Abstract
Background
Over 12,000 children are diagnosed with cancer every year in the United States. In addition to symptoms associated with their disease, children undergoing chemotherapy frequently experience significant pain, which is unfortunately often undertreated. The field of m-Health offers an innovative avenue for pain assessment and intervention in the home setting. The current study describes the development and initial evaluation of a tablet-based program, Pain Buddy, aimed to enhance pain management and foster improved quality of life in children ages 8–18 years undergoing cancer treatment.
Methods
An animated avatar-based tablet application was developed using state-of-the-art software. Key aspects of Pain Buddy include daily pain and symptom diaries completed by children, remote monitoring of symptoms by uploading patient’s data through internet to a cloud server, cognitive and behavioral skills training, interactive three-dimensional avatars that guide children through the program, and an incentive system to motivate engagement. Twelve children between the ages of 8 and 18 participated in a pilot study of Pain Buddy.
Results
Children were highly satisfied with the program. Pain and appetite disturbances were most frequently endorsed. Symptom trigger alerts to outside providers were largely related to clinically significant pain. Children infrequently used analgesics, and reported using some non-pharmacological pain management strategies.
Conclusion
Pain Buddy appears to be a promising tool to improve pain and symptom management in children undergoing cancer treatment. Results from the current study will inform future improvements to Pain Buddy, in preparation for a randomized controlled trial to assess the efficacy of this innovative treatment.
Keywords: Pediatric cancer, Cancer pain, Symptom management, Quality of life, Health information technology
1. Introduction
Over 12,000 children are diagnosed with cancer every year in the United States [1], and existing research indicates that the majority of these children experience troubling symptoms that include pain, fatigue, and nausea [2]. Unfortunately, children with cancer not only suffer from distressing symptoms related to the disease process, but also suffer from symptoms related to the treatment for the disease. Indeed, given the aggressive protocols children being treated with chemotherapy go through, they often experience painful conditions such as mucositis and peripheral neuropathy [3]. Uncontrolled pain not only has significant negative psychosocial effects, but also modulates the physiological pain response, resulting in sensitization and potentially deleterious effects on physiological and immune function [4]. Unrelieved symptoms related to either cancer or chemotherapy also lead to poorer quality of life, including increased distress [2]. Unfortunately, evidence suggests that cancer pain and symptoms are undertreated in most children [3,5–9], and there are few controlled studies in this area.
Although parents and children who suffer from cancer report that pain is a significant concern, pain assessment throughout cancer treatment is not performed systematically [3,10–12] and without accurate data, physicians are unable to intervene appropriately. Additional barriers to treatment of pain in children with cancer include misunderstanding of use of analgesia in children (i.e., fears of addiction) and lack of understanding of pain expression in children [10,12,13]. This is particularly relevant to management of symptoms in the home setting by parents, who have been shown to significantly under treat children’s pain [3,14]. Unfortunately, there is an extreme dearth of research into children’s cancer symptom management, particularly with regard to effective pain interventions. Moreover psychosocial interventions, particularly skills-based training, are effective for cancer pain and symptoms for adults and children [15,16], yet are not easily accessible by patients.
Recently there has been a national push toward involving information technology in health care, such as electronic medical records, personal health records, and real-time decision-support systems [17–19]. The growth in pervasive computing and wearable technology has led to the field of m-Health, defined as “mobile computing, medical sensor, and communications technologies for health-care” [20,21]. Accordingly, there is a growing literature of the impact of m-Health technologies, particularly involving the management of pain. Indeed, there is empirical evidence that the use of computer-based decision-support positively impacts management of chronic pain in adults [22] and can lead to significant improvements in overall clinical care [22–25]. Real-time pain assessment and decision-support guiding treatment implemented via mobile technology (e.g., smartphones, tablets) provides two innovative, key pathways to the translation of pain management guidelines to practice for cancer patients. First, the ability to communicate with children and families electronically (e.g., web-based assessment and intervention, text messaging, email notifications) is a simple, efficient system and over half of teens in the U. S. have mobile phones and over a third report using text messaging [26] and these numbers are increasing. Electronic communication is beginning to be used as a modality to engage teens in their healthcare [27–30], and such programs are very well-received [28]. Second, electronic means of assessment have been shown to greatly increase adherence to monitoring health information data [31].
