Abstract
The BDNF Val66Met polymorphism has been associated with sensitivity to stress and affective disorders. We therefore sought to model the inter-causality of these relationships under controlled laboratory conditions. We subjected humanized BDNF Val66Met (hBDNFVal66Met) transgenic mice to a history of stress, modeled by chronic late-adolescent corticosterone (CORT) exposure, before evaluating affective-related behavior using the forced-swim test (FST) in adulthood. While hBDNFMet/Met mice had a depression-like phenotype in the FST irrespective of CORT, hBDNFVal/Val wildtype mice had a resilient phenotype but developed an equally robust depressive-like phenotype following CORT. A range of stress-sensitive molecules were studied across the corticohippocampal axis, and where genotype differences occurred following CORT they tended to inversely coincide with the behavior of the hBDNFVal/Val group. Notably, tyrosine hydroxylase was markedly down-regulated in the mPFC of hBDNFVal/Val mice as a result of CORT treatment, which mimicked expression levels of hBDNFMet/Met mice and the FST behavior of both groups. The expression of calretinin, PSD-95, and truncated TrkB were also concomitantly reduced in the mPFC of hBDNFVal/Val mice by CORT. This work establishes BDNFVal66Met genotype as a regulator of behavioral despair, and identifies new biological targets of BDNF genetic variation relevant to stress-inducible disorders such as depression.
Introduction
Affective disorders, such as major depressive disorder, have an estimated lifetime prevalence of ~20.8%,1 and are projected to become a leading cause of long-term disability.2 The neurobiology of affective disorders has advanced rapidly, and has led to the identification of a number of core pathology that includes reduced neurotrophic support and synaptic remodeling3 within brain regions such as the hippocampus4, 5 and medial prefrontal cortex (mPFC).5
One environmental factor that has consistently been associated with the onset and maintenance of affective disorders is stress. Clinical markers of stress exposure, such as daily life stress, history of stressful life events, and trauma have all been shown to play a role in aspects of affective disorder symptomology or risk, and collectively support the assertion that both developmental and ongoing stress are capable of inducing depressive disorders.6 In rodent models, exposure to a broad range of stress paradigms has led to the identification of several remodeling events that putatively occur as a result of glucocorticoid stress hormones within the brain. These include widespread alterations within the hippocampus, including reductions in dendritic spine branching and complexity,7 changes in the expression of brain-derived neurotrophic factor (BDNF),8 NMDA receptor subunit reorganization9 and synaptic scaffolding proteins such as the excitatory postsynaptic molecule PSD-9510 and presynaptic marker synaptophysin.11 A similar pattern of reorganization following stress also occurs in other brain regions including the mPFC, which appears particularly vulnerable to stress-induced alterations in noradrenergic activity12, 13 and the maturation of inhibitory interneuron networks.14
BDNF has been widely studied as a susceptibility factor for both stress and affective dysregulation. BDNF plays a fundamental role in brain development, neuronal differentiation and synaptic plasticity.15 It has been suggested that BDNF is a transducer of antidepressant effects,16 principally because BDNF is recruited by antidepressant therapeutics (as well as other mood disorder treatments such as electroconvulsive shock17 and transcranial magnetic stimulation therapies18) and is suppressed by many risk factors for mood disorders including stress.19 In rodent models, BDNF also mediates behavioral endophenotypes of relevance to affective disorders,20, 21 while in clinical samples serum BDNF concentrations predict the effectiveness of selective-serotonin reuptake inhibitors in the treatment of depression.22
The BDNF Val66Met polymorphism, named after a Valine→Methionine substitution at codon 66 within the BDNF prodomain, has been widely studied as a risk factor for affective disorders due to its common frequency and established functionality.15 Specifically, the Val66Met substitution results in the diminished activity-dependent release of BDNF,23 deficient hippocampus-dependent memory function24 and a lack of response to antidepressant therapeutics in BDNFVal66Met knock-in mice.24, 25 However, the role of this gene variant as a risk factor for mood disorders and modulator of antidepressant response has been the source of much controversy given non-concordant results between association studies (see ref. 15 for extensive review). These inconsistent clinical findings strengthen the case for animal models in providing well controlled findings on the biological mechanisms which underpin stress responsivity, as well as antidepressant response. That said, a number of reports have emerged in recent years which suggest that the Val66Met variant may induce HPA axis dysfunction,25 which we previously hypothesized may lead to a long-lasting sensitivity to glucocorticoid stress hormones15 and thus vulnerability to affective dysregulation. In support of this hypothesis, it has been previously published that childhood adversity may unmask an effect of the 66Met allele on depression,26 while in otherwise healthy adults a history of sexual trauma has been shown to moderate the expression of depressive symptoms among 66Met allele carriers.27 Of concern, however, is a report which suggests that first-episode depression patients that carry BDNFVal/Met and BDNFMet/Met genotypes are more likely to have experienced stressful life events than BDNFVal/Val genotype controls,28 suggesting that this variant may induce a stress-sensitivity loop whereby 66Met allele carriers are not just more sensitive to the effects of stress but are also more likely to experience stressful life events.
Therefore, we sought to resolve what effect BDNFVal66Met genotype has on corticohippocampal molecular remodeling and depression-related behavioral despair using the forced-swim test (FST), and whether a phenotype was dependent on, or unmasked by, a history of chronic stress.
Materials and methods
Genetic construct of humanized BDNFVal66Met mice
The BDNF gene is highly conserved between species,29 and despite differences in promoter structure the coding exon of mouse Bdnf closely resembles that of human BDNF. To generate the Val66Met knock-in human-BDNF expressing (hitherto referred to as hBDNFVal66Met) mice, a 274bp region was amplified from one BDNFVal/Val and one BDNFMet/Met human donor and inserted into the mouse BDNF gene, replacing the respective murine sequence.30 Extensive procedural details (including details of recombination probes and targeting constructs) are available in a previous publication.30 Once generated, BDNFHuman Val/+ mice were crossed with BDNFHuman Met/+ mice to generate hBDNFVal/Met mice, which were subsequently used as breeders to produce hBDNFVal/Val and hBDNFMet/Met mice. All mice were maintained on a C57BL/6 background. Mice were bred and group-housed in individually ventilated cages under standard lighting conditions (12 h light cycle) and provided ad libitum access to food and water. All experimental procedures were approved by the Florey Institute of Neuroscience’s animal ethics committee, and conducted in accordance with guidelines set by the National Health and Medical Research Council of Australia.
Late-adolescent chronic CORT protocol
Chronic adolescent stress was simulated by treating pseudorandomly selected mice with 25 mg l−1 of the mouse stress hormone, corticosterone (CORT), which was dissolved in the animal’s drinking water, as previously described.31 This dosage was selected based on previous work which has shown that this low-dosage recapitulates depression-like brain and behavioral phenotypes without peripheral side-effects that may influence animal health.32 Importantly, chronic CORT delivered via drinking water also recapitulates several translationally relevant aspects of daily life stress, as it follows a diurnal cycle and is capable of inducing persistent alterations in anxiety- and affective-related behavior that can be rescued by antidepressant treatment.32 This model thus allows the long-term evaluation of stress-induced changes in brain chemistry or behavioral outputs that last beyond the disruption of the HPA axis during treatment,32, 33 as is similar to the lasting effects of early life stress on the chronicity of depression-related symptoms in humans.34 Animals assigned to the chronic CORT group received the treatment solution between weeks six to nine, a developmental period which is similar to late adolescence in humans as evidenced by a late spike in the production of sex-steroid hormones.35 Mice allocated to the control group received unaltered water over this timeframe. There was a two-week washout period following treatment, so that the HPA axis and circulating glucocorticoids levels could recover,33 before behavioral testing commenced.
