrodorsally, and the VHS was measured. All dogs underwent complete echocardiographic examination, including transthoracic 2-dimensional, M-mode, spectral, and color-flow Doppler, by three experienced echocardiographers. Experienced echocardiographers in the Department of Veterinary Medical Imaging at SNU VMTH performed the echocardiography with an ultrasound system (ProSound Alpha7; Hitachi, Japan) equipped with a 3–8 MHz phase array sector transducer (Hitachi). A lead II electrocardiogram was simultaneously displayed on the ultrasound monitor for timing purposes. All echocardiographic values were measured in same manner and according to the recommendations of the Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine by using standard methods [14,18]. Measurements of the M-mode left ventricular parameters were obtained from 2D guided M-mode echocardiography in the right parasternal short-axis view at the level of the papillary muscles [12]. End diastolic and end systolic volumes (EDV and ESV, respectively) of the left ventricle were calculated by using the Teichholz method [16]. The left ventricular ejection fraction (EF) was calculated by using EF = (EDV – ESV)/EDV × 100. Fractional shortening (FS) was calculated from the left ventricular end diastolic (LVDd) and systolic (LVDs) dimensions by using FS = (LVDd – LVDs)/LVDd × 100. Both EF and FS were determined on the short axis of the M-mode. Peak velocity of tricuspid regurgitation (TR) was obtained under color Doppler guidance. Systolic pulmonary pressure was diagnosed if the peak TR velocity was > 2.8 m/sec.
Assessment of the left atrial (LA) and aortic root (Ao) dimensions was performed from the right parasternal short-axis view at the heart base, and the ratio between LA and Ao was calculated (LA/Ao) [8]. All measurements were averaged from data during three cardiac cycles. All results were confirmed by a cardiologist of the Department of Veterinary Internal Medicine. Dogs with heart disease were classified into ISACHC heart failure (HF) class I, II, or III. All data including echocardiograms with digital recordings were reviewed by experienced investigators.
A telephone interview with the owner(s) of each enrolled dog was used to ascertain the animal's clinical or survival status. First, the owner was asked whether the dog had survived. If the dog had died, the date of death and its clinical status at the time of death were recorded. In cases where the owner could not be contacted, we contacted veterinarians in the local hospital that had overseen the care of the dog and asked about the animal's clinical status or survival.
Statistical analysis
Statistical analysis was performed by using R package, ver. 3.1.1. Numerical variables are reported as mean ± SD values. The effects of 22 clinical, electrocardiogram, echocardiographic, and Doppler variables on survival were evaluated. The clinical variables included sex, arrhythmia, syncope, dyspnea, PE, age > 11 years, weight > 5 kg, heart rate > 140 beats/min, murmur > II, SBP > 140 mmHg, ISACHC HF class, VHS > 10.5, LA/Ao > 1.5, EDV > 30 mL/m2, and ESV > 5 mL/m2.
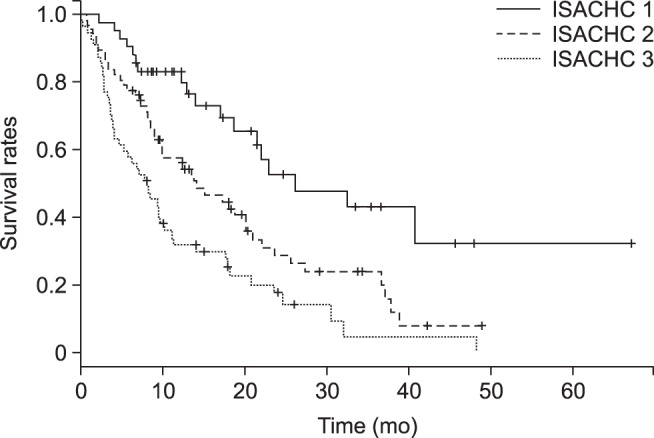
Univariate Cox survival analysis was used to evaluate the hazard ratio of an adverse event. Survival curves, median survival times, and 95% confidence intervals were obtained by applying the Kaplan-Meier method. Survival time was counted from the day of diagnosis of MMVD at the SNU VMTH to either the day of death or the end of the observation period. Differences in survival characteristics of each ISACHC HR class are shown in Fig. 1. All statistical analyses were conducted and confirmed by a statistician of the Department of Statistics, SNU, Korea.
