Summary
Crohn's disease (CD) is characterized by the expansion of mesenteric fat, also known as “creeping fat.” We explored the plasticity and immune properties of adipose-derived stem cells (ASCs) in the context of CD as potential key players in the development of creeping fat. Mesenteric CD-derived ASCs presented a more proliferative, inflammatory, invasive, and phagocytic phenotype than equivalent cells from healthy donors, irrespective of the clinical stage. Remarkably, ASCs from the subcutaneous depot of patients with CD also showed an activated immune response that was associated with a reduction in their immunosuppressive properties. The invasive phenotype of mesenteric CD ASCs was governed by an inflammasome-mediated inflammatory state since blocking inflammasome signaling, mainly the secretion of interleukin-1β, reversed this characteristic. Thus, CD alters the biological functions of ASCs as adipocyte precursors, but also their immune properties. Selection of ASCs with the best immunomodulatory properties is advocated for the success of cell-based therapies.
Keywords: creeping fat, cell therapy, interleukin 1B, immunity, invasion, migration, phagocytosis, lymphocytes, regulatory T cell, mesenchymal stem cells
Highlights
-
•
ASCs isolated from CD patients are highly proliferative, invasive, and phagocytic
-
•
Proliferative ASCs may be responsible for the development of creeping fat
-
•
ASCs from CD patients have dampened immunosuppressive properties
-
•
Selection of the best immunosuppressive ASCs for cell therapy is advocated
Serena and colleagues show that CD alters the biological function and immune properties of ASCs as adipocyte precursors. They propose that understanding how CD alters ASC functionality is critical for elucidating the complex pathophysiology of this disease, as well as for the success of cell-based therapies. Their work suggests that inhibiting the secretion of IL-1β may represent a novel therapeutic approach in CD.
Introduction
Crohn's disease (CD) is becoming increasingly common and represents a significant burden to healthcare systems (Kappelman et al., 2008). CD is characterized by persistent inflammation and ulceration of the small or large bowel with an undulating course of activity, and frequently recurs after periods of remission.
Hyperplasia of the mesenteric fat adjacent to the inflamed regions of the intestine, so-called creeping fat (CF), is a hallmark of CD and seems to be directly related to disease activity (Buning et al., 2015, Connelly et al., 2014, Fink et al., 2012, Li et al., 2015). Indeed, it has been recently described that reoperation rates among CD patients decreases dramatically, from 27% to 2.7%, when mesenteric adipose tissue (AT) is included during intestinal resection (Coffey and O'Leary, 2016). While its function and pathological significance for CD remains largely unknown, CF is clearly related to inflammatory response (Desreumaux et al., 1999, Peyrin-Biroulet et al., 2007, Zulian et al., 2012) and shows abundant macrophage and T cell infiltrates (Zulian et al., 2012). CF is considered an invasive form of mesenteric AT and, unlike obesity, its expansion surrounding inflamed intestinal tract tissue is dependent largely on adipocyte hyperplasia, with adipocytes 75% smaller than those from healthy subjects (Kredel and Siegmund, 2014, Zulian et al., 2012). AT hyperplasia results from the increase in adipocyte number via recruitment and differentiation of AT precursors termed AT-derived mesenchymal stem cells (ASCs) (Goncalves et al., 2015, Zulian et al., 2013). It is therefore possible that the origin of CF lies in the functional properties of ASCs. ASCs not only participate in turnover of mature adipocytes in humans (ca. 10% per year) but possess immunoregulatory properties that may be induced by the underlying pathological state (Pachon-Pena et al., 2016, Serena et al., 2016).
There is increasing data pointing to a connection between bacterial translocation and the development of CF (Batra et al., 2012, Peyrin-Biroulet et al., 2012). Because bacteria can trigger preadipocyte proliferation in vitro (Zulian et al., 2013), bacterial translocation might underlie AT hyperplasia. Moreover, it has been demonstrated that a pro-inflammatory stimulus such as tumor necrosis factor α (TNF-α) can stimulate the proliferation of adipocyte precursors (Zubkova et al., 2016). Accordingly, precursors may be conditioned by the systemic inflammatory environment characteristic of CD, as previously described in other inflammatory-based diseases, including obesity, whereby an activation of the ASC niche occurs through an increase in ASC proliferative capacities (Pachon-Pena et al., 2016, Serena et al., 2016) and a decrease in their sensitivity to apoptosis (Ejarque et al., 2017).
Given this background, we hypothesized that CF associated with CD is the consequence of an elevated proliferation and migration capacity of ASCs of mesenteric AT to migrate to the inflamed intestine and, once there, differentiate to mature adipocytes. However, as the disease progresses the local pro-inflammatory environment might disturb ASC function. To investigate this, we characterized the biological and immunological properties of mesenteric ASCs isolated from patients with CD, both in clinical relapse and in clinical remission, including migration and invasion capacities and also phagocytic capacity. We extended this analysis to ASCs isolated from subcutaneous fat depots to test whether CD patients have a predisposition to greater activation of the immune system in a constitutional way that affects AT in general, without limitation in its location. Our work shows that CD alters the biological function and immune properties of ASCs as adipocyte precursors.
Results
Mesenteric ASCs of Patients with CD Show Higher Proliferation Rates but Lower Adipogenic Capacities than Those of Healthy Donors
ASCs were isolated from visceral adipose tissue (VAT) of healthy subjects (n = 6) and patients with active (n = 10) or inactive (n = 5) CD. When ASCs were isolated from the same amount of mesenteric AT from patients with CD (CD ASCs) and from healthy individuals (healthy ASCs), a greater number of ASCs was obtained from CD patients than from healthy individuals. Correspondingly, the AT-cell number ratio was significantly higher in CD ASCs, both from active and inactive patients, than in healthy ASCs (Figure 1A), which suggests an increase in the number of adipocyte precursors in mesenteric AT of CD patients. Moreover, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 5-bromo-2′-deoxyuridine (BrdU) incorporation assays revealed a higher proliferation rate in mesenteric CD ASCs than in healthy ASCs (Figures 1B and 1C). To study the adipogenic potential of ASCs, we cultured them in well-defined adipogenic differentiation medium and evaluated lipid content and gene expression after 14 days. Neutral lipid content, measured by oil red O staining, was significantly lower in differentiated CD ASCs (independent of clinical stage) than in healthy ASCs (Figures 1D and 1E), concomitant with a decrease in the gene expression of typical adipogenic markers such as leptin, peroxisome proliferator activated receptor gamma (PPARG), fatty acid binding protein 4 (FABP4), and lipoprotein lipase (LPL) (Figure 1F). Collectively, these data reveal that similar to obesity (Pachon-Pena et al., 2016), CD modifies the plasticity of mesenteric ASCs.
Figure 1.
CD Increases Mesenteric ASC Proliferation and Reduces Their Adipogenic Differentiation Capacity
(A–C) AT-cell number ratio (A), MTT (B), and BrdU cell proliferation (C) assays were performed as detailed in Experimental Procedures to study the cell proliferation of mesenteric ASCs.
(D) Representative images of intracellular lipid enrichment in mature adipocyte (AD) from healthy subjects, active CD patients, and inactive CD individuals (magnification, ×200; scale bar, 200 μm).
(E) Quantification of oil red O staining of AD from healthy subjects, active CD patients, and inactive CD patients.