To date, m-Health programs have been developed with a specific focus on pain assessment [32]. Both children and adults show high adherence rates to completion of electronic pain diaries for a variety of illnesses that involve pain, including cancer [33,34]. There is some evidence that use of pain diaries to track chronic pain leads to improved patient adherence to treatment recommendations and additional data provided to health care providers to use for treatment decision-making [35]. Moreover, asking patients to recall their pain experience over the period of time leading up to their present medical visit (i.e., retrospective pain reporting) has been shown to be subject to bias and is inferior to real time measurement of pain using ecological momentary assessment approaches (i.e., electronic pain diaries) [36]. Despite the growth of m-Health programs focused on pain assessment; to date, little focus has been on capitalizing on pervasive computing technology to deliver interventions for pain management.
Because of changes in the U.S. healthcare system, there has been a shift in the management of care of cancer patients from the hospital to the home [37]. This shift has resulted in improved satisfaction among patients and families; however, it has come with an added burden of pain management by parents and caregivers in home setting, who often have little education regarding pain and pain management [38]. Pain management in children in the hospital setting has greatly improved in the past several decades [39], though in the home setting, there is growing evidence that children’s pain is poorly managed [3]. Thus, the under management of children’s pain in the home setting provides an avenue for targeted research that incorporates electronic means of assessment and intervention that allows children to stay in the home setting to maximize satisfaction and quality of life. Moreover, there is a dire need to focus on children undergoing cancer treatment, as this vulnerable population has been neglected in the literature on behavioral management of pain and stress.
2. Development of an electronic pain management program: Pain Buddy
It is clear that management of children’s cancer pain at home is in need of attention. The growing use of mobile technology, particularly among youth in the U.S., provides a promising means of merging engaging modalities for intervention with efforts to improve quality of life of children undergoing cancer treatment. Accordingly, the current focus of our program of research is development of an innovative m-Health application that provides remote monitoring of pain and symptoms and delivery of cognitive and behavioral skills training to children undergoing treatment for cancer. The conceptual framework of this newly developed program (Pain Buddy) [40,41] is grounded in social learning theory [42], which suggests that beliefs about self-efficacy, or beliefs about one’s capabilities, play a role in coping responses. Early research highlighted the role of self-efficacy in health behavior change and suggested that manipulations of perceived self-efficacy impacted health-related changes in behavior [43]. In particular, opportunities for skill rehearsal may increase self-efficacy [44], and there is evidence that providing feedback during web-based learning modules leads to increases in perceived self-efficacy [45]. Accordingly, Pain Buddy is based upon the premise that providing children with real time feedback and opportunities for rehearsal of pain management strategies will increase empowerment and self-efficacy of pain management, thereby leading to improved quality of life and reduced suffering during cancer treatment. Pain Buddy is an Android-based program that has been developed to be used on a seven-inch tablet by children ages 8–18 years undergoing cancer treatment.
Development of Pain Buddy follows a three-phase model [46]: Program development, formative evaluation, and outcome evaluation. In this manuscript, we describe the first two phases of the project. Specifically, we discuss the development of Pain Buddy, including the various components of the program, and then present feasibility and preliminary outcome data from a pilot study of the intervention.
3. Materials and methods
This project was approved by the Institutional Review Board at Children’s Hospital of Orange County (CHOC Children’s).
3.1. Phase I – development of Pain Buddy
3.1.1. Stakeholders
The components of Pain Buddy were developed with a multi-disciplinary task force that includes psychologists, oncologists, anesthesiologists, nurses, engineers, and computer scientists, all who possess expertise in pediatric cancer, pediatric pain management, health information technology, and web-based intervention development and delivery. Children receiving cancer treatment were also included throughout the development process to provide input and feedback into the various components of the program. Utilizing a collaborative approach with patient stakeholders ensures appropriate input throughout development to generate a program that meets the needs of both patients and healthcare providers.
3.1.2. Procedures
The task force meets biweekly to review project development, relevant literature and developments in the fields, and to conduct beta testing of Pain Buddy through each phase of development.