Fig. 1. Survival of 168 dogs classified according to International Small Animal Cardiac Health Council (ISACHC) criteria. One-hundred-fifteen of the 168 dogs died during the observation period. Nineteen dogs were ISACHC class I, 47 dogs were ISACHC class II, and 49 dogs were ISACHC class III at initial examination. Survival duration of the ISACHC class I dogs (mean survival time: 461 ± 322 days) was higher than those of the dogs in classes II and III (274 ± 289 days and 376 ± 315 days, respectively).
Results
Basic characteristics
One hundred and sixty-eight dogs from 16 breeds were included in this study (Table 1). There were 85 females and 83 males; the mean age was 10.8 ± 2.5 years (range, 5–17 years), and mean weight was 5.2 ± 2.6 kg (range, 1.7–14.5 kg). Ninety-six dogs weighed ≤ 5 kg, and 72 dogs weighed > 5 kg. The majority of the dogs included in the study were Maltese (n = 51; 30.4%) followed by Shi-Tzu (n = 43; 25.6%).
Table 1. List of dogs included in study by breed and numbers.
History and clinical symptoms
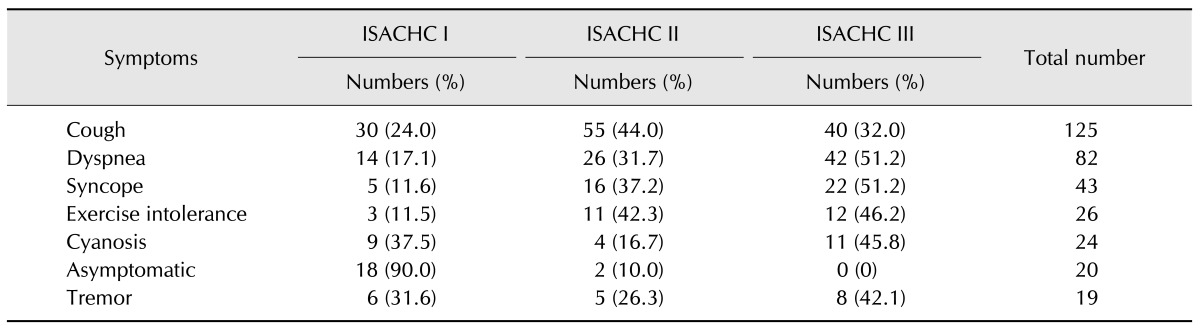
Analysis of the clinical symptoms revealed that 125 dogs had cough, 82 had dyspnea, 43 had a case history of syncope, and 24 had cyanosis (Table 2). There were 20 asymptomatic dogs. With regard to concurrent cardiac diseases, 125 dogs (74.4%) had TVI and 32 (19%) had PE. Fifty-nine (35.1%) dogs had TC, and 59 (47.2%) presented with a cough.
Table 2. Presenting signs and clinical findings for each International Small Animal Cardiac Health Council (ISACHC) class among 168 dogs with myxomatous mitral valve degeneration.
Electrocardiogram
Forty dogs (23.8%) exhibited arrhythmia, which was identified as sinus arrhythmia.
Radiography and echocardiography
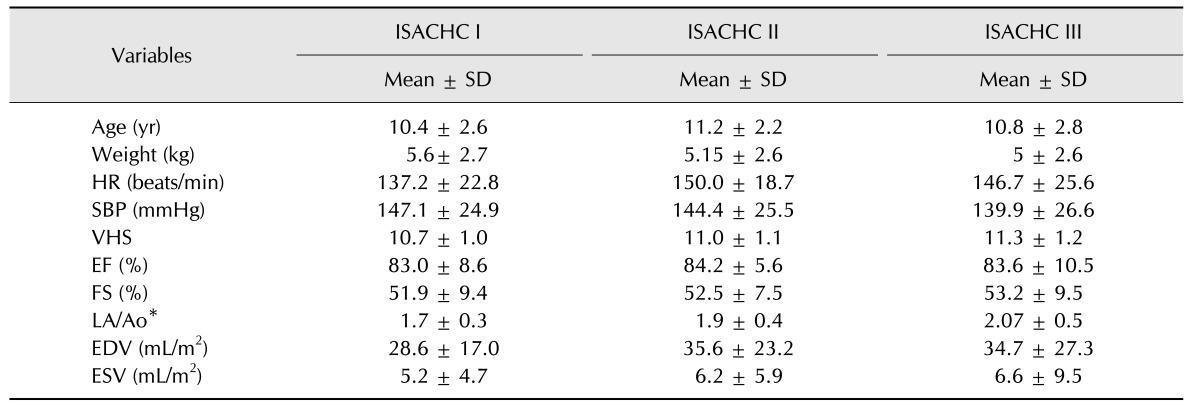
The clinical and echocardiographic variables for each of the ISACHC classes are summarized in Tables 3 and 4. The mean VHS (n =168) was 11 ± 1.1. The mean end diastolic volume was 33.5 ± 23.81, and the mean, end systolic volume (n = 167) was 6.1 ± 7.13 mL/m2. The mean LA/Ao ratio (n = 167) was 1.89 ± 0.4. The mean EF was 83.7 ± 8.4%, and the mean FS was 52.6 ± 8.7%. In addition, LA/Ao ratio was significantly increased in ISACHC class III subjects (one-way ANOVA with Tukey's range test, p < 0.0001).