(F) Gene expression of adipogenic markers was analyzed by RT-PCR in AD and undifferentiated ASCs from healthy subjects, active CD patients, and inactive CD patients. n = 5–10 per group as explained in Experimental Procedures.
∗p < 0.017 versus healthy ASCs; #p < 0.017 as indicated in the figure.
Mesenteric ASCs from Patients with CD Present an Inflammasome-Mediated Inflammatory Response
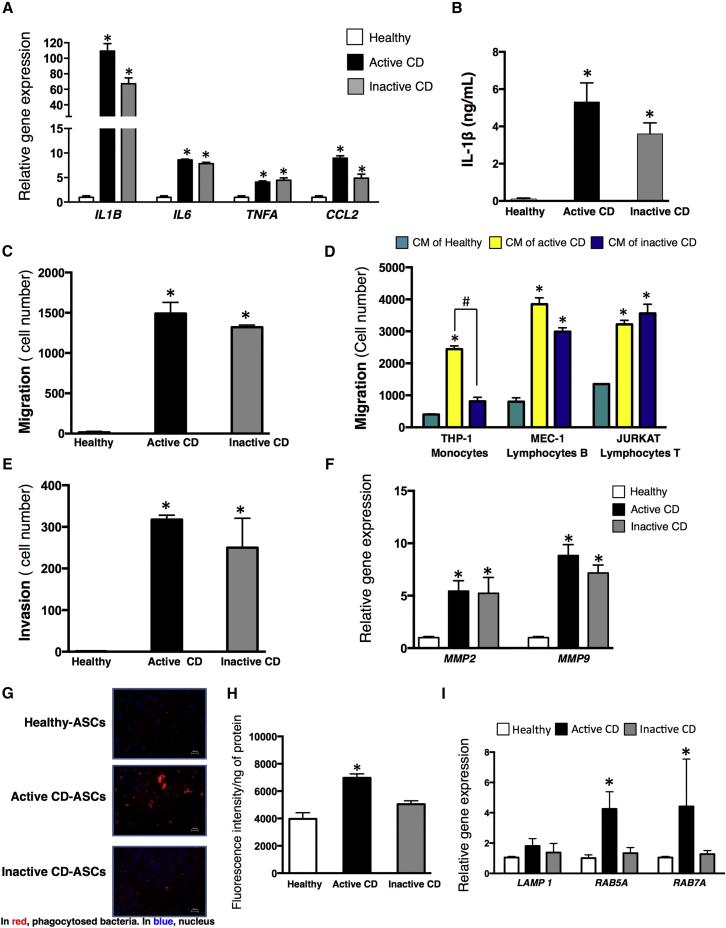
It is known that CF exhibits a high level of inflammatory cytokine secretion (Kredel and Siegmund, 2014). Consistent with this, we observed that gene expression of typical inflammatory markers (IL6, TNFA, CCL2, and IL1B) was higher in mesenteric CD ASCs from active patients than from healthy donors (Figure 2A). Of note, an inflammatory phenotype was also detected in CD ASCs from inactive patients (Figure 2A). As previously described for mesenteric AT of active CD patients (Zulian et al., 2012), the expression of anti-inflammatory markers such as IL10 and adiponectin was also increased in CD ASCs (Figure 2A). Interestingly, expression of these markers was significantly lower in inactive CD ASCs than in active CD ASCs, revealing a decrease in anti-inflammatory mediators but not pro-inflammatory mediators in the former.
Figure 2.
CD Triggers an Inflammasome-Mediated Inflammatory Response in Mesenteric ASCs and Increases Their Metabolic Activity
(A) Expression of IL6, TNFA, CCL2, IL1B, IL10, and adiponectin were analyzed by qPCR in ASCs isolated from VAT of healthy subjects, active CD patients, and inactive CD patients.
(B) Secretion of IL-1β was analyzed by ELISA from conditioned medium (CM) of ASCs from healthy subjects, active CD patients, and inactive CD patients.
(C) Gene expression of different components of the inflammasome, NLRP1, NLRP3, and CASP1 were analyzed by qPCR.
(D) Gene expression of glucose and lipid metabolism genes was analyzed by qPCR in VAT ASCs.
(E and F) Lactate (E) and succinate (F) levels in CM of ASCs isolated from healthy subjects, active CD patients, and inactive CD patients.
n = 5–10 per group as explained in Experimental Procedures, with the exception of metabolic data (n = 4 for all groups). ∗p < 0.017 versus healthy ASCs; #p < 0.017 as indicated in the figure.
Because of the important role of interleukin-1β (IL-1β) in host response (Grant and Dixit, 2013), we next examined inflammasome-mediated immune responses in ASCs. In accordance with the gene expression data, IL-1β protein expression in conditioned medium (CM) of ASCs was significantly higher in CD ASCs than in healthy ASCs, and was significantly higher in active CD ASCs than in inactive CD ASCs (Figure 2B). Gene expression levels of critical inflammasome components that drive IL-1β secretion (Grant and Dixit, 2013) were also measured in ASCs from healthy, inactive CD, and active CD subjects. In line with data on IL-1β secretion, gene expression of NLRP1, NLRP3, and CASP1 was higher in mesenteric CD ASCs (both active and inactive) than in healthy ASCs. No significant differences in the gene expression of these inflammasome components were found between ASCs from active and inactive CD patients, with the exception of NLRP1 (Figure 2C).
Given the close link between inflammasome activation and glucose homeostasis (Grant and Dixit, 2013, Serena et al., 2016), we compared the metabolic gene expression profile of ASCs isolated from CD patients and healthy controls (n = 4 for all groups). Compared with healthy ASCs, both active and inactive CD ASCs displayed a glycolytic phenotype that was characterized by significantly higher expression of SLC2A1, hexokinase-2 (HK2), phosphofructokinase (PFKM), and lactate dehydrogenase-b (LDHB), and significantly lower expression of pyruvate dehydrogenase kinase-4 (PDK4) (Figure 2D). Interestingly, the level of SLC2A4 mRNA was significantly lower in CD ASCs from active patients than from inactive patients (Figure 2D), presumably due to the greater systemic inflammation in the former (Papa et al., 1997, Poletto et al., 2015). The glycolytic phenotype of CD ASCs was accompanied by an increase in the amount of lactate and succinate released into CM (n = 4 for all groups) (Figures 2E and 2F), which agreed well with the detected lower and higher mRNA levels of SDHB and OGDH, respectively, in these cells (Figure 2D). CD ASCs also presented an increase in the expression of the β-oxidation markers CPT1B and SLC25A2 (Figure 2D). Taken together, our data suggest that CD provokes glycolytic and fatty acid oxidation metabolism in mesenteric ASCs, as previously described in some tumor cells (Altundag et al., 2005). For all these experiments, ASCs were isolated from VAT of healthy subjects (n = 6), active CD patients (n = 10), and inactive CD patients (n = 5).