3.1.3. Base technology
The infrastructure of the Pain Buddy application has been implemented using the following technologies: (1) A mobile device, (2) an application acting as a serious game to collect patients’ data, (3) a remote server consisting of a database (4), a web portal (5), and a set of algorithms (6) to synchronize, monitor, and analyze patients’ data collected from the mobile device. Further information on each itemized technology is provided as follows:
The selected mobile device is a seven-inch tablet (model ASUS Google Nexus 7) with 16 Gb of internal memory and a screen resolution of 1920 × 1200 pixel (960dp × 600dp). This device features Wi-Fi, which is used to connect to Internet and send data to the Pain Buddy server. The version of the operative system was Android 4.4 (KitKat).
The development of the Pain Buddy app was designed as a serious game. Many technologies have been used to design and develop this game, but the main tool used for implementation was Unity 3D, using C# as the programing language.
The computer used as a server was a PowerEdge R220 Rack Server, Intel® Xeon® E3-1220 v3 3.1 GHz with 8 Gb RAM and dual HDD 500 GB Raid 1. The operative system was Windows Server 2012.
To handle client–server data, the Pain Buddy server integrated MySQL version 5.6 as an open-source relational database management system.
In order to provide a friendly interface for system administrators and healthcare providers to manage and visualize patients’ data, a web portal was implemented using HTML 5.0 and PHP version 4.5. It also made use of JavaScript technologies to draw relevant patient data into interactive charts.
The program synchronizing and monitoring data have been implemented on the server’s side. The languages used for implementing the program are MySQL and PHP, and this program includes algorithms to handle data and send notifications if needed.
3.2. Phase II – formative evaluation of Pain Buddy
3.2.1. Participants
Children and adolescents between the ages of 8 and 18 who were diagnosed with cancer and currently undergoing outpatient cancer treatment were invited to participate in this study. One of each child or adolescent’s parents was also invited to participate. Participants were required to be English-speaking and must not have a cognitive or developmental delay.
3.2.2. Measures
3.2.2.1. Demographic and medical record abstraction form
Parents provided baseline demographics, including gender, race, ethnicity, parents’ education, occupation, age, and income. Treatment-related information was also collected from patient medical records, including age, weight, diagnosis, and person completing study measures.
3.2.2.2. Content and usability measure
Assessment of content and usability were collected using methods similar to those used by other investigators in the study of utility of electronic interventions [29,47]. Ratings of content of components were provided in response to queries such as “How useful or helpful was the information provided?” on a Likert-type scale with anchors of Not at All and Very Much. Likewise, patients were asked to provide a rating of how easy or difficult the section was to use on a Likert-type scale, with anchors Very Easy to Very Difficult. In reviewing each section, patients were asked to respond to open-ended questions querying “What did you like best about this component?” and “What did you like least about this component?”.
3.2.2.3. Memorial Symptom Assessment Scale (MSAS) [48,49]
The MSAS is a child self-report instrument that evaluates whether or not the child experienced a particular symptom since a prior diary entry. If the child reports “yes” to having experienced any of the symptoms, then the child is prompted to describe the frequency (i.e., a very short time, a medium amount, or a lot), severity (i.e., a little, a medium amount, or a lot), and distress (i.e. not at all, a little, a medium amount, or very much) experienced due to the symptom. There are two versions of the MSAS: (1) MSAS 7–12 for children who are 7–12 years old, and (2) MSAS 10–18 for children who are 10–18 years old. In the current study, participants age 9 or younger completed the MSAS 7–12; participants age 10 or older completed the MSAS 10–18. The MSAS 7–12 consists of 8 symptoms, whereas the MSAS 10–18 contains 30 symptoms. On the MSAS 7–12, participants rate each symptom frequency, intensity, and level of distress on a scale ranging from 0 (“Not at all”) to 3 (“Very much or a lot”); each rating was re-scaled so that the maximum score for each of these items is 10. On the MSAS 10–18, participants rate each symptom frequency, intensity, and level of distress on a scale ranging from 0 (“Not at all”) to 4 (“Very much”); each rating was scaled so that the maximum score for each of these items is also 10.