Table 3. Mean ± SD of several variables for dogs with myxomatous mitral valve degeneration in each International Small Animal Cardiac Health Council (ISACHC) class.
HR, heart rate; SBP, systolic blood pressure; VHS, vertebral heart score; EF, ejection fraction; FS, fractional shortening; LA/Ao, left atrium aortic root ratio; EDV, end diastolic volume; ESV, end systolic volume. Differences were considered significant if p < 0.0001 compared with each group and was determined by one-way ANOVA with Tukey's range test. *p < 0.0001.
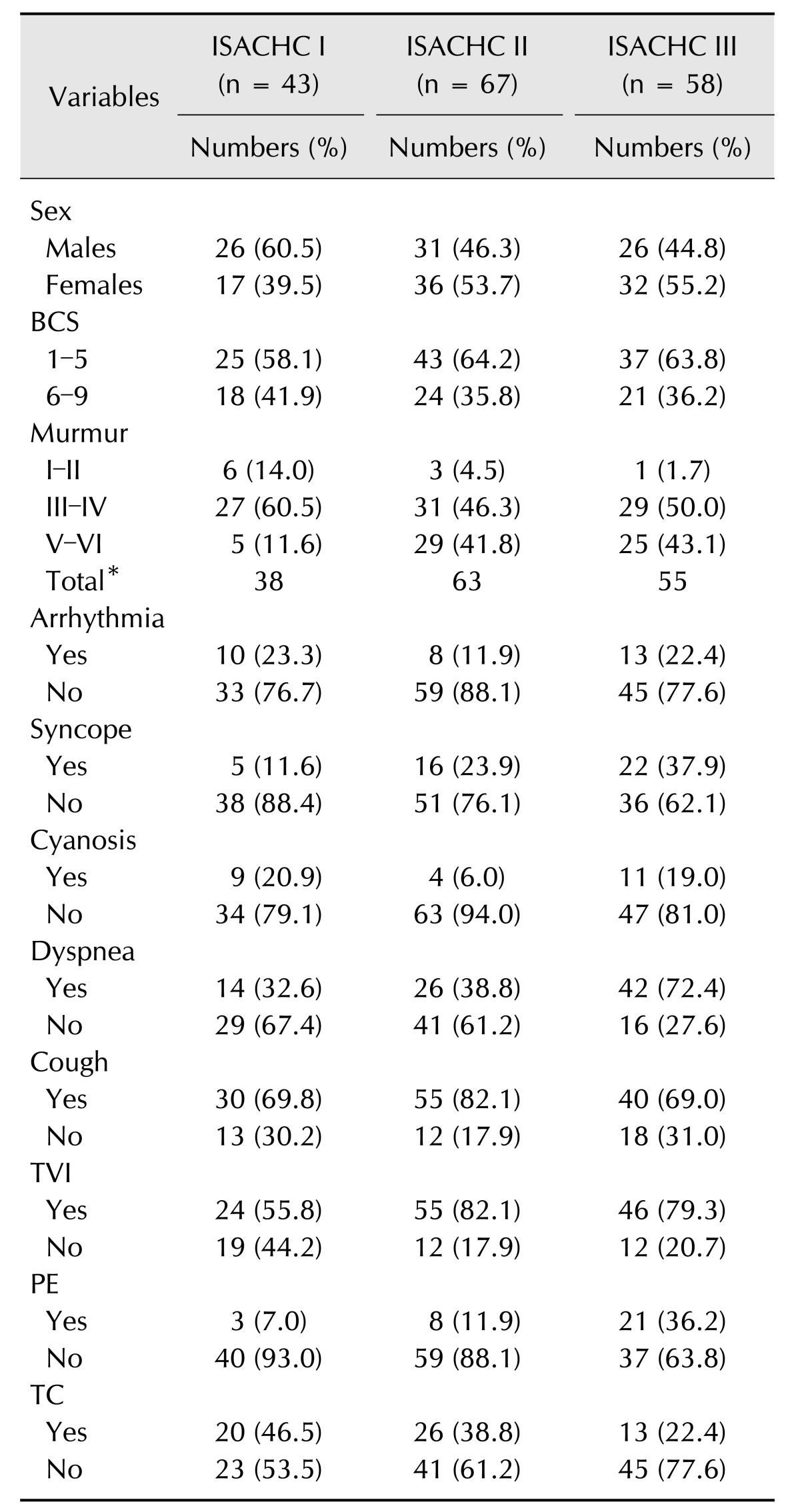
Table 4. Distribution of dogs to each International Small Animal Cardiac Health Council (ISACHC) class according to discrete study variables.