Mesenteric ASCs from Patients with CD Have an Exacerbated Macrophage-like Phenotype
The inflammatory response, including immune cell migration, is essential both for host defense and tissue repair. We previously showed that ASCs can act as non-professional phagocytes, a capacity that is boosted in inflammatory settings such as obesity and type 2 diabetes (Serena et al., 2016). We used ASCs from VAT of healthy subjects (n = 6) and active (n = 10) and inactive (n = 5) CD patients for all experiments, with the exception of phagocyte data (n = 4 patients for all groups). We first evaluated ASC migration using Transwell assays. Remarkably, the 24-hr CM from explants of CF VAT of active CD patients triggered the strong migration of healthy ASCs, active CD ASCs, and also T Jurkat cells (Figure 3A), which agrees well with our hypothesis that ASCs migrate to CF. Furthermore, the basal migration of ASCs was significantly higher for CD ASCs, both from active and inactive patients, than for healthy ASCs (Figure 3B). We also examined the migration of monocytes (THP-1 cells), B lymphocytes (MEC-1 cells), and T lymphocytes (Jurkat cells) to ASC CM placed in the bottom chamber of the Transwell system, and detected that the migration of all cells was significantly greater to CM generated from active CD ASCs than from healthy ASCs, whereas CM from inactive CD ASCs significantly stimulated the migration of B and T lymphocytes only and was moderately less effective than CD ASC CM in stimulating B cells (Figure 3C). Using Matrigel invasion assays, we found that CD ASCs exhibited a robust invasion capacity in basal non-stimulated conditions relative to healthy ASCs (Figure 3D). Consistent with the observed increase in cell migration/invasion, gene expression of two matrix metalloproteinase (MMP) family proteins, MMP2 and MMP9, was significantly higher in CD ASCs than in healthy ASCs (Figure S1A) and correlated with increased MMP2 and MMP9 activity measured by gelatin zymography (Figure 3E). We previously demonstrated that an inflammatory environment such as that found in obesity and type 2 diabetes increases ASC phagocytic capacity (Serena et al., 2016). Since cell migration and invasion are crucial steps for phagocytosis, we hypothesized that ASCs may respond to the bacterial translocation within mesenteric AT described in CD patients (Kruis et al., 2014) by increasing their phagocytic capacity. We therefore studied this parameter in CD ASCs and healthy ASCs. Remarkably, the phagocytic cell response against bacteria was significantly higher in CD ASCs than in healthy ASCs, as measured by the ingestion of pHrodo-labeled Escherichia coli BioParticles (Figures 3F and 3G). In line with this finding, gene expression of the typical phagocytic/endocytosis markers RAB7A and RAB5A was significantly higher in CD ASCs than in healthy ASCs (Figure S1B). Of note, neither CD ASCs nor healthy ASCs were able to phagocytose yeast (data not shown), indicating specific phagocytic capabilities for ASCs. Collectively, these data indicate that mesenteric ASCs isolated from CD patients are immune activated, even when the disease is in clinical remission.
Figure 3.
CD Changes the Functional Properties of Mesenteric ASCs
(A) The migratory capacity of healthy ASCs, active CD ASCs, and immune cells (Jurkat cells) into 24-hr CM of CF of active CD patients or VAT of healthy individuals were assessed in Transwell assays.
(B) The migratory capacity of basal ASCs isolated from VAT of healthy subjects, active CD patients, and inactive CD patients was assessed in Transwell assays. Representative toluidine blue-stained cells are shown below the graph (magnification, ×200; scale bar, 200 μm).
(C) CM of VAT from healthy subjects, active CD patients, and inactive CD patients was tested to ascertain if it promotes the migration of immune cells (monocytes, THP-1 cell line; B lymphocytes, MEC-1 cell line; and T lymphocytes, Jurkat cell line) using the Transwell system.
(D) Invasion capacity was studied in ASCs by adding Matrigel to the upper Transwell chamber. Representative toluidine blue-stained cells are shown below the graph (magnification, ×200; scale bar, 200 μm).
(E) Zymographic analysis of MMP2/9 activities using gelatin as substrate. Representative zymogram and densitometric analysis are shown.
(F) Phagocytosis assay was performed using a rhodamine-based red dye conjugated to E. coli bacteria, which turns bright red upon lysosomal acidification. Phagocytic activity of cells is marked in red and the cell nucleus is marked in blue (DAPI). Representative images of ASCs from healthy subjects, active CD patients, and inactive CD patients (magnification, ×200; scale bar, 200 μm).
(G) Phagocytosis was quantified using the Varioskan LUX multimode microplate reader. Fluorescence intensity was normalized to total protein content.
n = 5–10 per group as explained in Experimental Procedures, with the exception of phagocytic data (n = 4 for all groups). ∗p < 0.05 versus healthy ASCs; #p < 0.01 as indicated in the figure.
ASCs Isolated from Subcutaneous Fat Depots of Patients with CD Also Show an Activation of the Immune Response
To determine whether the pro-inflammatory effects of CD on ASCs was restricted to visceral fat depots or was a global response, we next examined ASCs isolated from subcutaneous adipose tissue (SAT) depots. We used ASCs from SAT of healthy subjects (n = 6), active CD patients (n = 10), and inactive CD patients (n = 6) for all experiments, with the exception of phagocyte data (n = 4 patients for all groups). We found that ASCs from SAT, both from active and inactive CD patients, largely recapitulated the phenotype of equivalent VAT-derived ASCs, including a significantly higher AT-cell number ratio and proliferative activity (Figures S2A and 2B), and a reduced adipogenic differentiation capacity (Figures S2C and 2D) compared with their healthy counterparts. Moreover, gene expression of inflammatory markers (IL1B, IL6, TNFA, and CCL2) was significantly higher in ASCs isolated from SAT of active and inactive CD patients than in cells from healthy donors (Figure 4A). Also, the secretion of IL-1β into CM was significantly higher for CD ASCs than for healthy ASCs (Figure 4B). Overall, these results indicate that ASCs from SAT of CD patients exhibit an inflammatory profile, even during clinical remission of the disease.
Figure 4.
CD Alters the Functional Properties of Subcutaneous ASCs
(A) Expression of IL1B, IL6, TNFA and CCL2 was analyzed by qPCR in ASCs isolated from SAT of healthy subjects, active CD patients, and inactive CD patients.
(B) Secretion of IL-1β was analyzed by ELISA from CM of ASCs from healthy subjects, active CD patients, and inactive CD patients.
(C) Migratory capacity of ASCs isolated from healthy subjects, active CD patients, and inactive CD patients from SAT was assessed using the Transwell system.
(D) CM of SAT from healthy subjects, active CD patients, and inactive CD patients was tested to ascertain if it promotes the migration of immune cells (monocytes, THP-1 cell line; B lymphocytes, MEC-1 cell line; and T lymphocytes, Jurkat cell line) using the Transwell system.
(E) Invasion capacity was studied in ASCs by adding Matrigel to the upper Transwell chamber.
(F) MMP2 and MMP9 gene expression was analyzed by qPCR in ASCs from healthy subjects, active CD patients, and inactive CD patients.
(G) Phagocytosis assay was performed using a rhodamine-based red dye conjugated to E. coli bacteria, which turns bright red upon lysosomal acidification. Phagocytic activity of cells is marked in red, and the cell nucleus is marked in blue (DAPI). Representative images of ASCs from healthy subjects, active CD patients, and inactive CD patients (magnification, ×200; scale bar, 200 μm).
(H) Phagocytosis was quantified using the Varioskan LUX multimode microplate reader. Fluorescence intensity was normalized to total protein content.
(I) Gene expression of different phagocytic markers, LAMP1, RAB5A, and RAB7A, was analyzed by qPCR.
n = 6–10 per group as explained in Experimental Procedures, with the exception of phagocytic data (n = 4 for all groups). ∗p < 0.017 versus healthy ASCs; #p < 0.017 as indicated in the figure.