The MSAS 10–18 comprises three subscales: psychological (PSYCH; e.g., “difficulty concentrating or paying attention”), physical (PHYS; e.g., “pain”) and general distress index (GDI; e.g., “how much did it [the symptom] bother you?”). The alpha coefficients of the PSYCH, PHYS, and GDI subscales have been reported at 0.83, 0.87 and 0.85 respectively, suggesting high internal consistency within each subscale.
3.2.2.4. Adolescent Pediatric Pain Tool (APPT) [50]
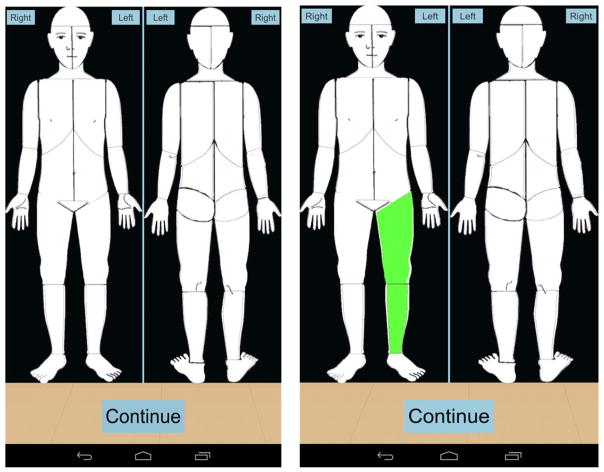
The APPT is a self-report multidimensional pain instrument that is reliable and valid for use in children and adolescents ages 8 to 17. The first part of the measure includes a body outline for the front of the body and another outline for the back of the body (Fig. 1). Patients are asked to mark any areas where they are experiencing pain. Secondly, pain intensity is assessed on a word-graphic rating scale, which is a 100-mm horizontal line that has the anchors of “no pain,” “little pain,” “medium pain,” “large pain,” and “worst pain.” Lastly, the third part of the measure contains a list of 67 words representing four domains of pain. These domains include: sensory (represents pressure or pain sensation, such as “aching” and “stinging”), affective (represents unpleasant emotions of pain, such as “screaming” and “deadly”), evaluative (represents intensity of pain, such as “miserable” and “uncontrollable”), and temporal (represents duration and pattern of pain, such as “constant” and “on and off”). A percentage is calculated for each domain (by dividing the words circled by the number of words possible in each category), as well as a total score for the number of words recorded by participants out of 67 possible words. Participants were also asked to indicate the level of functional impairment associated with their pain in the domains of school activities, social activities, extracurricular activities, chores, and sleep.
Fig. 1.
Body map of the adolescent pediatric pain tool in the pain buddy symptom diary.
Only participants who endorsed experiencing pain on the MSAS were directed to complete the APPT.
3.2.2.5. Coping interventions questionnaire
This questionnaire assessed participants’ strategies for coping with and decreasing their pain. Specific coping strategies assessed include use of pain medication, relaxation techniques, distraction, use of heat packs, massage, and social support. With regard to medication, children were asked to indicate whether a medication was taken, the medication type, dosage, and frequency. With regard to non-pharmacological strategies, participants were asked to indicate the number of times they engaged in each coping activity. For all interventions, participants were asked to indicate how helpful each strategy was using a 0–100 mm VAS where 0=“Did not help at all” and 100=“Helped a lot.” Finally, two items from the Coping Strategies Questionnaire [51] were used to measure coping efficacy. The items, used in prior pain diary studies [52], are answered on a 7-point scale and are: 1) “Based on all the things you did to cope, or deal with your pain today, how much control do you feel you had over it?” and 2) “Based on all the things you did to cope, or deal with your pain today, how much were you able to decrease your pain?”.
Only participants who endorsed experiencing pain on the MSAS were directed to complete the Coping Interventions Questionnaire, after they completed the APPT.
3.2.3. Procedures
3.2.3.1. Recruitment
Parents and their child undergoing cancer treatment were recruited from a pediatric hospital to participate in the current study. Potential participants were identified through review of the Outpatient Infusion Center (OPI) and/or Cancer Institute appointment schedules at the hospital. Those determined to be eligible through review of electronic medical records were mailed a letter of introduction that describes the purpose of the study, eligibility and study requirements, contact information for study personnel, as well as a study information sheet. Within two weeks of sending out the letter, a research associate contacted the family by telephone to answer any questions they may have and invite their participation. Interested families were invited to participate during an in-person encounter at their regularly scheduled visit at OPI, Cancer Institute, or Oncology floor, by a trained research associate.