BCS, body condition score; TVI, tricuspid valve insufficiency; PE, pulmonary edema; TC, tracheal collapse. *Murmur status of some dogs was not available due to medical record loss.
Survival analysis
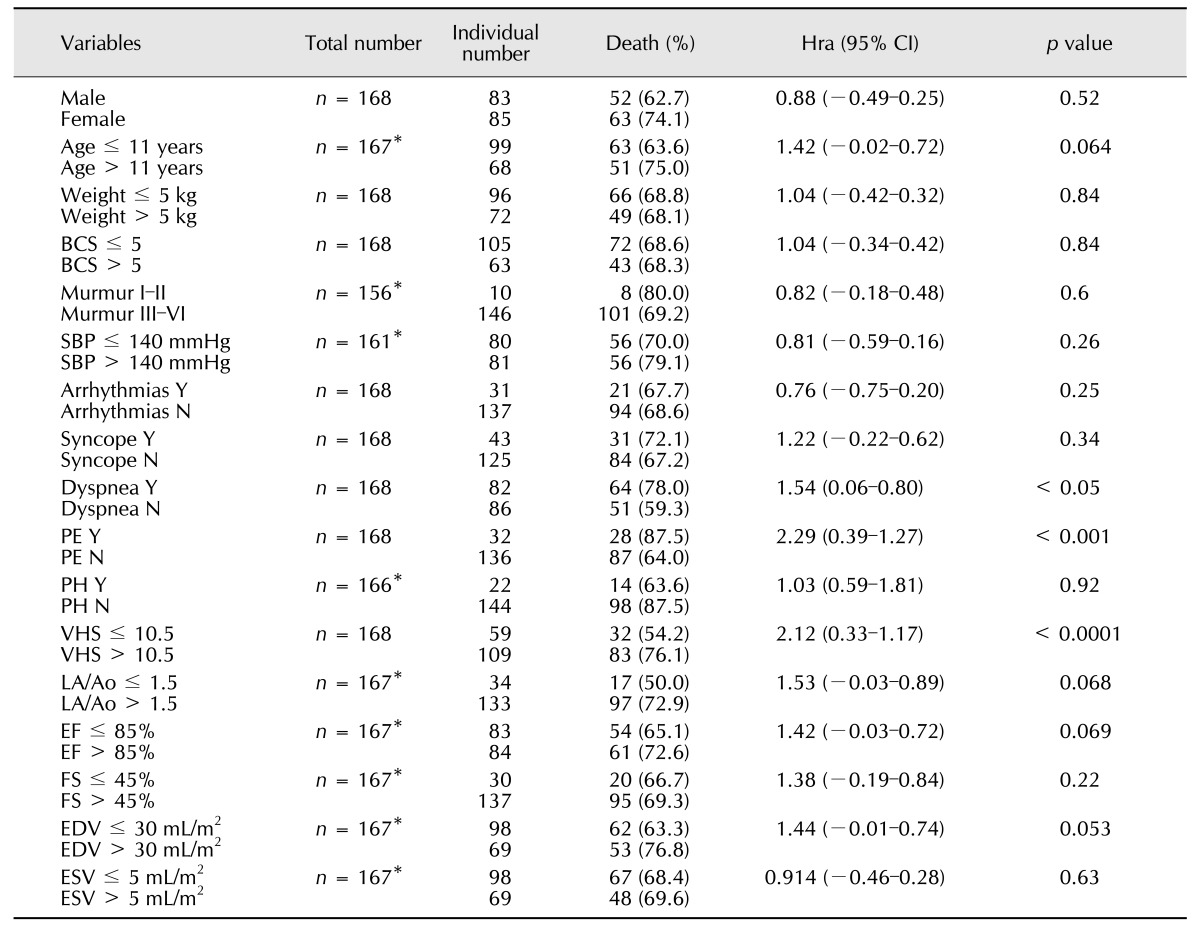
One hundred and fifteen dogs died during the observation period, and their mean survival time was 345 ± 309 days. Of the 16 variables that were used as predictors in the Kaplan-Meier analysis, dyspnea, PE, and VHS were significantly associated (p < 0.05) with overall survival time (Table 5). Of the 115 mortalities, 19 were ISACHC class I, 47 were ISACHC class II, and 49 were ISACHC class III at initial examination. The survival time of the ISACHC class I dogs was higher than those of the class II and III dogs; approximately 30% of ISACHC class I dogs were alive 60 months after the initial diagnosis (Fig. 1).
Table 5. Probability of adverse effect on survival of variables included in the univariate analysis.