The migratory properties of SAT-derived ASCs from patients with CD also mirrored those of VAT-derived ASCs with respect to basal migration (Figure 4C), migration of monocytes and B and T lymphocytes to CM (Figure 4D), invasion (Figure 4E), and gene expression of MMP2/9 (Figure 4F). However, contrasting with the results for VAT-derived ASCs, only active CD ASCs showed a significantly higher phagocytic capacity than their healthy counterparts (Figures 4G and 4H), which was accompanied by significantly higher expression of RAB7A and RAB5A (Figure 4I). Collectively, these findings indicate that while the inflammatory response is also activated in ASCs from the SAT of patients with CD, only those from active CD present a phagocyte-like phenotype.
Loss of Immunosuppressive Properties in SAT ASCs from Patients with CD
We next explored whether ASCs isolated from SAT of patients with CD retain their typical immunoregulatory properties (Melief et al., 2013). We previously demonstrated that protective immunosuppressive properties, including elevated TGF-β1 secretion, promotion of M2 macrophage polarization, and inhibition of T and B cell proliferation, all of which are mainly ascribed to SAT-derived ASCs, are perturbed in ASCs isolated from an obesity-induced inflammatory milieu (Serena et al., 2016). Similar to that observed in obesity, TGFB1 expression was lower both in active (n = 10) and inactive (n = 6) CD ASCs than in healthy ASCs (n = 6), and this was significant for levels both in inactive and active CD ASCs (Figure 5A). Moreover, the level of TGF-β1 protein in CM was significantly lower from inactive CD ASCs than from healthy ASCs (Figure 5B). We also observed that the capacity to promote the M2 macrophage phenotype was blunted in ASCs isolated from CD patients. Accordingly, human THP1 PMA-activated macrophages (M0) were incubated with CM from healthy, active CD, and inactive CD ASCs (n = 6–10 for all groups), and gene expression of typical M2 markers was quantified. Whereas expression of IL10, CD163, and MRC1 in THP1 cells was significantly increased after addition of CM from healthy ASCs, this increase was less evident when M0 macrophages were cultured with CM from inactive or active CD ASCs (Figure 5C). We also analyzed the expression of M1 markers and found that CM of healthy ASCs, but not of CD ASCs, significantly decreased the expression of a panel of M1 markers (Figure 5C). We next evaluated the capacity of ASCs to inhibit T and B cell proliferation. Whereas CM from healthy ASCs suppressed the proliferation of T and B cells, CM from CD ASCs had the opposite effect and significantly increased T and B cell proliferation (Figure 5D). Our results show that the typical immunosuppressive properties of ASCs are blunted in CD patients.
Figure 5.
CD Reduces the Immunosuppressive Properties of ASCs
(A) ASCs were isolated from SAT of healthy subjects, active CD patients, and inactive CD patients, and the expression of TGFB1 was analyzed by qPCR.
(B) Secretion of TGF-β1 was analyzed by ELISA in CM of ASCs from healthy subjects, active CD patients, and inactive CD patients.
(C) CM of ASCs from SAT of healthy subjects, active CD patients, and inactive CD patients, was added to THP-1 PMA-activated macrophage, and gene expression of M1/M2 phenotype markers was analyzed by qPCR.
(D) Cell proliferation of Jurkat T cells and MEC-1 B cells was measured after adding CM of ASCs from SAT of healthy subjects, active CD patients, and inactive CD patients.
(E) Gene expression of Th1/Th2/Treg markers were studied in naive T lymphocytes that were co-cultured with healthy or active CD ASCs for 48 hr.
(F) Secretion of G-CSF was analyzed by ELISA in culture supernatant of co-cultured naive T lymphocytes with healthy ASCs or active CD ASCs.
(G) Gene expression of GCSF in ASCs previously co-cultured with naive T lymphocytes.
n = 6–10 per group. ∗p < 0.017 versus healthy ASCs; #p < 0.017 versus control (CM) as indicated in the figure.
To further examine the inflammatory capacity of ASCs, we performed co-culture experiments with naive T lymphocytes. We found that naive T lymphocytes that were co-cultured with active CD ASCs presented a significant increase in the gene expression of the T helper cell 1 (Th1) marker TNFA, and a significant decrease of the Th2 marker GATA3 and of the regulatory T cell (Treg) marker (TGFB1), compared with those co-cultured with healthy ASCs (Figure 5E). Remarkably, when we studied the secretion of soluble factors, the amount of granulocyte colony-stimulating factor (G-CSF) was significantly lower in the supernatant of naive T lymphocytes co-cultured with active CD ASCs than with healthy ASCs (Figure 5F). It is known that G-CSF mediates immune regulation, including the ability to promote Treg differentiation and has beneficial effects in the prevention of inflammatory bowel diseases in animal models (Rutella et al., 2005). Finally, we separated ASCs and naive T lymphocytes after 48 hr of co-culture and analyzed gene expression of GCSF in the two cell types by qPCR. We found that G-CSF expression was significantly lower in active CD ASCs than in healthy ASCs (Figure 5G). This result strongly suggests that healthy ASCs may produce G-CSF, which promotes Treg differentiation (Rutella et al., 2005). Conversely, the lower gene expression and production of G-CSF in active CD ASCs co-cultured with naive T cells may lead to a decrease Treg differentiation. Thus, the co-culture between naive T cells and ASCs is necessary to secrete G-CSF. Accordingly, CD ASCs in contact with naive T lymphocytes in vivo may lead to a change in the phenotype of these cells in CD patients and decrease the T regulatory lymphocyte population.
Inflammasome Inhibition Counters the Invasive and Glycolytic Phenotype of ASCs from CD Patients
Both TGF-β1 and IL-1β have been previously revealed as key players in the dysfunctional behavior of ASCs in metabolic diseases linked to inflammation (Barbagallo et al., 2017, Pourgholaminejad et al., 2016, Serena et al., 2016). We therefore attempted to reverse the phenotype of ASCs isolated from CD patients using different strategies to inhibit inflammasome components. To do this, we treated ASCs (n = 4 for all groups) with the IL-1 receptor antagonist (IL1RA), the caspase-1 inhibitor YVAD-CHO, or TGF-β1, alone or in combination. Only combined treatment of IL1RA plus YVAD-CHO was effective in significantly reducing the invasion capacity of ASCs isolated from the SAT and mesenteric AT depots of active and inactive CD patients (Figures 6A and 6B), which was accompanied by a significant reduction of MMP2/9 gene expression (Figures 6C and 6D). Moreover, this combination resulted in a decrease in the gene expression of inflammatory and inflammasome markers and also phagocyte markers (Figures 6E and 6F). Surprisingly, the combined treatment of IL1RA plus YVAD-CHO reversed the effects of the CD environment on glycolytic but not β-oxidation markers (Figures 6E and 6F), suggesting that the inflammasome directs the invasive and glycolytic phenotype of ASCs in the context of CD.
Figure 6.
Inflammasome Inhibition of CD ASCs Reverses the Immune-Activated Phenotype
(A–D) Invasion capacity was studied in (A) SAT or (B) VAT CD ASCs treated with 40 ng/mL interleukin 1 receptor antagonist (IL1RA), 20 ng/mL TGF-β1, 10 μM YVAD-CHO (caspase-1 inhibitor), or the combined treatments at the same doses described in the figure. MMP2/9 gene expression was analyzed by qPCR in ASCs from (C) SAT and (D) VAT isolated from healthy subjects, active CD patients, and inactive CD patients.