Forced-swim test
The forced-swim test was used as our representative model of learned helplessness and behavioral despair. Mice were ~15 weeks of age at testing. Four swim chambers (maximum volume of 2 l) were filled with 1.7 l of water pre-heated to 21 °C, and were separated by white dividers. Before experimentation, mice were habituated for one hour in the test room. Following habituation, mice were individually placed into a swim chamber and allowed to swim for six minutes. Behavior was recorded for offline analysis, with time spent immobile – defined as a lack of swimming movement of a duration equal to or exceeding one second – being quantified by two observers who were unaware of group allocations. The primary outputs were latency to immobility, the time-course of immobility over the test session and the average immobility over the last 4 minutes of testing.
Preparation of brain lysates and western blot
Experimental mice were killed via cervical dislocation one week after completing behavior. Brains were snap-frozen on dry ice, stored at −80 °C and later dissected with a brain matrix according to the Paxinos and Watson atlas.36 The dorsal hippocampus (DHP), ventral hippocampus (VHP) and mPFC (which included the prelimbic, infralimbic and cingulate cortices) were chosen as our primary regions of interest given their involvement in mood disorders. Once dissected, protein was extracted and aliquoted into 50 μg protein samples before undergoing SDS–PAGE and transfer to nitrocellulose membrane, as previously described.31 Membranes were incubated in 5% bovine serum albumin in Tris-buffered saline with primary antibody overnight (β-Actin: 1:10000, Sigma-Aldrich, Sydney, NSW, Australia; Calretinin: 1:1000, Swant, Switzerland cr7697; fl. and tr.TrkB: 1:1000, Santa Cruz Biotechnology, Dallas, TX, USA H181; pTrkBY515: 1:1000, Abcam, Cambridge, UK ab109684; Gephyrin: 1:1000, Abcam ab32206; NR2A: 1:1000, Abcam ab14596; NR2B/NR1: 1:200, Abcam ab110; PSD-95: 1:1,000, Abcam ab18258; Synaptophysin: 1:400, Sigma-Aldrich s5768; Tyrosine Hydroxylase: 1:1,000, Millipore, Bayswater, VIC, Australia ab152). Blots were imaged using a LAS-4000 Luminescence Analyzer (Fuji Film Life Science, Stamford, CT, USA) and analyzed using the TotalLab Quant Analysis Software (Total Lab, Newcastle, UK). Western blots were repeated between two to four times to confirm results.
BDNF Enzyme-Linked immunosorbent assay
BDNF expression was quantified using the BDNF Emax ImmunoAssay System (Promega, Madison, WI, USA), so that an exact concentration per region could be derived between hBDNFVal66Met genotype groups. According to the manufacturer, the kit has a sensitivity as low as 15.6 pg μl−1, with <3% cross-reactivity to other neurotrophins. Assays were performed according to manufacturer’s instructions. Briefly, a 96-well plate was coated with 100 μl of anti-BDNF antibody diluted in carbonate-coating buffer (10 μl:9.99 ml) and incubated overnight. The following morning, plates were blocked for 1 h with supplied buffer before 100 μl of BDNF standards and experimental samples were plated in duplicate. After incubating with shaking for 2 h, 100 μl of an anti-BDNF polyclonal antibody solution (1:500 diluted in blocking buffer) was added per well and incubated for a further 2 h. Following washing, 100 μl of diluted anti-IgY HRP conjugate (1:200 in blocking buffer) was added to wells and incubated for 1 h. Absorption was correspondingly developed by adding 100 μl of supplied TMB solution per well and and incubating for 10 min, and terminated with 100 μl per well of 1 N hydrochloric acid. Absorption was read at 450 nm using a plate reader.
Statistical analysis
The total sample size of the current study was 166 hBDNFVal66Met mice, comprising 26 control and 25 CORT-treated hBDNFVal/Val mice, 40 control and 28 CORT-treated hBDNFVal/Met mice, and 23 control and 24 CORT-treated hBDNFMet/Met mice. Sampling was based on our prior investigations using this mouse line, which have been adequately powered to detect medium-to-large as well as more subtle effects upon pooling (see below; refs. 31, 37). Data analysis was undertaken using the IBM SPSS and Graphpad Prism packages. For tests that only involve between-group comparisons a 3 (genotype) × 2 (sex) × 2 (treatment) Analysis of Variance (ANOVA) was conducted, with assumptions being screened for. Within-group comparisons were analyzed using a Mixed Model ANOVA. SEMs were used as our measure of variance for all graphing. As no significant interaction involving the main effect of sex was observed on any given behavioral or molecular measure, denoting no effect of sex on the main effects of genotype and treatment, data from male and female mice were analyzed together to increase power as consistent with previous investigations.31, 37 No exclusion criteria were applied, except for outliers (defined as values falling outside of ± 2 s.d.). Statistical significance was set at P=0.05, as per Fisher’s tables, while all between-group comparisons were corrected for multiple comparisons using Tukey’s or Holm-Sidak’s method depending on observed power.
Results
hBDNFVal66Met genotype determines vulnerability to stress-related despair
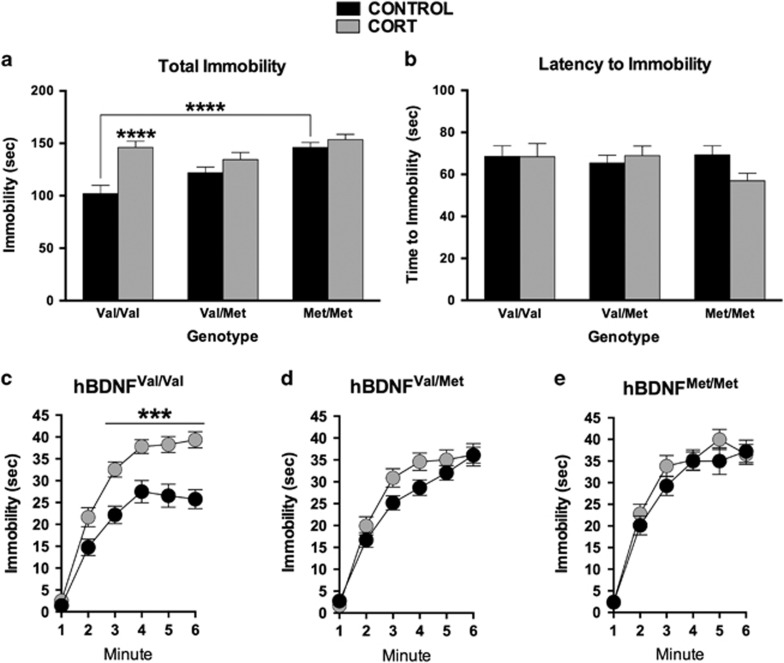
The FST was used as our model of behavioral despair. Analysis revealed main effects of hBDNFVal66Met Genotype (F(2,160)=9.7, P=0.0001) and chronic CORT (F(1,160)=17.5, P<0.0001), as well as their interaction (F(2,160)=5.0, P=0.0077), on immobility. Post-hoc comparisons of the significant main effects revealed that mice carrying the hBDNFMet/Met genotype were immobile for significantly longer than hBDNFVal/Val mice at baseline (P<0.0001), while mice treated with CORT were also immobile for significantly longer than those allocated to the control group (P<0.0001). Post-hoc analysis of the genotype × treatment interaction revealed that the chronic CORT treatment selectively increased the immobility of hBDNFVal/Val mice relative to controls (P<0.0001). No effect of CORT was detected among the other genotype groups. No significant differences were observed between groups for latency to immobility.