CI, confidence interval; Hra, hazard ratio; BCS, body condition score; SBP, systolic blood pressure; PE, pulmonary edema; PH, pulmonary hypertension; VHS, vertebral heart score; LA/Ao, left atrium aortic root ratio; EF, ejection fraction; FS, fractional shortening; EDV, end diastolic volume; ESV, end systolic volume; Y, yes; N, No. *Data for some dogs were not available due to medical record loss.
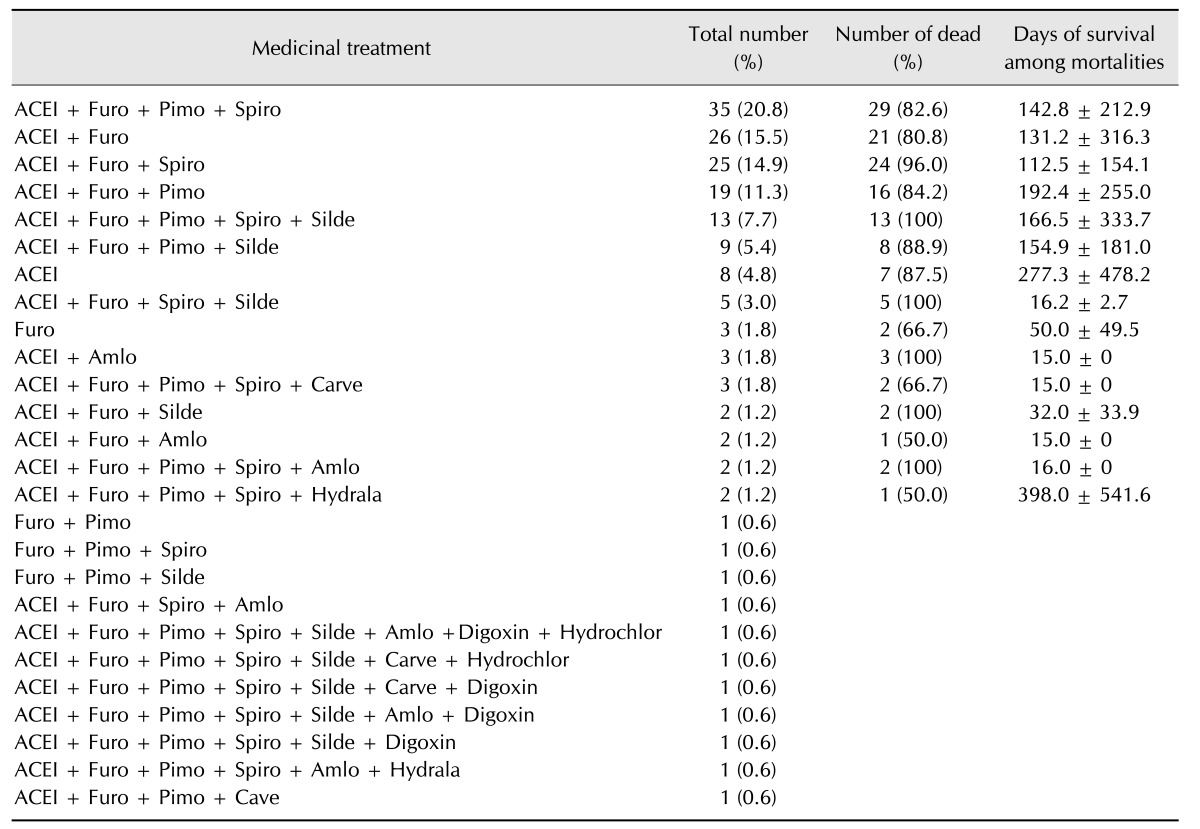
Medicinal treatments
The prescribed medicinal treatments at the time of death or, among the live dogs, the last prescribed treatments of the 168 dogs, along with the mean survival rate and survival duration among the mortalities, are summarized in Table 6. Most dogs were treated with angiotensin converting enzyme inhibitors, but some received furosemide alone or in combination with other diuretics or pimobendan. The drug combinations were notably diverse among the168 dogs, therefore, statistical analysis of survival rate and survival duration among the mortalities could not be carried out.