(E and F) SAT (E) and VAT (F) relative gene expression of inflammasome, phagocytic, and metabolic markers was determined in untreated CD ASCs (basal) or those treated with IL1RA plus YVAD-CHO.
n = 4 for all groups. ∗p < 0.017 versus untreated ASCs (basal); #p < 0.017 as indicated in the figure.
Discussion
CD is a complex multifactorial inflammatory disorder. AT is an important source of immune-competent cells, including ASCs. Here we show that mesenteric CF contains ASCs with highly proliferative and invasive capacities and an altered immune profile. Remarkably, most of the features of ASCs from patients with an active stage of the disease are also observed in patients with clinical remission, both in mesenteric and subcutaneous depots.
CF is traditionally viewed as an unusual growth of mesenteric AT that secretes pro-inflammatory cytokines, chemokines, and acute phase proteins; however, whether it is a contributor to chronic inflammation, an initiator, or simply a bystander remains unknown. To date, no studies have focused on ASCs as active immune-competent cells in CD. We show that ASCs both from mesenteric CF and subcutaneous fat have an activated inflammasome. Classically, inflammasome-driven caspase-1 activation and IL-1β secretion occur as an innate immune response to protect the host against pathogens (Carracedo et al., 2013). In addition to this pro-inflammatory response, we found that ASCs isolated from the CF of CD patients have higher gene expression levels of anti-inflammatory molecules such as adiponectin or IL-10 than those isolated from VAT of healthy individuals. These data seem contradictory, but are in agreement with previous data from Yamamoto et al. (2005) who demonstrated that adiponectin production is higher in hyperplasic mesenteric AT contiguous with the involved intestine of CD patients than in healthy mesenteric AT. Indeed, the authors suggest that adiponectin secretion by mesenteric adipocytes could negatively regulate some of the inflammatory reactions observed in CD. We speculate that this may due in part to the small size of adipocytes in the CF, as it has recently been shown that adiponectin secretion correlates negatively with adipocyte size (Meyer et al., 2013). Accordingly, the small adipocytes characteristic of CF of CD patients may secrete higher amounts of adiponectin. In line with inflammasome activation (Grant and Dixit, 2013, Serena et al., 2016), CD ASCs showed a strong glycolytic metabolic phenotype, presumably due to high metabolic demand for ATP to maintain their proliferative, migratory, and invasive capacities, akin to the Warburg effect in cancer cells. Moreover, the β-oxidation markers CPT1B and SLC25A2 are increased in CD ASCs. We hazard that this increase in oxidative metabolism reflects their proliferative activity, as de novo synthesis of fatty acids is required to maintain cellular membrane synthesis in a manner similar to what has been recently described in certain tumor cells (Carracedo et al., 2013).
Of particular interest was our finding that subcutaneous ASCs from patients with CD present an elevated inflammatory threshold despite residing beyond the inflamed AT compartment, and this is also seen in patients in clinical remission of the disease. Previous studies have reported that the SAT of patients with CD in clinical remission is completely free from the disease, with a gene expression and inflammatory cytokine secretion profile similar to that found in SAT of healthy individuals (Zulian et al., 2012). While our data show that ASCs isolated from CD SAT have an inflammatory phenotype, we believe that these results are not contradictory. We speculate that these differences may be attributable to the cellular plasticity of AT resident cells, specifically macrophages, which can modify their functional phenotype depending on environmental cues (Leavy, 2011). Indeed, adipose tissue macrophages (ATMs) and monocytes derived from peripheral blood mononuclear cells are highly plastic in CD, as shown by the dramatic decrease in the expression of inflammatory markers once the disease is in clinical remission (Figure S3). Nevertheless, we found that ASCs from inactive CD patients remained primed to immunomodulatory dysfunction as evidenced by the largely similar phenotype to that found in active CD ASCs, indicating that ASCs are not equally as plastic as ATMs and maintain an altered functionality. Thus, inflammation induces irreversible changes to CD ASCs, shifting them toward an inflammatory profile even in periods of clinical disease remission.
We found that ASCs from patients with CD had a robust invasion capacity and bacterial phagocytic activity, pointing to a role for these cells in the inflammatory events occurring in damaged intestinal tissue. We hypothesize that upon microbial dysregulation, a hallmark of CD, ASCs migrate from mesenteric AT and specifically home to intestinal lesions. This event, which would be beneficial initially, also induces an accumulation of AT CF surrounding the intestine, which is a consequence of the natural fate of ASCs to differentiate into adipocytes. Furthermore, our data suggest that the hyperplasia of adipocytes observed in the CF of CD patients could potentially be a result of the ASCs inherent to this compartment, and not from other adipose depots.
A limitation to our study is the potential confounding effects that may have been caused by systemic steroid therapy, mainly in the active disease cohort (Table 1). One would expect a decrease in the immune response because of the immunosuppressant properties of corticosteroids; however, we found that the immune response of ASCs from these patients was clearly increased in parallel with markers of disease activity, suggesting little or no effect associated with systemic steroid treatment.
Table 1.
Anthropometric and Biochemical Variables from the Cohort
| Healthy | Inactive CD | Active CD | |
|---|---|---|---|
| n (SAT/VAT) | 6/6 | 6/5 | 10/10 |
| Sex (male/female) | 3/3 | 3/3 | 5/5 |
| Age (years) | 43 ± 8.2 | 41.2 ± 5.4 | 39.8 ± 12.4 |
| BMI (kg/m2) | 23.15 ± 2.1 | 24.59 ± 3.3 | 23.2 ± 3.8 |
| Glucose (mg/dL) | 81.1 ± 4.9 | 87 ± 12.5 | 72 ± 9.7 |
| Cholesterol (mg/dL) | 105 ± 17.3 | 131 ± 23.4 | 150 ± 20.3a |
| HDLc (mg/dL) | 38.9 ± 4.6 | 46.6 ± 4.2a | 42.9 ± 5.4 |
| Triglycerides (mg/dL) | 100 ± 5.3 | 116 ± 13.1a | 96 ± 9.9 |
| Insulin (μIU/mL) | 2.07 ± 0.9 | 5.25 ± 1.2a | 7.5 ± 1.4a,b |
| HOMA-IR | 0.49 ± 0.1 | 1.2 ± 0.5 | 1.45 ± 0.35a |
| Age at diagnosis (years) | – | 38 ± 8.6 | 28.5 ± 9.4 |
| Last attack (months) | – | 24 ± 15.7 | – |
| Indication for surgery | CoH | CoH | 5 SCD/5 FCD |
| Preoperative immunomodulator use | – | 6/6 | 10/10 |
| Preoperative biological agent treatment | – | 0/6 | 0/10 |
| Preoperative steroid treatment | – | 3/6 | 8/10 |
| C-reactive protein (mg/dL) | 0.1 ± 0.09 | 0.39 ± 0.15 | 6.14 ± 3.81a,b |
SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; BMI, body mass index; HDLc, high-density lipoprotein cholesterol; CoH, cholecystectomy or hernia; SCD, stenotic Crohn's disease; FCD, fistulizing Crohn's disease.