3.2.3.2. Training on Pain Buddy
After providing consent, parent–child dyads participated in an in-person training session to receive instructions on using Pain Buddy. A trained research associate provided a tutorial on accessing different features on Pain Buddy. Participants also practiced completing the MSAS, APPT, and coping interventions questionnaire. Parents signed a release form for the tablet, and provided contact information.
3.2.3.3. Using Pain Buddy
Each family was provided with one tablet that contained the Pain Buddy diary application. Each child was provided with an individual protected pin to enter in the log in screen (Fig. 2), and all information entered into the mobile device was immediately encrypted.
Fig. 2.
Pain Buddy log on screen.
It is important to note that Pain Buddy uses the Wi-Fi feature of the tablet (model ASUS Google Nexus 7) to send patient’s data to the Pain Buddy server when an internet connection is available. The connection to internet must be achieved through Wi-Fi, as the Asus Google Nexus 7 does not include cellular network. Further information on the Pain Buddy connectivity features can be found under the “Electronic Communication” section.
Participants were asked to complete the diary twice a day for 10 days. However, they were not required to return their tablet immediately after 10 days, as this may not have been feasible for the participants and families. They were also not explicitly told to refrain from continuing to use the Pain Buddy tool after 10 days, and therefore, some participants chose to continue completing diaries after the initial 10 days were over. Each child was provided with an individual protected pin to enter in the log in screen (Fig. 2), and all information entered into the mobile device was immediately encrypted.
Once a participant completed a section on the electronic diary device, that screen is refreshed to the next section with no ability to “turn back” the page to the previous section. If a participant suspended their diary entry sometime in the sequence of item presentations, the electronic diary device timed-out after 30 minutes, reset the screen, and returned them to the main menu of the program. Children provided pain ratings using the MSAS twice a day – once in the morning and once in the evening. If participants endorsed experiencing pain on the MSAS at each diary entry, they also completed the APPT and intervention questionnaire to further assess their pain and use of coping tools to manage pain. Children received cognitive-behavioral skills training to manage pain and symptom-related distress, when 1) ratings met pre-determined symptom criteria, 2) the child initiated training, and/or 3) the application prompted the child to complete a selected skill every two days. The cognitive-behavioral skills training was delivered via an interactive avatar (Fig. 3). To encourage adherence to the diary schedule, research assistants contacted patients who missed two diaries in a row to troubleshoot and remind them to complete diaries at their scheduled times. Patients who did adhere to the diary schedule were called at the five-day mark, and research assistants provided reinforcement for participants’ diary completion.
Fig. 3.
Pain Buddy cognitive and behavioral skills training menu.
3.2.4. Analysis
Analyses included descriptive statistics and one-sample Wilcoxon signed rank tests. One-sample Wilcoxon signed rank tests were performed to determine whether the observed median is equal to the middle value of the scale for each test. All analyses were performed using the SPSS version 23.0 statistical analysis software.
4. Results
4.1. Phase I – development of Pain Buddy
Development of Pain Buddy resulted in an application that comprises:
A symptom diary;
An electronic communication tool;
Cognitive and behavioral skills training for pain and symptom management;
A three-dimensional (3-D) avatar that guides the child throughout the program.
Each of the components of Pain Buddy will be detailed in the following sections.