Table 6. Distribution of medicinal treatments administered to the study's 168 dogs.
ACEI, angiotensin converting enzyme inhibitor; Cave, carvediolol; Furo, furosemide; Pimo, pimobendan; Spiro, Spironolacton; Silde, sildenafile; Hydrala, Hydralazine; Hydrochlor, hydrochlorothiazide; Amlo, amlodipine.
Discussion
Twenty-two prognostic variables of dogs with MMVD were investigated in this study to allow assessment of their survival-related characteristics. The most significant clinical and echocardiographic factors related to survival duration were dyspnea, PE, and VHS (p < 0.05). The mean survival time of the dogs with dyspnea (327 ± 293 days) was shorter than that of dogs without dyspnea (365 ± 331 days). The presence of PE also increased the dogs with and without PE demonstrated mean survival times of 237 ± 219 days and 379 ± 327 days, respectively.
During HF progress due to MMVD, pulmonary congestion and edema appear. PE can be detected before clinical signs such as dyspnea occur, and it can, directly and indirectly, affect the survival of animals with MMVD. Therefore, early detection and appropriate management of PE could help to prevent dyspnea and deterioration of CHF.
Cardiomegaly has been associated with decreased survival time in dogs with MMVD [3,9]. In this study, a VHS over 10.5 vertebrae had a hazard ratio of 2.12 (Table 5), suggesting that the possibility of death in dogs with a VHS > 10.5 is two-fold higher than that in dogs with a VHS ≤ 10.5. As demonstrated in Table 3, the mean VHS of dogs in ISACHC class III tended to be higher than that of dogs in ISACHC classes I and II; however, the differences were not significant. When other prognostic variables, such as BCS, age, heart rate, syncope, cyanosis, cough, TVI, TC, arrhythmia, and echocardiographic results (i.e., EF, FS, PH, LA/Ao, EDV, and ESV) were examined, only age > 11 years, LA/Ao > 1.5, EF > 85, and EDV > 30 mL/m2, were significantly correlated with overall survival times (p < 0.1). These prognostic factors are correlated with cardiomegaly and disease progress in mitral valve regurgitation. Although statistically not significant, EDV and ESV were higher in dogs in ISACHC classes II and III, and an EDV > 30 mL/m2 of EDV tended to be associated with a higher possibility of death (p = 0.053). The EDV is a preload that stretches the left ventricle of the heart to its greatest dimensions under a variable physiologic demand. When excessive, ventricular volume overload occurs and may result in HF [10]. In addition, the LA/Ao ratio can be an important prognostic variable. With MMVD progression, the LA dimension increases, and left atrium size is reflective of disease severity. Most of the echocardiographic and radiographic results in this study reflected the appropriate ISACHC classification. Based on echocardiographic results, the higher ISACHC classes were accompanied by increased heart size and increased enlargement of the left atrium. Overall, mitral regurgitation in MMVD can produce a pressure overload on the right heart by increasing pulmonary vascular pressure [7], and VHS increases during the progression of valvular disease [5]. Monitoring heart size at initial diagnosis and regularly afterward could assist in assessing the prognosis of dogs with MMVD.