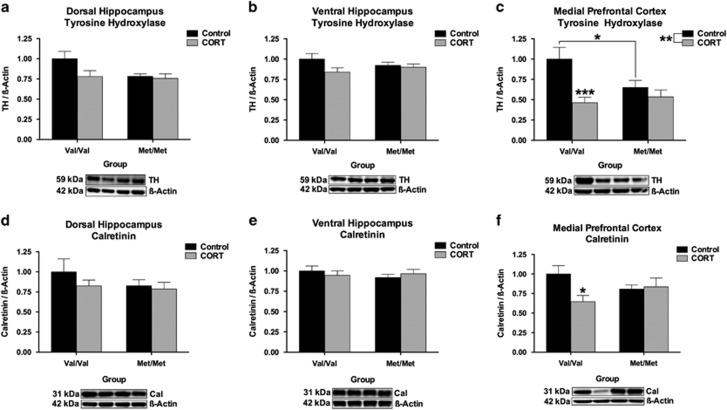
Late-adolescent CORT exposure suppresses tyrosine hydroxylase and calretinin expression in the mPFC of Adult hBDNFVal/Val Mice
Expression of tyrosine hydroxylase (TH), the rate-limiting enzyme involved in the biosynthesis of catecholamine neurotransmitters such as noradrenaline,38 has been implicated in suicidality,39 is altered in depression40, 41 and is responsive to antidepressant therapeutics.42 In addition, gene variants within the TH gene have also been associated with mood disorder symptomology,43 while chronic stress may regulate the release of noradrenaline within the hippocampus44 and prefrontal cortex.12, 45 To this end, we examined whether expression of TH was altered in our three regions of interest in our hBDNFVal66Met mouse line and whether a history of chronic CORT treatment alters the expression of this enzyme in adulthood. There was no effect of hBDNFVal66Met genotype or history of adolescent CORT exposure on the expression of TH in the DHP or VHP. However, within the mPFC a significant hBDNFVal66Met genotype × history of CORT treatment (F(1, 49)=4.42, P=0.041) interaction was observed, whereby chronic CORT reduced the expression of TH in hBDNFVal/Val mice by >50% (P<0.001) to levels that were consistent with vehicle and CORT-treated hBDNFMet/Met mice, which did not differ from one another. A further main effect of CORT was also detected in the mPFC (F(1, 49)=10.6, P=0.002), however no other main effects reached significance.
While TH expression in the mPFC largely represents terminal TH present in projections from the midbrain, there also remains a subset of cortical TH-immunoreactive cells that are predominantly bipolar type inhibitory interneurons that selectively express the calcium-channel binding protein calretinin.46 As such, we next examined whether the expression of calretinin was altered across our regions of interest. There were no significant group differences in the DHP or VHP. However, in the mPFC a hBDNFVal66Met genotype × CORT treatment (F(1, 49)=4.54, P=0.038) interaction was once more observed where, like TH, the expression of calretinin was selectively decreased among the hBDNFVal/Val group as a function of CORT treatment (P<0.05). No further main effects or interactions reached significance.
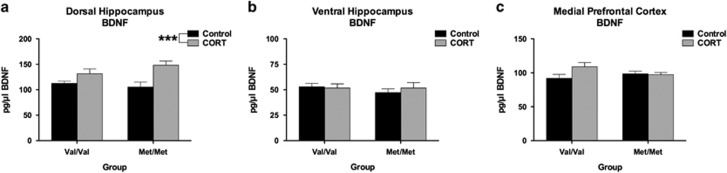
No Effect of hBDNFVal66Met Genotype on Basal BDNF Levels in Corticohippocampal Regions
BDNF levels were quantified via ELISA so to determine an absolute concentration of basal BDNF between groups. BDNF expression in the DHP was not significantly different between hBDNFVal/Val and hBDNFMet/Met mice, however a significant effect of prior CORT treatment was detected in this brain region (F(1,30)=13.73, P=0.0009) reflecting that this treatment increased BDNF expression irrespective of genotype. BDNF expression in the VHP and mPFC did not significantly differ between the various treatment and genotype groups. No other main effects or comparisons reached significance.
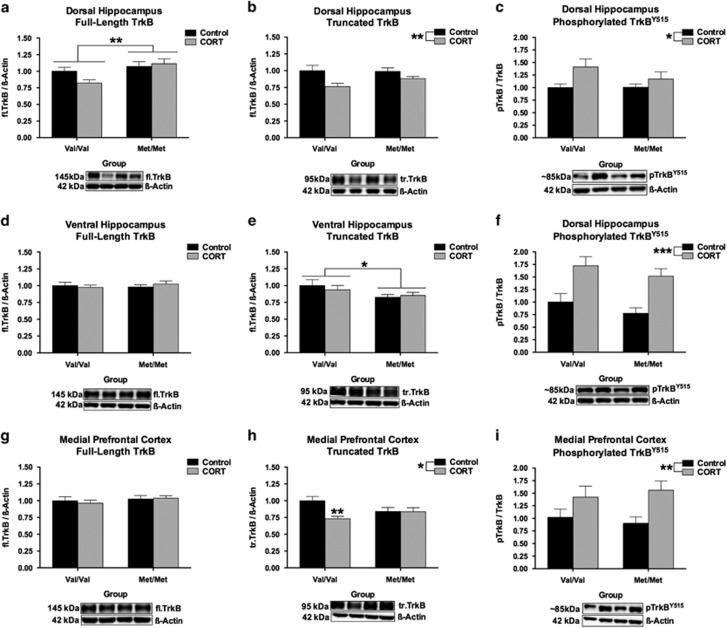
Region-Specific Effects of hBDNFVal66Met Genotype and CORT on TrkB Receptors
We also screened for an effect of hBDNFVal66Met genotype and history of CORT treatment on the expression of BDNF’s cognate receptor TrkB. This included assessments of the functional full-length TrkB (fl.TrkB) receptor, the catalytic domain-lacking and dominant-negative truncated TrkB (tr.TrkB) receptor,47, 48 and the phosphorylation of TrkBY515. In the DHP, a significant effect of hBDNFVal66Met genotype was detected for fl.TrkB (F(1, 52)=8.13, P=0.0062). Post-hoc testing revealed that hBDNFMet/Met mice had higher fl.TrkB expression than hBDNFVal/Val mice in this brain region (P<0.01). While this genotype effect appeared to be moderated by an inhibitory effect of CORT on the expression of TrkB protein in the DHP of hBDNFVal/Val mice, there was no statistical support for a genotype × CORT interaction (P=0.10) in spite of our relatively large group sizes (in this region, per group, n=14). Contrary to this, fl.TrkB was not altered in the VHP or mPFC. The analysis of tr.TrkB receptor expression revealed a significant main effect of chronic CORT treatment (F(1, 51)=9.48, P=0.0033) in the DHP, which decreased tr.TrkB expression irrespective of genotype in this region. In the VHP, a significant main effect of hBDNFVal66Met genotype (F(1, 52)=4.7, P=0.035) emerged, whereby hBDNFMet/Met mice had lower expression of the tr.TrkB isoform than hBDNFVal/Val mice (P<0.05). In the mPFC, a significant main effect of chronic CORT treatment (F(1, 48)=6.03, P=0.018), as well as a hBDNFVal66Met genotype × chronic CORT treatment interaction (F(1, 48)=5.57, P=0.022) emerged for tr.TrkB availability. Post-hoc analysis revealed that while chronic CORT treatment tended to decrease the expression of tr.TrkB irrespective of genotype, the magnitude change in tr.TrkB expression following CORT treatment was more pronounced among hBDNFVal/Val mice (P<0.01). Lastly, the only main effect to reach significance for pTrkBY515 was a history of CORT treatment, which emerged in the DHP (F(1, 51)=5.74, P=0.0203), VHP (F(1, 52)=22.51, P<0.0001) and mPFC (F(1, 40)=8.67, P=0.0054). Chronic CORT increased basal pTrkBY515, which was normalized to fl.TrkB levels, in these regions.