4.1.1. Symptom diary
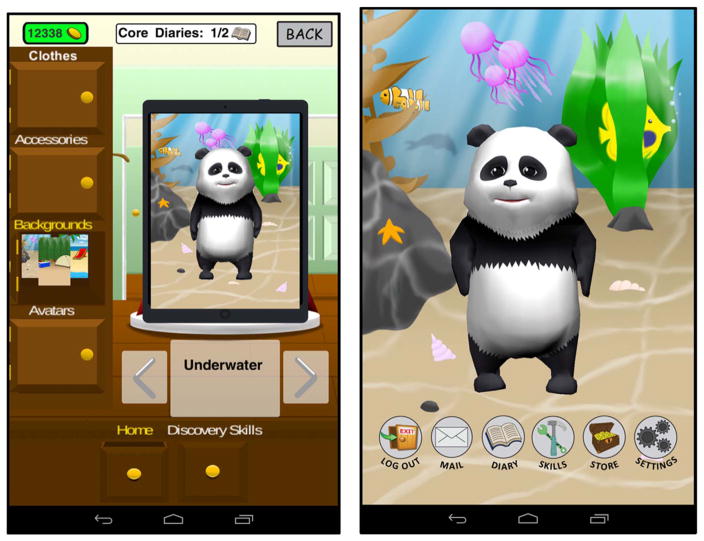
A primary challenge with optimal pain management in the home setting is that pain and symptoms are not assessed consistently during cancer treatment. In order to address this gap in care, Pain Buddy includes a symptom diary that comprises validated measures of pain and symptoms experienced during cancer treatment in children. The diaries are designed to be completed twice per day and can be completed in just a few minutes each day. There is a section in the diary about overall pain and symptoms common during cancer treatment (Memorial Symptom Assessment Scale 7–12/10–18, MSAS) [49,53], a section about pain severity and characteristics for any child who endorses pain on the MSAS (Adolescent Pediatric Pain Tool, APPT) [54], and a final section asking about any interventions (including both pharmacological and nonpharmacological) used for pain management. In order to enhance engagement with the diary, children earn Pain Buddy “coins” that are banked for use in the Pain Buddy store, which provides an opportunity for children to customize the program even further. In the store, they can purchase new avatars and/or customize the avatar selected with new accessories (Fig. 4), including hats, glasses, clothing, and can purchase additions to the design layout of Pain Buddy, change background colors, and change artwork displayed within the layout, etc. (Figs. 5 and 6).
Fig. 4.
Panda bear and penguin three-dimensional avatars.
Fig. 5.
Avatar accessory options in the Pain Buddy store.
Fig. 6.
Background options in the Pain Buddy store.
4.1.2. Electronic communication
A second challenge with pain management is that health care providers do not have access to real time data with regard to pain and symptoms. In order to address this gap in care, Pain Buddy uses a central server to handle communication of the symptom diary to health care providers. Moreover, rather than inundate providers with constant streams of data, Pain Buddy has symptom alert algorithms programmed in the server that will alert health care providers via email or text when a child reports symptoms that meet pre-determined thresholds signaling that intervention might be warranted (see Table 1).
Table 1.
Algorithm for symptom alert triggers in the Pain Buddy symptom diary.
| Symptom | Ratings |
|---|---|
| MSAS 7–12 | |
| Overall pain rating | ≥7.5 |
| Pain | ≥A medium amount (Frequency) AND ≥A medium amount (Severity) AND ≥A medium amount (Bothersome/Distressing) |
| Tired | Three consecutive entries of: |
| Almost all the time (Frequency) AND Very tired (Severity) AND Very much (Bothersome) | |
| Itching | Three consecutive entries of: |
| Almost all the time (Frequency) AND Very itchy (Severity) AND Very much (Bothersome/Distressing) | |
| Vomiting | Three consecutive entries of: |
| ≥A medium amount (Frequency) AND ≥A medium amount (Bothersome/Distressing) | |
| Sleep | Five consecutive entries of: |
| ≥A medium amount (Bothersome/Distressing) | |
| Feeling Sad | Five consecutive entries of: |
| ≥A medium amount (Frequency) AND ≥A medium amount (Severity) AND ≥A medium amount (Bothersome/Distressing) | |
| Worried | Five consecutive entries of: |
| ≥A medium amount (Frequency) AND | |
| Very worried (Severity) AND ≥A medium amount (Bothersome/Distressing) | |
| MSAS 8–18 | |
| Overall pain rating | ≥7.5 |
| Pain | ≥A lot (Frequency) AND ≥Moderate (Severity) AND ≥Somewhat (Bothersome/Distressing) |
| Drowsiness, Difficulty Swallowing | ≥A lot (Frequency) AND ≥Severe (Severity) AND ≥Quite a Bit (Bothersome/Distressing) |
| Problems with Urination | ≥A lot (Frequency) AND ≥Severe (Severity) AND ≥Somewhat (Bothersome/Distressing) |