Results are presented as mean ± SD. ANOVA followed by post hoc Bonferroni test was used to compare means between groups.
p < 0.01 versus healthy.
p < 0.05 versus inactive CD.
ASCs are considered as regenerative cells that promote tissue repair and regeneration in vivo (Baptista et al., 2015). In the present study we demonstrate that, similar to what is observed in obesity (Serena et al., 2016), ASCs of CD patients are immune activated, are inhibited in their adipogenic differentiation potential, and have dampened immunosuppressive properties. This persistent immune activation in patients may help to explain that while autologous transplants may be effective, they do not always prevent relapse or improve the disease outcome. Moreover, a recent report on allogeneic ASC transplantation reported an efficacy of 26% (Panes et al., 2016), which might be due to the metabolic phenotype of the donor. Our data offer a new perspective to better interpret the discrepancies in clinical assays using ASCs in patients with CD. We propose that ASCs with the best immunomodulatory properties should be chosen, or/and alternatively treated ex vivo to restore their immunosuppressive properties (as shown here with an IL-1β antagonist), before their use in cell therapy both for autologous and allogeneic transplantation. This information may be important for clinical trial design. Clinicians should be aware of possible autologous cell transplant contraindications (reduced anti-inflammatory properties of ASCs in CD patients, including those in remission) and select the best donor regarding also the metabolic phenotype if an allogeneic cell transplant is chosen. While further studies are necessary to fully characterize the molecular events leading to immune activation of resident ASCs, the results shown here highlight their importance in CD and support their role in the formation of creeping fat. Moreover, our findings indicate a path for increasing the therapeutic benefit of stem cell transplantation in CD. Future studies in animal models will be needed to demonstrate in vivo the reduced anti-inflammatory properties associated with CD ASCs.
Experimental Procedures
Study Subjects
Subjects were recruited at the University Hospital Joan XXIII (Tarragona, Spain) and University Hospital Vall d'Hebrón (Barcelona, Spain) in accordance with the tenets of the Helsinki Declaration. The corresponding hospital ethics committees approved the study, and written informed consent was obtained from all participants before entering in the study. Donors were classified as those in relapse (active) or in remission (inactive) following the Crohn's Disease Activity Index criteria (Best et al., 1976, Van Assche et al., 2010). Healthy subjects (n = 6) and inactive CD donors (n = 6) were recruited from age- and gender-matched subjects undergoing non-acute surgical interventions such as hernia or cholecystectomy, in a scheduled routine surgery. Active CD patients (n = 10) were recruited from those undergoing surgery for symptomatic complications. VAT from mesenteric origin and SAT were obtained from the same individual during the surgical procedure, with the exception of one inactive CD patient, in which only SAT was available. Clinical data, anthropometric, and biochemical variables from the cohort are presented in Table 1.
ASC Isolation and Culture
ASCs were isolated as described previously (Dubois et al., 2008, Gimble and Guilak, 2003). In brief, SAT and VAT was washed extensively with PBS to remove debris and treated with 0.1% collagenase in PBS and 1% BSA for 1 hr at 37°C with gentle agitation. Digested samples were centrifuged at 300 × g at 4°C for 5 min to separate adipocytes from stromal cells. The cell pellet containing the stromal fraction was resuspended in stromal culture medium consisting of DMEM/F12, 10% fetal bovine serum (FBS), and 1% antibiotic/antimycotic solution. To prevent spontaneous differentiation, primary cultures of ASCs at passage 0 (P0) were grown to 90% confluence and harvested with trypsin-EDTA, and aliquots (1 × 106 cells) were cryopreserved in liquid nitrogen until required (Pachon-Pena et al., 2011). ATMs were also isolated from the stromal vascular fraction of AT biopsies as described previously (Ceperuelo-Mallafre et al., 2016, Serena et al., 2016, Titos et al., 2011). The AT-cell number ratio was defined as the ratio of the number of cells proliferating at P0 per gram of AT digested.
Human ASC Immunophenotyping
ASCs (2 × 105 cells) were incubated with a panel of primary antibodies described in Table S1 (Pachon-Pena et al., 2016). After isolation, the minimal functional and quantitative criteria, established by the International Society of Cell Therapy and the International Federation for Adipose Therapeutics and Science, were routinely confirmed by flow cytometry as described previously (Pachon-Pena et al., 2016, Serena et al., 2016). All experiments were performed in cells at P3–P7.
Cell Migration Assay
Basal migratory capacity of ASCs was analyzed using a Transwell system (8-μm pore polycarbonate membrane) as described previously (Baek et al., 2011, Corcione et al., 2006, Serena et al., 2016). The migratory capacity of human monocytes (THP-1 cell line) and T and B lymphocytes (Jurkat and MEC-1 cells, respectively) in response to application of 24-hr CM from undifferentiated ASCs was performed using 5-μm pore polycarbonate Transwell inserts as described by Serena et al. (2016). A similar experiment was performed to examine the migratory capacity of ASCs and T cells (Jurkat) in response to 24-hr CM from VAT explants of active (CF origin) and from healthy individuals (mesenteric origin).
Cell Invasion Assay
Invasion capacity was determined as for migration except that the membrane was first coated with Matrigel (Sigma, St. Louis, MO, USA) in PBS for 2 hr at 37°C. ASCs were added to the upper chamber and incubated for 24 hr at 37°C. Cells invading the lower surface of the Transwell membrane were stained and counted.
Zymography
To determine MMP-2 and MMP-9 activity, near-confluent ASCs (80%) were deprived of serum for 24 hr and the CM was electrophoresed in 8% SDS-polyacrylamide gels polymerized with 0.1% gelatin under non-reducing conditions. Gels were washed with 2.5% Triton X-100 (30 min) to remove SDS, rinsed with substrate buffer (0.2 M NaCl, 5 mM CaCl2, 1% Triton X-100, 0.02% NaN3, 50 mM Tris [pH 7.5]) and incubated in this buffer at 37°C overnight to allow protein renaturation and MMP activation. Gels were stained with Coomassie brilliant blue (Bio-Rad, Richmond CA, USA) to visualize gelatin degradation.
Phagocytosis Assay
The pHrodo Escherichia coli (bacteria) and Zymosan (yeast) BioParticles Phagocytosis Kits (Invitrogen Molecular Probes, Eugene, OR, USA) were used to assess the phagocytic capacity of undifferentiated ASCs (seeded at a density of 20,000 cells/cm2). Phagocytic activity was quantified using the Varioskan LUX multimode microplate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Cell Proliferation Assays
MTT Assay
Cell proliferation was determined by a standard colorimetric MTT assay. In brief, ASCs were seeded in 96-well plates and allowed to attach for 24 hr; after which the MTT assay (day +1) was performed to count the initial number of cells. After 7 days, a second MTT assay was performed (day +7), and the difference in absorbance between day +7 and day +1 was considered the proliferation of the ASCs.
BrdU Assay
ASC proliferation was also assessed by incorporation of BrdU using the BrdU Cell Proliferation Assay Kit (Millipore, Billerica, MA, USA). Cells (10,000/well) were cultured in 96-well plates containing DMEM/F12 medium with 10% (v/v) FBS at 37°C in 5% CO2 and allowed to attach for 24 hr. BrdU was added to the medium and cells were incubated for a further 18 hr. Cells were then fixed, and BrdU incorporation was determined with an anti-BrdU specific antibody with detection by spectrophotometry at 450 nm. Proliferation of Jurkat and MEC-1 cells in response to CM from ASCs was performed in the same manner.