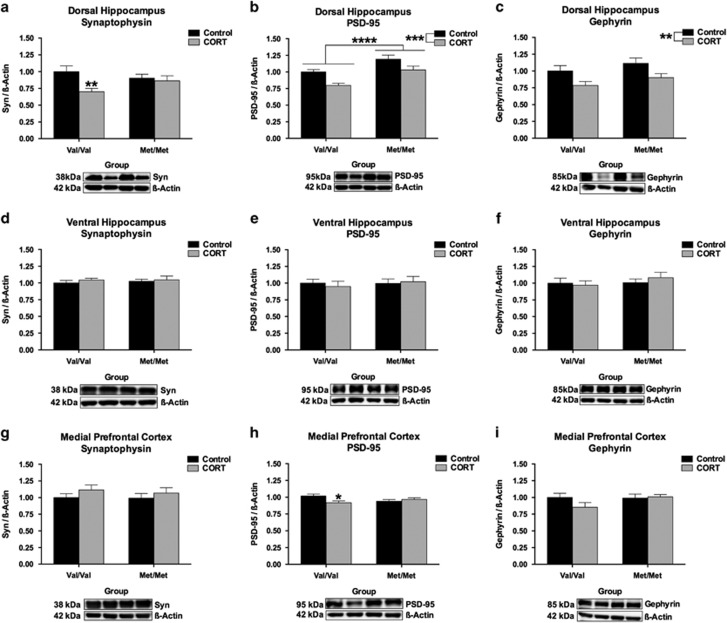
hBDNFVal/Val Mice are more vulnerable than hBDNFMet/Met mice to synaptic protein reorganization following chronic CORT exposure
To assess the effect of hBDNFVal66Met genotype and chronic CORT exposure on synaptic integrity, expression levels of the excitatory and inhibitory terminal scaffolding proteins PSD-95 and gephyrin, as well as the presynaptic vesicle transport molecule, synaptophysin, were screened in the DHP, VHP and mPFC. In the DHP, a significant hBDNFVal66Met genotype x CORT interaction was observed for the expression of synaptophysin (F(1, 43)=4.34, P=0.043), whereby chronic CORT reduced its expression in the DHP of hBDNFVal/Val mice but not hBDNFMet/Met mice (P<0.01). In addition to this, the expression of PSD-95 was found to significantly depend on hBDNFVal66Met genotype in the DHP (F(1, 50)=18.66, P<0.0001); reflecting that hBDNFMet/Met mice had higher expression levels of PSD-95 than hBDNFVal/Val mice in this region. A history of chronic CORT exposure in adolescence also significantly decreased the expression of PSD-95 (F(1, 50)=13.76, P=0.0005), but did not interact with genotype. Similarly, no effect of hBDNFVal66Met genotype was detected on the expression of gephyrin in the DHP, which was reduced irrespective of genotype by history of CORT exposure (F(1, 52)=9.21, P=0.0037). In the VHP, none of the main effects reached significance nor did they interact to determine the expression of synaptophysin, PSD-95 or gephyrin, suggesting that the DHP but not VHP is selectively sensitive to the effect of hBDNFVal66Met genotype and the long-term effect of chronic adolescent CORT treatment. In the mPFC, none of the main effects reached significance for the expression of our synaptic proteins of interest. However, a subtle but significant hBDNFVal66Met genotype × chronic CORT treatment interaction emerged for the expression of PSD-95 (F(1, 48)=6.12, P=0.017). Post-hoc testing revealed that, once more, it was the hBDNFVal/Val wildtype genotype which was associated with reduced PSD-95 expression following the chronic CORT treatment (P<0.05). All other comparisons failed to reach significance.
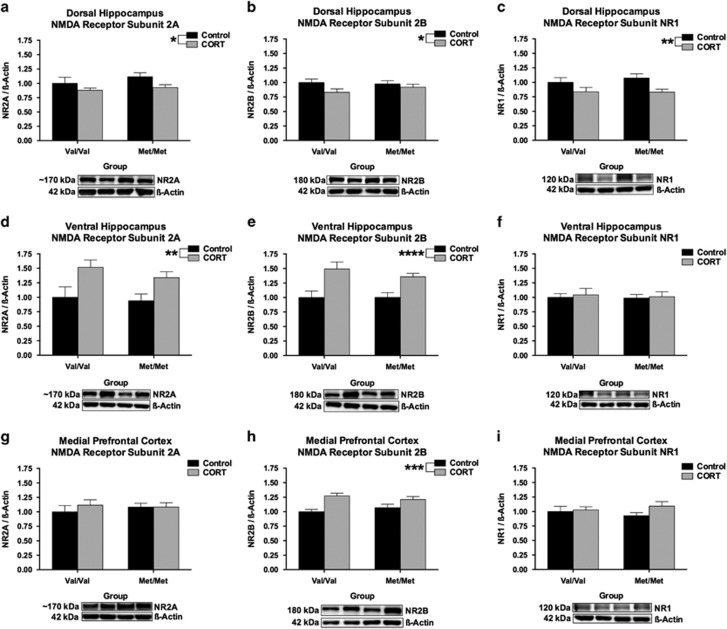
hBDNFVal66Met genotype does not alter NMDA receptor subunit expression
Given the role of PSD-95 as a scaffolding protein involved in securing NMDA receptors to the cell membrane,49 that BDNFVal66Met genotype is associated with altered NMDA receptor physiology50, 51, 52 and that NMDA receptor subunits may modulate FST performance,53, 54, 55 we next screened for differences in expression of the NMDA receptor NR2A, NR2B and NR1 subunits. In the DHP, there was a main effect of chronic CORT treatment on NR2A (F(1, 52)=4.73, P=0.034), NR2B (F(1, 52)=4.06, P=0.049) and NR1 (F(1, 46)=7.97, P=0.007) subunit expression, where a history of CORT decreased the expression of these subunits irrespective of genotype. In the VHP a main effect of CORT was observed for NR2A (F(1, 52)=11.55, P=0.001) and NR2B (F(1, 51)=19.27, P<0.0001) expression, albeit in the opposite direction to the effect seen in the DHP. No effect of hBDNFVal66Met genotype or history of CORT treatment emerged for NR1 within the VHP. Lastly, in the mPFC, the only effect to reach significance was that of CORT on the expression of the NR2B subunit (F(1, 52)=17.25, P=0.0001), where expression was increased as a result of CORT treatment irrespective of hBDNFVal66Met genotype. No other main effects or interactions reached significance.