Some of the factors assessed in this study were not significant predictors of survival in small-breed dogs. Body weight, BCS, SBP, arrhythmia, syncope, FS, and end systolic volume were not significantly associated with the risk of death (p > 0.2). In addition, the sex of the animal with MMVD was not associated with survival duration. An earlier study reported that male dogs are prone to MMVD [16], but the proportions of each sex in that study population were similar. Moreover, our univariate analysis of sex showed that it did not influence the survival of the dogs with MMVD. In addition, weight did not influence the life expectancy of the dogs. This result is different from that in a study of large-breed dogs [2]. Although we used a 1 kg difference to stratify the dogs by weight, weight was not a significant factor. In addition, heart murmur intensity was not significantly associated with survival duration of the dogs with MMVD in this study. However, the number of dogs in the low-intensity murmur group (murmur I–II) was too small to allow a statistically powerful comparison with the number of dogs in the higher intensity murmur group (murmur III–VI). Finally, our analysis also showed that an SBP >140 mmHg was not significantly associated with survival. These results differ from those reported by Borgarelli et al. [2] whose study included large-breed dogs.
As a retrospective study, there are some study limitations. As we focused on small-breed dogs, many dogs with MMVD that were brought to the SNU VMTH had to be excluded. In addition, we were unable to ascertain the survival status of some dogs, and other dogs had to be excluded because they did not meet the inclusion criteria. Moreover, some of the small-breed dogs had undergone only one cardiology consultation, so there was insufficient information for them to be included in the study. Therefore, the results of the current study may not represent a larger population of small-breed dogs. In addition, more than half of the dogs included in the present study were Maltese and Shih Tzu. The results could be biased to those two breeds, thereby limiting the generalizability of the findings to the entire small-breed dog population. Moreover, the predisposition of Maltese, the most popular dog breed in Korea, to MMVD has not been investigated. To determine whether this breed is prone to MMVD, a large percentage of Maltese population in Korea should be studied.
The survival rates and survival duration of dogs that died were analyzed, but the SD of survival duration varied, thereby limiting the statistical analysis. In addition, the drug treatment combinations in the study population were too diverse, and the sample sizes of some groups were too small to allow meaningful statistical analysis. Thus, additional detailed studies with larger study populations are needed; particularly studies of medicinal treatment combinations administered in each of the ISACHC classes.
In conclusion, respiratory distress, directly or indirectly associated with MMVD, is likely to affect survival time in small-breed dogs. The univariate analysis in our study showed that, rather than assessing body weight, BCS, SBP, arrhythmia, syncope, FS, or ESV, clinical assessment of dyspnea, PE, and VHS can be useful in evaluating the survival of dogs with MMVD and can be useful factors when providing prognostic information to owners of small-breed dogs. Among the methods discussed in the current study, measuring the VHS via lateral chest radiographs might be a relatively convenient way of evaluating an animal's prognosis in clinics where echocardiography is not available. In addition, intensive monitoring of VHS after the initial diagnosis should be employed in clinical practice in order to assess the prognosis and modulate the treatment of animals with MMVD.
Acknowledgments
We thank the Research Institute for Veterinary Science, and the BK21 PLUS Program for Creative Veterinary Science, Seoul National University in Korea.
Footnotes
Conflict of Interest: The authors declare no conflict of interests.