Co-culture of Naive T Lymphocytes and ASCs
Co-culture experiments between human naive T lymphocytes and ASCs isolated from healthy subjects or CD patients were performed to measure T cell responses. In brief, 60,000 ASCs/well from healthy or active CD patients were seeded in 12-well plates and allowed to attach overnight; after which 800,000 T cells/well were added. After 48 hr, the supernatant was collected to determine T cell subsets (Th1/Th2/Treg) using the Multi-analyte ELISA Array Kit, MEH:003A (Qiagen, Germany), and the gene expression of T cells subset markers were assessed by qPCR.
Statistical Analysis
For in vitro data, experimental results are presented as mean ± SD from 4–10 independent experiments (independent donors) performed at least in duplicate. Comparisons among the three groups were performed using the non-parametric Kruskal-Wallis test and between two groups using the Mann-Whitney U test with Bonferroni adjustment (significance, p < 0.017). For clinical and anthropometrical variables, normally distributed data are expressed as mean ± SD and for variables with no Gaussian distribution, values are expressed as median (interquartile range). Statistical analysis was performed with the Statistical Package for the Social Sciences software, version 15 (SPSS, Chicago, IL, USA). For details of further experiments, see Supplemental Experimental Procedures.
Author Contributions
C.S., N.K., A.M., E.M.-M., M.E., and M.T.-P. carried out the experiments and generated data. E.E., M. Martí, N.B., F.G., M. Menacho, and M. Millan carried out part of the study selection and human sample processing. A.Z., M. Millan, and S.F.-V. contributed to the discussion and reviewed the manuscript. C.S. and J.V. conceived the study, discussed data, and wrote the manuscript, and are the guarantors of this work. N.K. and A.M. contributed equally.
Acknowledgments
This study was supported by grants from the Spanish Ministry of Economy and Competitiveness (MINECO) and Instituto de Salud Carlos III (PI15/00143 to C.S.; PIE14/00045 (Inflammes), PI14/00228, and CB07708/0012 to J.V.; and SAF2015-65019-R to S.F.-V.) and a unrestricted grant supported by Menarini Lab SA. C.S. acknowledges support from the “Ramón y Cajal' program from MINECO (RYC2013-13186), and S.F.-V. from the Miguel Servet tenure-track program (CPII16/00008) from the Fondo de Investigación Sanitaria. A.M. acknowledges support from MINECO (FJCI-2014-23060). All were co-financed by the European Regional Development Fund. The authors thank Kelly Roche and Cati Nuñez for excellent technical assistance and Dr. McCreath for helpful comments on the manuscript.
Published: September 28, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, three figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.07.014.
Contributor Information
Carolina Serena, Email: carolserena@gmail.com.
Joan Vendrell, Email: jvo@comt.es.
Supplemental Information
References
- Altundag K., Altundag O., Baptista M.Z., Akyurek S. Heparanase activity and bone loss in postmenopausal breast cancer patients. J. Clin. Oncol. 2005;23:8916–8917. doi: 10.1200/JCO.2005.04.0121. author reply 8917–8918. [DOI] [PubMed] [Google Scholar]
- Baek S.J., Kang S.K., Ra J.C. In vitro migration capacity of human adipose tissue-derived mesenchymal stem cells reflects their expression of receptors for chemokines and growth factors. Exp. Mol. Med. 2011;43:596–603. doi: 10.3858/emm.2011.43.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista L.S., Silva K.R., Borojevic R. Obesity and weight loss could alter the properties of adipose stem cells? World J. Stem Cells. 2015;7:165–173. doi: 10.4252/wjsc.v7.i1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo I., Li Volti G., Galvano F., Tettamanti G., Pluchinotta F.R., Bergante S., Vanella L. Diabetic human adipose tissue-derived mesenchymal stem cells fail to differentiate in functional adipocytes. Exp. Biol. Med. (Maywood) 2017;242:1079–1085. doi: 10.1177/1535370216681552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra A., Heimesaat M.M., Bereswill S., Fischer A., Glauben R., Kunkel D., Scheffold A., Erben U., Kuhl A., Loddenkemper C. Mesenteric fat - control site for bacterial translocation in colitis? Mucosal Immunol. 2012;5:580–591. doi: 10.1038/mi.2012.33. [DOI] [PubMed] [Google Scholar]
- Best W.R., Becktel J.M., Singleton J.W., Kern F., Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- Buning C., von Kraft C., Hermsdorf M., Gentz E., Wirth E.K., Valentini L., Haas V. Visceral adipose tissue in patients with Crohn's disease correlates with disease activity, inflammatory markers, and outcome. Inflamm. Bowel Dis. 2015;21:2590–2597. doi: 10.1097/MIB.0000000000000527. [DOI] [PubMed] [Google Scholar]
- Carracedo A., Cantley L.C., Pandolfi P.P. Cancer metabolism: fatty acid oxidation in the limelight. Nat. Rev. Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceperuelo-Mallafre V., Ejarque M., Serena C., Duran X., Montori-Grau M., Rodriguez M.A., Yanes O., Nunez-Roa C., Roche K., Puthanveetil P. Adipose tissue glycogen accumulation is associated with obesity-linked inflammation in humans. Mol. Metab. 2016;5:5–18. doi: 10.1016/j.molmet.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J.C., O'Leary D.P. The mesentery: structure, function, and role in disease. Lancet Gastroenterol. Hepatol. 2016;1:238–247. doi: 10.1016/S2468-1253(16)30026-7. [DOI] [PubMed] [Google Scholar]
- Connelly T.M., Juza R.M., Sangster W., Sehgal R., Tappouni R.F., Messaris E. Volumetric fat ratio and not body mass index is predictive of ileocolectomy outcomes in Crohn's disease patients. Dig. Surg. 2014;31:219–224. doi: 10.1159/000365359. [DOI] [PubMed] [Google Scholar]
- Corcione A., Benvenuto F., Ferretti E., Giunti D., Cappiello V., Cazzanti F., Risso M., Gualandi F., Mancardi G.L., Pistoia V. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Desreumaux P., Ernst O., Geboes K., Gambiez L., Berrebi D., Muller-Alouf H., Hafraoui S., Emilie D., Ectors N., Peuchmaur M. Inflammatory alterations in mesenteric adipose tissue in Crohn's disease. Gastroenterology. 1999;117:73–81. doi: 10.1016/s0016-5085(99)70552-4. [DOI] [PubMed] [Google Scholar]
- Dubois S.G., Floyd E.Z., Zvonic S., Kilroy G., Wu X., Carling S., Halvorsen Y.D., Ravussin E., Gimble J.M. Isolation of human adipose-derived stem cells from biopsies and liposuction specimens. Methods Mol. Biol. 2008;449:69–79. doi: 10.1007/978-1-60327-169-1_5. [DOI] [PubMed] [Google Scholar]