Discussion
The aim of the current study was to examine the effect of the BDNF Val66Met polymorphism on behavioral despair at baseline, and whether a depression-like phenotype was unmasked or modulated by a history of chronic stress. The FST was used as our representative paradigm for behavioral despair, while a CORT exposure paradigm was chosen as our model of stress due to its specificity in receptor action in the brain and diurnal cycle which recapitulates the core features of chronic life stress. Our data supported our hypothesis that the hBDNFVal66Met genotype gates learned helplessness in adulthood, and that a history of CORT exposure during late adolescence acts to regulate this effect. Specifically, we report that while hBDNFMet/Met mice have a robust learned helplessness phenotype at baseline, following chronic CORT the hBDNFVal/Val genotype group develops a behavioral despair phenotype that is just as robust as that of the hBDNFMet/Met group (Figure 1).
Figure 1.
hBDNFVal66Met genotype and history of CORT treatment regulate immobility in the Forced-Swim Test (FST). (a) A main effect of genotype was detected at baseline for immobility, where hBDNFMet/Met mice were immobile for significantly longer than hBDNFVal/Val mice. An interaction between genotype and CORT treatment was also observed, where the hBDNFVal/Val mice were most susceptible to the effects of CORT on immobility. (b) No main effect or interaction was detected for average latency to immobility. (c–e) An analysis of immobility as a time-course confirmed the selective effect of CORT on this genotype group. All data are presented as mean±s.e.m.; ***P<0.001, ****P<0.0001. Per group, n=23–40.
The presence of a 66Met-derived despair phenotype at baseline in our hBDNFVal66Met model is consistent with the broader literature on the role of BDNF in animal models of depression, where deficient BDNF expression results in social defeat and learned helplessness phenotypes,20, 21 and likely occurs via the disrupted activity-dependent release of BDNF induced by the 66Met substitution. In favor of the construct validity of this model, our behavioral data are also consistent with clinical data from population genetic studies which have reported evidence that the 66Met allele may be associated with affective disorders, as well as clinical components of these disorders.15 However, this clinical evidence has been inconsistent,15 possibly due to the involvement of interaction factors such as vulnerability to stress. In this respect, our observation that this enduring learned helplessness phenotype occurs at baseline our hBDNFMet/Met genotype group, but that the hBDNFVal/Val wildtype genotype produces a convergent phenotype following a history of simulated stress exposure, provides support for a role of the BDNFVal66Met variant in mood disorders, while also providing an explanation for why results may have failed to replicate between some studies (as a long-term effect of prior stress or trauma may not have been stratified as covariates). In addition to this, the current data also extend our prior report using this mouse line and model of stress31 by establishing that glucocorticoid stress hormones differentially interact with BDNFVal66Met genotype to alter behavior depending on the system being studied and construct being measured. Specifically, we previously replicated a gene-dosage effect of the hBDNFMet/Met genotype on fear conditioned memory and short-term spatial memory which we found could be ‘rescued’ by chronic CORT,31 while in other behavioral paradigms, such as prepulse inhibition, chronic CORT exposure unveiled a heterozygote disadvantage phenotype among hBDNFVal/Met mice relative to both homozygous genotype groups.37 In this respect, we can confirm that for behavioral despair on the FST an effect of hBDNFVal66Met genotype emerges at baseline, and that chronic CORT exposure induces a deficit among hBDNFVal/Val mice, while having no further effect on hBDNFVal/Met mice or hBDNFMet/Met mice (see Figure 1). Thus, hBDNFMet/Met mice perform on the FST similarly to CORT-treated hBDNFVal/Val mice, implicating that the Val66Met variant induces vulnerability to behavioral despair.
Our investigation of the mechanism that underscores this convergence in FST phenotype between hBDNFMet/Met and CORT-treated hBDNFVal/Val mice revealed several differences in the long-term molecular adaptation to chronic CORT treatment between hBDNFVal/Val and hBDNFMet/Met mice. Specifically, in the mPFC, a history of chronic CORT treatment decreased the expression of TH in hBDNFVal/Val mice to levels consistent with the hBDNFMet/Met genotype group (see Figure 2c), which inversely corresponded with the FST performance of both genotype groups. TH within the cortex is believed to largely originate from terminals of catecholamine projections that emanate from within the midbrain. These projections originate from brain regions responsible for producing dopamine and noradrenaline, which have both been implicated in the adaptation to stress56 and mood disorders.57 Therefore, our TH phenotype may be related to both dopamine and/or noradrenaline activity. However, as BDNF+/Met mice have increased noradrenergic transporter expression within the locus coeruleus,25 and considering that the noradrenergic agent desipramine (but not the SSRI fluoxetine) rescues the FST phenotype of BDNF+/Met mice following restraint stress,25 the possibility that our TH phenotype is linked to alterations in noradrenergic activity remains a tantalizing possibility. Noradrenaline plays an important role in modulating the behavioral response to stress, and it is of note that various stress treatments increase noradrenaline release12 and sensitization58, 59 within the mPFC. Importantly, TH is found within noradrenergic terminals in the PFC,60, 61 and selective noradrenaline reuptake inhibitors (but not selective serotonin reuptake inhibitors) reduce Fos-like immunoreactivity in the mPFC following testing on the FST.62 As hyporeactivity of the mPFC in response to stress may provide a foundation for stress-related behavioral traits,63 it would be of interest to consider measurements of catecholamine activity within the mPFC of hBDNFVal66Met mice, both at baseline and following stress, in future studies. That said, other than neuronal terminal TH, a subset of TH-immunoreactive cells may also exist in the PFC64 and these cells are believed to be inhibitory interneurons based on their morphology and colocalization with both GAD and calretinin.46 The functionality of these neurons is unknown.46 Nevertheless, we also quantified calretinin within the mPFC and found it too was concomitantly reduced selectively in the hBDNFVal/Val genotype group as a result of CORT treatment (Figure 2f). As such, it is possible that the significant reduction in TH within the mPFC of CORT-treated hBDNFVal/Val mice may arise from an alteration in terminal TH and noradrenergic innervation by ascending projections, a selective loss of TH-immunoreactive calretinin interneurons within the mPFC, or a combination of both outcomes.
Figure 2.
hBDNFVal66Met genotype and chronic CORT exposure interact to selectively reduce the expression of Tyrosine Hydroxylase (TH) and calretinin in the mPFC but not hippocampus. While there was no effect of genotype or chronic CORT treatment on TH (a, b) or calretinin (d, e) expression in the DHP or VHP, the expression of both TH (c) and calretinin (f) was found to be reduced in hBDNFVal/Val mice following CORT in the mPFC. All data are presented as mean±s.e.m.; *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. Per group, n=13–14.