References
- 1.Boon JA. Manual of Veterinary Echocardiography. Chichester: Wiley; 1998. Evaluation of size, function, and hemodynamics; pp. 153–266. [Google Scholar]
- 2.Borgarelli M, Savarino P, Crosara S, Santilli RA, Chiavegato D, Poggi M, Bellino C, La Rosa G, Zanatta R, Haggstrom J, Tarducci A. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med. 2008;22:120–128. doi: 10.1111/j.1939-1676.2007.0008.x. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan JW. Vertebral scale system to measure heart size in radiographs. Vet Clin North Am Small Anim Pract. 2000;30:379–393. [PubMed] [Google Scholar]
- 4.Disatian S. Myxomatous degenerative mitral valve disease: an update. Thail J Vet Med. 2010;40:151–157. [Google Scholar]
- 5.Guglielmini C, Diana A, Pietra M, Di Tommaso M, Cipone M. Use of the vertebral heart score in coughing dogs with chronic degenerative mitral valve disease. J Vet Med Sci. 2009;71:9–13. doi: 10.1292/jvms.71.9. [DOI] [PubMed] [Google Scholar]
- 6.Häggström J, Boswood A, O'Grady M, Jöns O, Smith S, Swift S, Borgarelli M, Gavaghan B, Kresken JG, Patteson M, Ablad B, Bussadori CM, Glaus T, Kovacević A, Rapp M, Santilli RA, Tidholm A, Eriksson A, Belanger MC, Deinert M, Little CJ, Kvart C, French A, Rønn-Landbo M, Wess G, Eggertsdottir AV, O'Sullivan ML, Schneider M, Lombard CW, Dukes-McEwan J, Willis R, Louvet A, DiFruscia R. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: the QUEST study. J Vet Intern Med. 2008;22:1124–1135. doi: 10.1111/j.1939-1676.2008.0150.x. [DOI] [PubMed] [Google Scholar]
- 7.Häggström J, Kvart C, Pedersen H. Acquired valvular heart disease. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine: Diseases of the Dog and Cat. 6th ed. Oxford: Elsevier Saunders; 2005. pp. 1022–1039. [Google Scholar]
- 8.Hansson K, Häggström J, Kvart C, Lord P. Left atrial to aortic root indices using two-dimensional and M-mode echocardiography in cavalier King Charles spaniels with and without left atrial enlargement. Vet Radiol Ultrasound. 2002;43:568–575. doi: 10.1111/j.1740-8261.2002.tb01051.x. [DOI] [PubMed] [Google Scholar]
- 9.Lamb CR, Tyler M, Boswood A, Skelly BJ, Cain M. Assessment of the value of the vertebral heart scale in the radiographic diagnosis of cardiac disease in dogs. Vet Rec. 2000;146:687–690. doi: 10.1136/vr.146.24.687. [DOI] [PubMed] [Google Scholar]
- 10.Nelson RW, Couto CG. Small Animal Internal Medicine. 5th ed. St. Louis: Elsevier; 2014. Cardiovascular system disorders; pp. 1–216. [Google Scholar]
- 11.Parker HG, Kilroy-Glynn P. Myxomatous mitral valve disease in dogs: does size matter? J Vet Cardiol. 2012;14:19–29. doi: 10.1016/j.jvc.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 13.Serres FJ, Chetboul V, Tissier R, Carlos Sampedrano C, Gouni V, Nicolle AP, Pouchelon JL. Doppler echocardiography-derived evidence of pulmonary arterial hypertension in dogs with degenerative mitral valve disease: 86 cases (2001–2005) J Am Vet Med Assoc. 2006;229:1772–1778. doi: 10.2460/javma.229.11.1772. [DOI] [PubMed] [Google Scholar]
- 14.Simpson KE, Devine BC, Gunn-Moore DA, French AT, Dukes-McEwan J, Koffas H, Moran CM, Corcoran BM. Assessment of the repeatability of feline echocardiography using conventional echocardiography and spectral pulse-wave Doppler tissue imaging techniques. Vet Radiol Ultrasound. 2007;48:58–68. doi: 10.1111/j.1740-8261.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith PJ, French AT, Van Israël N, Smith SGW, Swift ST, Lee AJ, Corcoran BM, Dukes-McEwan J. Efficacy and safety of pimobendan in canine heart failure caused by myxomatous mitral valve disease. J Small Anim Pract. 2005;46:121–130. doi: 10.1111/j.1748-5827.2005.tb00302.x. [DOI] [PubMed] [Google Scholar]
- 16.Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence or absence of asynergy. Am J Cardiol. 1976;3:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 17.The BENCH (BENazepril in Canine Heart disease) Study Group. The effect of benazepril on survival times and clinical signs of dogs with congestive heart failure: results of a multicenter, prospective, randomized, double-blinded, placebo-controlled, long-term clinical trial. J Vet Cardiol. 1999;1:7–18. doi: 10.1016/S1760-2734(06)70025-X. [DOI] [PubMed] [Google Scholar]
- 18.Thomas WP, Gaber CE, Jacobs GJ, Kaplan PM, Lombard CW, Moise NS, Moses BL Echocardiography Committee of the Specialty of Cardiology; American College of Veterinary Internal Medicine. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. J Vet Intern Med. 1993;7:247–252. doi: 10.1111/j.1939-1676.1993.tb01015.x. [DOI] [PubMed] [Google Scholar]