- Ejarque M., Ceperuelo-Mallafre V., Serena C., Pachon G., Nunez-Alvarez Y., Terron-Puig M., Calvo E., Nunez-Roa C., Oliva-Olivera W., Tinahones F.J. Survivin, a key player in cancer progression, increases in obesity and protects adipose tissue stem cells from apoptosis. Cell Death Dis. 2017;8:e2802. doi: 10.1038/cddis.2017.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C., Karagiannides I., Bakirtzi K., Pothoulakis C. Adipose tissue and inflammatory bowel disease pathogenesis. Inflamm. Bowel Dis. 2012;18:1550–1557. doi: 10.1002/ibd.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J., Guilak F. Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy. 2003;5:362–369. doi: 10.1080/14653240310003026. [DOI] [PubMed] [Google Scholar]
- Goncalves P., Magro F., Martel F. Metabolic inflammation in inflammatory bowel disease: crosstalk between adipose tissue and bowel. Inflamm. Bowel Dis. 2015;21:453–467. doi: 10.1097/MIB.0000000000000209. [DOI] [PubMed] [Google Scholar]
- Grant R.W., Dixit V.D. Mechanisms of disease: inflammasome activation and the development of type 2 diabetes. Front. Immunol. 2013;4:50. doi: 10.3389/fimmu.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelman M.D., Rifas-Shiman S.L., Porter C.Q., Ollendorf D.A., Sandler R.S., Galanko J.A., Finkelstein J.A. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredel L.I., Siegmund B. Adipose-tissue and intestinal inflammation—visceral obesity and creeping fat. Front. Immunol. 2014;5:462. doi: 10.3389/fimmu.2014.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis T., Batra A., Siegmund B. Bacterial translocation—impact on the adipocyte compartment. Front. Immunol. 2014;4:510. doi: 10.3389/fimmu.2013.00510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavy O. Immunotherapy: stopping monocytes in their tracks. Nat. Rev. Immunol. 2011;11:715. doi: 10.1038/nri3096. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhu W., Gong J., Zhang W., Gu L., Guo Z., Cao L., Shen B., Li N., Li J. Visceral fat area is associated with a high risk for early postoperative recurrence in Crohn's disease. Colorectal Dis. 2015;17:225–234. doi: 10.1111/codi.12798. [DOI] [PubMed] [Google Scholar]
- Melief S.M., Schrama E., Brugman M.H., Tiemessen M.M., Hoogduijn M.J., Fibbe W.E., Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980–1991. doi: 10.1002/stem.1432. [DOI] [PubMed] [Google Scholar]
- Meyer L.K., Ciaraldi T.P., Henry R.R., Wittgrove A.C., Phillips S.A. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte. 2013;2:217–226. doi: 10.4161/adip.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachon-Pena G., Yu G., Tucker A., Wu X., Vendrell J., Bunnell B.A., Gimble J.M. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J. Cell. Physiol. 2011;226:843–851. doi: 10.1002/jcp.22408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachon-Pena G., Serena C., Ejarque M., Petriz J., Duran X., Oliva-Olivera W., Simo R., Tinahones F.J., Fernandez-Veledo S., Vendrell J. Obesity determines the immunophenotypic profile and functional characteristics of human mesenchymal stem cells from adipose tissue. Stem Cells Transl. Med. 2016;5:464–475. doi: 10.5966/sctm.2015-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panes J., Garcia-Olmo D., Van Assche G., Colombel J.F., Reinisch W., Baumgart D.C., Dignass A., Nachury M., Ferrante M., Kazemi-Shirazi L. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. doi: 10.1016/S0140-6736(16)31203-X. [DOI] [PubMed] [Google Scholar]
- Papa P.C., Seraphim P.M., Machado U.F. Loss of weight restores GLUT 4 content in insulin-sensitive tissues of monosodium glutamate-treated obese mice. Int. J. Obes. Relat. Metab. Disord. 1997;21:1065–1070. doi: 10.1038/sj.ijo.0800517. [DOI] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Chamaillard M., Gonzalez F., Beclin E., Decourcelle C., Antunes L., Gay J., Neut C., Colombel J.F., Desreumaux P. Mesenteric fat in Crohn's disease: a pathogenetic hallmark or an innocent bystander? Gut. 2007;56:577–583. doi: 10.1136/gut.2005.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin-Biroulet L., Gonzalez F., Dubuquoy L., Rousseaux C., Dubuquoy C., Decourcelle C., Saudemont A., Tachon M., Beclin E., Odou M.F. Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease. Gut. 2012;61:78–85. doi: 10.1136/gutjnl-2011-300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletto A.C., David-Silva A., Yamamoto A.P., Machado U.F., Furuya D.T. Reduced Slc2a4/GLUT4 expression in subcutaneous adipose tissue of monosodium glutamate obese mice is recovered after atorvastatin treatment. Diabetol. Metab. Syndr. 2015;7:18. doi: 10.1186/s13098-015-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourgholaminejad A., Aghdami N., Baharvand H., Moazzeni S.M. The effect of pro-inflammatory cytokines on immunophenotype, differentiation capacity and immunomodulatory functions of human mesenchymal stem cells. Cytokine. 2016;85:51–60. doi: 10.1016/j.cyto.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Rutella S., Zavala F., Danese S., Kared H., Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J. Immunol. 2005;175:7085–7091. doi: 10.4049/jimmunol.175.11.7085. [DOI] [PubMed] [Google Scholar]
- Serena C., Keiran N., Ceperuelo-Mallafre V., Ejarque M., Fradera R., Roche K., Nunez-Roa C., Vendrell J., Fernandez-Veledo S. Obesity and type 2 diabetes alters the immune properties of human adipose derived stem cells. Stem Cells. 2016;34:2559–2573. doi: 10.1002/stem.2429. [DOI] [PubMed] [Google Scholar]
- Titos E., Rius B., Gonzalez-Periz A., Lopez-Vicario C., Moran-Salvador E., Martinez-Clemente M., Arroyo V., Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- Van Assche G., Dignass A., Reinisch W., van der Woude C.J., Sturm A., De Vos M., Guslandi M., Oldenburg B., Dotan I., Marteau P. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: special situations. J. Crohns Colitis. 2010;4:63–101. doi: 10.1016/j.crohns.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kiyohara T., Murayama Y., Kihara S., Okamoto Y., Funahashi T., Ito T., Nezu R., Tsutsui S., Miyagawa J.I. Production of adiponectin, an anti-inflammatory protein, in mesenteric adipose tissue in Crohn's disease. Gut. 2005;54:789–796. doi: 10.1136/gut.2004.046516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubkova E.S., Beloglazova I.B., Makarevich P.I., Boldyreva M.A., Sukhareva O.Y., Shestakova M.V., Dergilev K.V., Parfyonova Y.V., Menshikov M.Y. Regulation of adipose tissue stem cells angiogenic potential by tumor necrosis factor-alpha. J. Cell. Biochem. 2016;117:180–196. doi: 10.1002/jcb.25263. [DOI] [PubMed] [Google Scholar]
- Zulian A., Cancello R., Micheletto G., Gentilini D., Gilardini L., Danelli P., Invitti C. Visceral adipocytes: old actors in obesity and new protagonists in Crohn's disease? Gut. 2012;61:86–94. doi: 10.1136/gutjnl-2011-300391. [DOI] [PubMed] [Google Scholar]
- Zulian A., Cancello R., Ruocco C., Gentilini D., Di Blasio A.M., Danelli P., Micheletto G., Cesana E., Invitti C. Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn's disease. An in vivo and in vitro study. PLoS One. 2013;8:e78495. doi: 10.1371/journal.pone.0078495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.