Importantly, long-lasting changes in basal BDNF expression following CORT does not appear to underscore these behavioral and molecular phenotypes. The DHP was the only structure that exhibited a change in BDNF expression across our groups (Figure 3a), where an indiscriminate effect of prior CORT exposure increased total BDNF. While it is unclear why only the DHP exhibited this shift in expression, it is possible that the DHP is developmentally more sensitive to the effects of CORT than the VHP. This explanation is consistent with genotype-specific alterations in the developmental expression of GRs within the DHP of hBDNFVal66Met mice, as we have previously reported (see supplementary data of ref. 31), which establishes that differences in the developmental trajectory of the hippocampal longitudinal axis may result in critical periods of vulnerability to stress. Likewise, neither fl.TrkB expression nor phosphorylation (Figure 4) appeared to be associated with the behavioral and molecular phenotypes reported here. However, tr.TrkB receptors were found to be selectively down-regulated in the mPFC of hBDNFVal/Val mice following CORT (Figure 4h). As tr.TrkB receptors have dominant-negative effects upon fl.TrkB signaling,47, 48 the down-regulation of this isoform in the mPFC of CORT-treated hBDNFVal/Val mice may serve to indirectly increase BDNF binding to fl.TrkB during activity-dependent processing. This result thus represents a novel effect of stress hormones upon the BDNF-TrkB signaling pathway that has not been previously described in this brain region as a result of BDNF genetic variation and stress exposure. In addition to a decrease in synaptophysin within the DHP of hBDNFVal/Val mice (Figure 5a), expression of the postsynaptic marker PSD-95 within the mPFC also corresponded with the FST performance of the hBDNFVal/Val group (Figure 5h). However, these subtle genotype-mediated changes in synaptic reorganization did not appear to influence NMDA receptor subunits (Figure 6), despite PSD-95 anchoring this receptor and previous studies finding that the Val66Met substitution alters synaptic physiology that requires this receptor in the hippocampus50 and infralimbic cortex.51 These experiments thus yield evidence of subtle synaptic reorganization as would be expected following a history of CORT or stress exposure, but implicate that these changes are mostly independent of the Val66Met variant.
Figure 3.
No effect of hBDNFVal66Met genotype on basal BDNF protein levels in the (a) DHP, (b) VHP or (c) mPFC. The only main effect to reach significance in these regions was a history of chronic CORT treatment in the DHP, which increased BDNF expression levels independent of hBDNFVal66Met genotype. All data are presented as mean±s.e.m.; ***P<0.001. Per group, n=8–11.
Figure 4.
Effects of hBDNFVal66Met genotype and prior CORT exposure on TrkB isoforms. (a) hBDNFMet/Met mice have (subtly) higher fl.TrkB expression relative to hBDNFVal/Val mice in the DHP, while chronic CORT tends to (b) decrease tr.TrkB expression while (c) increasing TrkBY515 phosphorylation in this brain region. In the VHP, (d) fl.TrkB was unchanged however (e) hBDNFMet/Met mice tended to have lower tr.TrkB expression than hBDNFVal/Val mice. (f) CORT treatment increased pTrkBY515 phosphorylation in the VHP. In the mPFC, (g) fl.TrkB was once more unchanged. (h) However, a significant genotype × treatment interaction emerged for tr.TrkB expression whereby hBDNFVal/Val mice selectively responded to chronic CORT. Following CORT, expression levels of tr.TrkB in the mPFC of hBDNFVal/Val mice were reminiscent of those observed in the mPFC of hBDNFMet/Met mice. (i) Lastly, as in the DHP and VHP, a history of CORT also increased pTrkBY515 phosphorylation in the mPFC. All data presented as mean±SEM; *P<0.05, **P<0.01, and ***P<0.001. Per group, n=9–14.
Figure 5.
hBDNFVal66Met genotype alters synaptic protein expression within the DHP and mPFC but not VHP. (a) Within the DHP, the expression of synaptophysin was labile to remodeling by chronic CORT as a function of hBDNFVal66Met genotype, where hBDNFVal/Val mice were selectively vulnerable to the long-term effect of CORT. (b) The expression of PSD-95 in the DHP was found to be dependent on hBDNFVal66Met genotype. hBDNFMet/Met mice had significantly higher PSD-95 expression, an excitatory post-synaptic marker and scaffolding protein, than hBDNFVal/Val mice. While an effect of CORT on the expression of PSD-95 in the DHP was also detected, which decreased the expression of this scaffolding protein, this effect was independent of genotype. (c) This effect of CORT was recapitulated for the expression of inhibitory terminal marker gephyrin. There were no significant differences in synaptic protein reorganization in the VHP (d-f) In the mPFC, there were no detectable alterations in the expression of synaptophysin (g) or gephyrin (i), however a subtle hBDNFVal66Met × chronic CORT treatment interaction emerged for the expression of PSD-95 which resulted in a selective decrease of this marker among the hBDNFVal/Val group as a consequence of CORT treatment (h). All data are presented as mean±SEM; *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001. Per group, n=13–14.
Figure 6.
Chronic CORT alters the expression of the NMDA NR2A, NR2B and NR1 subunit composition independent of hBDNFVal66Met genotype. Chronic CORT treatment was found to decrease (a) NR2A, (b) NR2B and (c) NR1 subunit expression in the DHP. In the VHP, chronic CORT increased the expression of (d) NR2A and (e) NR2B but had no effect on the expression of (f) NR1. In the mPFC, the only group difference to emerge was an effect of CORT on (h) NR2B, which also increased the expression of this subunit. No modulatory effect of hBDNFVal66Met genotype emerged for the (g) NR2A or (i) NR1 subunits in this region. All data presented as mean±s.e.m.; *P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001. Per group, n=13–14.
Nonetheless, the current study has also yielded several surprising observations worthy of discussion. The first of these was a lack sex differences on the FST response, either as a product of prior CORT treatment or hBDNFVal66Met genotype. This is incongruent with data that chronic CORT exposure may elicit sex-specific effects in rodents on the FST,65 although it is worth noting that not all studies have observed such differences.66 Nonetheless, the Val66Met polymorphism has been associated with sex-specific risk of depression,67 albeit inconsistently too (for review, see ref. 15). While we have observed sex-specific effects in our hBDNFVal66Met polymorphic mice (for example, startle reactivity, see ref. 37, and other unpublished data not relevant to this manuscript), we do not observe differences for many other phenotypes (for example, hippocampus-dependent behavior, see ref. 31). This may suggest that the interaction between the BDNFVal66Met polymorphism, stress and sex may be paradigm/phenotype specific, or gated by other factors. While we did not observe a sex-specific phenotype on the FST here, both at baseline and following CORT, this report is nonetheless a clue that the BDNFVal66Met variant may influence risk of mood disorders via an effect on endogenous HPA axis homeostasis. It would be interesting for further investigations to examine whether hBDNFVal66Met genotype alters endogenous HPA axis reactivity, both before and following a history of stress in male and female mice. Prior studies using this mouse line have reported no differences in CORT levels following restraint stress among females,68 however other reports have reported increases in circulating CORT, ACTH and hypothalamic CRH mRNA expression following restraint stress in BDNF+/Met mice relative to wildtype controls.25 Data implicating genotype differences in response to early life stress,69, 70 as well as HPA axis reactivity have also been reported in humans.69, 71, 72, 73 While the aim of the current study was to evaluate and describe the long-term effects of chronic CORT exposure on the brain, it would be of interest to conduct an environmentally salient stress challenge or dexamethasone challenge following termination and wash-out of our CORT treatment to examine if lasting change in HPA-axis reactivity from prior stress are gated by hBDNFVal66Met genotype, as this may provide additional insight into prior reports that hBDNFVal66Met mice may be more vulnerable to stress in adulthood31, 37 well after the effects of CORT have washed-out.33
In summary, our data suggests that the BDNFVal66Met polymorphism gates stress-related learned helplessness and that this is likely to occur via divergent pathways between the BDNFVal/Val and BDNFMet/Met genotype groups. While there may be overlap in these pathways, it appears there are separable effects of chronic CORT treatment between the two genotype groups. Our results implicate that TH expression within the mPFC and behavioral despair on the FST is disrupted in hBDNFMet/Met mice at baseline, and that chronic CORT recapitulates these phenotypes in hBDNFVal/Val wildtype mice. Further, we also identify that long-term and genotype-specific effects of chronic CORT treatment are more pronounced in hBDNFVal/Val mice whereby, in addition to TH and behavioral despair, TrkB isoforms and select synaptic markers are impacted within the corticohippocampal axis. These data thus have important implications for the clinical literature, where an effect of the BDNFVal66Met variant may be obscured should a history of stress not be stratified for and analyzed as a covariate. Furthermore, the molecular data reported here suggest that TH expression is perturbed in the mPFC of hBDNFMet/Met mice, who have a robust despair phenotype irrespective of CORT treatment on the FST, and that in hBDNFVal/Val mice chronic CORT is sufficient to recapitulate this TH expression profile and phenotype on the FST. This knowledge suggests that catecholamine innervation of the mPFC, likely by noradrenaline, may be a therapeutic target that can be exploited to treat depression-related symptoms in human BDNFVal66Met carriers. In particular, these data suggest that it may be of value to more extensively trial the effectiveness of selective noradrenergic reuptake inhibitors on depression symptomology among BDNFVal66Met carriers. As such, further research which seeks to delineate the effects of stress in BDNFVal66Met carriers may eventually lead to advances in our knowledge of how common coding polymorphisms influence mood disorders, which could be utilized to predict risk and tailor intervention strategies.
Acknowledgments
MJN completed all experiments, and was supported by an APA scholarship awarded by the University of Melbourne. XD assisted with ELISAs, and was supported by a NHMRC-ARC Dementia Research Development Fellowship. RH was supported by a NHMRC Career Development Fellowship and a NARSAD Young Investigator Award. MvdB was supported by a NHMRC Senior Research Fellowship.
Footnotes
The authors declare no conflict of interest.
References
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51: 8–19. [DOI] [PubMed] [Google Scholar]
- Dewa CS, Lin E. Chronic physical illness, psychiatric disorder and disability in the workplace. Soc Sci Med 2000; 51: 41–50. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism 2005; 54: 20–23. [DOI] [PubMed] [Google Scholar]
- Masi G, Brovedani P. The hippocampus, neurotrophic factors and depression. CNS Drugs 2011; 25: 913–931. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 2003; 60: 804–815. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci Signal 2004; 225: 1–11. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005; 53: 129–139. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology 2009; 34: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JW, Louneva N, Han LY, Hodes GE, Wilson RS, Bennett DA et al. Chronic corticosterone exposure alters postsynaptic protein levels of PSD‐95, NR1, and synaptopodin in the mouse brain. Synapse 2011; 65: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J, Pesold B, Baader M, Hu M, Gewirtz JC, Duman RS et al. Stress differentially regulates synaptophysin and synaptotagmin expression in hippocampus. Biol Psychiatry 2001; 50: 809–812. [DOI] [PubMed] [Google Scholar]
- Finlay J, Zigmond M, Abercrombie E. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: effects of diazepam. Neuroscience 1995; 64: 619–628. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S et al. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry 2005; 29: 1214–1224. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff W, Poeggel G, Braun K. Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience 2008; 152: 18–28. [DOI] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: progress & controversy. Mol Psychiatry 2015; 20: 916–930. [DOI] [PubMed] [Google Scholar]
- Björkholm C, Monteggia LM. BDNF–a key transducer of antidepressant effects. Neuropharmacology 2016; 102: 72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res 2008; 1211: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H-Y, Crupi D, Liu J, Stucky A, Cruciata G, Di Rocco A et al. Repetitive transcranial magnetic stimulation enhances BDNF–TrkB signaling in both brain and lymphocyte. J Neurosci 2011; 31: 11044–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønli J, Bramham C, Murison R, Kanhema T, Fiske E, Bjorvatn B et al. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol Biochem Behav 2006; 85: 842–849. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006; 311: 864–868. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131: 391–404. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Wolf J, Shelly W, Rosser R, Burke HM, Lerner GK et al. Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112: 257–269. [DOI] [PubMed] [Google Scholar]
- Chen Z-Y, Jing D, Bath KG, Ieraci A, Khan T, Siao C-J et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006; 314: 140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci: Off J Soc Neurosci 2012; 32: 4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco E, Åslund C, Oreland L, Nilsson KW. Three-way interaction effect of 5-HTTLPR, BDNF Val66Met, and childhood adversity on depression: a replication study. Eur Neuropsychopharmacol 2013; 23: 1300–1306. [DOI] [PubMed] [Google Scholar]
- Aguilera M, Arias Sampériz B, Wichers M, Barrantes Vidal N, Moya Higueras J, Villa Martín E et al. Early adversity and 5-HTT-BDNF genes: new evidences of gene-environment interactions on depressive symptoms in a general population. Psychol Med 2009; 39: 1425–1432. [DOI] [PubMed] [Google Scholar]
- Drachmann Bukh J, Bock C, Vinberg M, Werge T, Gether U, Vedel Kessing L. Interaction between genetic polymorphisms and stressful life events in first episode depression. J Affect Disord 2009; 119: 107–115. [DOI] [PubMed] [Google Scholar]
- Anastasia A, Deinhardt K, Chao MV, Will NE, Irmady K, Lee FS et al. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat Commun 2013; 4: 2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Dhilla A, Mukai J, Blazeski R, Lodovichi C, Mason CA et al. Genetic modulation of BDNF signaling affects the outcome of axonal competition in vivo. Curr Biol 2007; 17: 911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaras M, Hill R, van den Buuse M. BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. Mol Psychiatry 2016; 21: 730–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression‐like syndrome in rodents. Curr Protoc Neurosci 2009; 9: 32, 1–9.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder A, Buret L, Hill RA, van den Buuse M. Gene–environment interaction of reelin and stress in cognitive behaviours in mice: implications for schizophrenia. Behav Brain Res. 2015; 287: 304–314. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene–environment interactions, and epigenetics. Exp Neurol. 2012; 233: 102–111. [DOI] [PubMed] [Google Scholar]
- Hill RA, Wu YWC, Kwek P, van den Buuse M. Modulatory effects of sex steroid hormones on brain‐derived neurotrophic factor‐tyrosine kinase B expression during adolescent development in C57Bl/6 Mice. J Neuroendocrinol 2012; 24: 774–788. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates. Gulf Professional Publishing, USA, 2004. [Google Scholar]
- Notaras M, Hill R, Gogos J, van den Buuse M. BDNF Val66Met genotype interacts with a history of simulated stress exposure to regulate sensorimotor gating and startle reactivity. Schizophr Bull 2017; 43: 665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers R, de Rijk-Van Andel J, Bräutigam C, Geurtz B, Van den Heuvel L, Steenbergen-Spanjers G et al. A review of biochemical and molecular genetic aspects of tyrosine hydroxylase deficiency including a novel mutation (291delC). J Inherit Metab Dis 1999; 22: 364–373. [DOI] [PubMed] [Google Scholar]
- Biegon A, Fieldust S. Reduced tyrosine hydroxylase immunoreactivity in locus coeruleus of suicide victims. Synapse 1992; 10: 79–82. [DOI] [PubMed] [Google Scholar]
- Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry 2004; 9: 609–620. [DOI] [PubMed] [Google Scholar]
- Baumann B, Danos P, Diekmann S, Krell D, Bielau H, Geretsegger C et al. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is reduced in depressed non-suicidal patients but normal in depressed suicide patients. Eur Arch Psychiatry Clin Neurosci 1999; 249: 212–219. [DOI] [PubMed] [Google Scholar]
- Brady LS, Gold PW, Herkenham M, Lynn AB, Whitfield HJ. The antidepressants fluoxetine, idazoxan and phenelzine alter corticotropin-releasing hormone and tyrosine hydroxylase mRNA levels in rat brain: therapeutic implications. Brain Res 1992; 572: 117–125. [DOI] [PubMed] [Google Scholar]
- Serretti A, Macciardi F, Verga M, Cusin C, Pedrini S, Smeraldi E. Tyrosine hydroxylase gene associated with depressive symptomatology in mood disorder. Am J Med Genet B Neuropsychiatr Genet 1998; 81: 127–130. [PubMed] [Google Scholar]
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J Neurosci 1991; 11: 1478–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem 1995; 65: 111–116. [DOI] [PubMed] [Google Scholar]
- Asmus SE, Anderson EK, Ball MW, Barnes BA, Bohnen AM, Brown AM et al. Neurochemical characterization of tyrosine hydroxylase-immunoreactive interneurons in the developing rat cerebral cortex. Brain Res 2008; 1222: 95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide FF, Vining ER, Eide BL, Zang K, Wang X-Y, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci 1996; 16: 3123–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner BM. Truncated TrkB: beyond a dominant negative receptor. Cytokine Growth Factor Rev 2012; 23: 15–24. [DOI] [PubMed] [Google Scholar]
- Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci 1996; 16: 2157–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninan I, Bath KG, Dagar K, Perez-Castro R, Plummer MR, Lee FS et al. The BDNF Val66Met polymorphism impairs NMDA receptor-dependent synaptic plasticity in the hippocampus. J Neurosci 2010; 30: 8866–8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Perez-Castro R, Lee FS, Chao MV, Ninan I. The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci 2012; 32: 2410–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing D, Lee FS, Ninan I. The BDNF Val66Met polymorphism enhances glutamatergic transmission but diminishes activity-dependent synaptic plasticity in the dorsolateral striatum. Neuropharmacology 2016; 112: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic-and antidepressant-like effects in mice. Neuropsychopharmacology 2006; 31: 2405–2414. [DOI] [PubMed] [Google Scholar]
- Poleszak E, Stasiuk W, Szopa A, Wyska E, Serefko A, Oniszczuk A et al. Traxoprodil, a selective antagonist of the NR2B subunit of the NMDA receptor, potentiates the antidepressant-like effects of certain antidepressant drugs in the forced swim test in mice. Metab Brain Dis 2016; 31: 803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xu T, Wang S, Yu L, Liu D, Zhan R et al. NMDA GluN2B receptors involved in the antidepressant effects of curcumin in the forced swim test. Prog Neuropsychopharmacol Biol Psychiatry 2013; 40: 12–17. [DOI] [PubMed] [Google Scholar]
- Stone EA, McCarty R. Adaptation to stress: tyrosine hydroxylase activity and catecholamine release. Neurosci Biobehav Rev 1983; 7: 29–34. [DOI] [PubMed] [Google Scholar]
- Lambert G, Johansson M, Ågren H, Friberg P. Reduced brain norepinephrine and dopamine release in treatment-refractory depressive illness: evidence in support of the catecholamine hypothesis of mood disorders. Arch Gen Psychiatry 2000; 57: 787–793. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Stress‐induced sensitization of dopamine and norepinephrine efflux in medial prefrontal cortex of the rat. J Neurochem 1994; 63: 575–583. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Sved AF, Zigmond MJ, Finlay JM. Sensitization of norepinephrine release in medial prefrontal cortex: effect of different chronic stress protocols. Brain Res 1999; 830: 211–217. [DOI] [PubMed] [Google Scholar]
- Erickson SL, Gandhi AR, Asafu-Adjei JK, Sampson AR, Miner L, Blakely RD et al. Chronic desipramine treatment alters tyrosine hydroxylase but not norepinephrine transporter immunoreactivity in norepinephrine axons in the rat prefrontal cortex. Int J Neuropsychopharmacol 2011; 14: 1219–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci: Off J Soc Neurosci 2006; 26: 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Knapp DJ, Johnson KB, Breese GR. Functional classification of antidepressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther 1996; 277: 1076–1089. [PubMed] [Google Scholar]
- Kalisch R, Salomé N, Platzer S, Wigger A, Czisch M, Sommer W et al. High trait anxiety and hyporeactivity to stress of the dorsomedial prefrontal cortex: a combined phMRI and Fos study in rats. Neuroimage 2004; 23: 382–391. [DOI] [PubMed] [Google Scholar]
- Braun K, Lange E, Metzger M, Poeggel G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience 1999; 95: 309–318. [DOI] [PubMed] [Google Scholar]
- Brotto LA, Gorzalka BB, Barr AM. Paradoxical effects of chronic corticosterone on forced swim behaviours in aged male and female rats. Eur J Neuropharmacology 2001; 424: 203–209. [DOI] [PubMed] [Google Scholar]
- Kalynchuk LE, Gregus A, Boudreau D, Perrot-Sinal TS. Corticosterone increases depression-like behavior, with some effects on predator odor-induced defensive behavior, in male and female rats. Behav Neurosci 2004; 118: 1365. [DOI] [PubMed] [Google Scholar]
- Verhagen M, Van Der Meij A, Van Deurzen P, Janzing J, Arias-Vasquez A, Buitelaar J et al. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry 2010; 15: 260–271. [DOI] [PubMed] [Google Scholar]
- Madra M, Zeltser L. BDNF-Val66Met variant and adolescent stress interact to promote susceptibility to anorexic behavior in mice. Transl Psychiatry 2016; 6: e776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Molendijk ML, Voshaar RCO, Bus BA, Prickaerts J, Spinhoven P et al. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology 2011; 214: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt J, Nemeroff C, Dobson-Stone C, Paul R, Bryant R, Schofield P et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry 2009; 14: 681–695. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Van der Does AW, Kouwenhoven C, Elzinga BM, Hommel B. BDNF Val 66 Met polymorphism is associated with higher anticipatory cortisol stress response, anxiety, and alcohol consumption in healthy adults. Psychoneuroendocrinology 2011; 36: 1562–1569. [DOI] [PubMed] [Google Scholar]
- Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D et al. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology 2009; 34: 382–388. [DOI] [PubMed] [Google Scholar]
- Alexander N, Osinsky R, Schmitz A, Mueller E, Kuepper Y, Hennig J. The BDNF Val66Met polymorphism affects HPA-axis reactivity to acute stress. Psychoneuroendocrinology 2010; 35: 949–953. [DOI] [PubMed] [Google Scholar]