| Shortness of Breath | ≥Sometimes (Frequency) AND ≥Moderately (Severity) AND |
| ≥Somewhat (Bothersome/Distressing) | |
| Cough | Three consecutive entries of: |
| ≥A lot (Frequency) AND ≥Severe (Severity) AND ≥Somewhat (Bothersome/Distressing) | |
| Numbness | Three consecutive entries of: |
| Almost Always (Frequency) AND Very Severe (Severity) AND | |
| ≥Quite a bit (Bothersome/Distressing) | |
| Vomiting, Diarrhea, Dizziness, Itching | Three consecutive entries of: |
| ≥A lot (Frequency) AND ≥Severe (Severity) AND ≥Quite a Bit (Bothersome/Distressing) | |
| Nausea | Three consecutive entries of: |
| ≥A lot (Frequency) AND ≥Moderate (Severity) AND ≥Quite a Bit (Bothersome/Distressing) | |
| Constipation | Three consecutive entries of: |
| ≥Moderately (Severity) AND ≥Quite a Bit (Bothersome/Distressing) | |
| Headache | Three consecutive entries of: |
| ≥Sometimes (Frequency) AND ≥Moderately (Severity) AND | |
| ≥Somewhat (Bothersome/Distressing) | |
| Mouth Sores | Three consecutive entries of: |
| ≥Moderately (Severity) AND ≥Somewhat (Bothersome/Distressing) | |
| Lack of Energy | Five consecutive entries of: |
| ≥Sometimes (Frequency) AND ≥Moderately (Severity) AND | |
| ≥Somewhat (Bothersome/Distressing) | |
| Nervousness | Five consecutive entries of: |
| ≥A lot (Frequency) AND Very Severe (Severity) AND ≥Quite a Bit (Bothersome/Distressing) | |
| Feelings of Sadness | Five consecutive entries of: |
| Almost Always (Frequency) AND Very Severe (Severity) AND Very Much (Bothersome/Distressing) | |
| Sweats | Five consecutive entries of: |
| Almost Always (Frequency) AND Very Severe (Severity) AND ≥Quite a Bit (Bothersome/Distressing) | |
| Swelling in Arms or Legs | Five consecutive entries of: |
| Very Severe (Severity) AND Very Much (Bothersome/Distressing) | |
| Changes in Skin | Five consecutive entries of: |
| Very Severe (Severity) AND ≥Quite a Bit (Bothersome/Distressing) | |
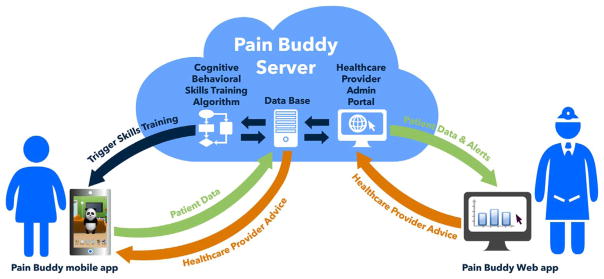
Providers can access the data in real time through a health care provider web portal that communicates with the Pain Buddy server and they can view all patient data or select easy-to-read graphs that will display changes in symptoms and identify the symptoms that prompted the alert to be triggered (Fig. 7). This allows healthcare providers to contact children and families in real time to provide treatment recommendations for pain and symptom management before symptoms continue to escalate. Pain Buddy will also notify children when these symptom thresholds have been met and that their healthcare provider has been notified. Pain Buddy can also be used offline (i.e., without Internet connectivity). In that situation, the application will store and encrypt all patients data in the mobile device until an Internet connection is available, which will then be used to send the data stored in the device to the server. Therefore, all data reported by children are sent to the server as soon as the reporting device has access to Internet through Wi-Fi connection. It is important to note that the Pain Buddy app handles communication efficiently, checking for connectivity after completion of a diary and sending data to the server when connectivity is detected. The average size of an “intensive” session may be up to 67 KB, including diary, store and cognitive training. Assuming patients use Pain Buddy as much as three times a day exploring most of the options available in the application, the average data usage by each patient for a month would be as much as 6.00 MB, which is fifty times less than the lowest data plan that most Internet providers currently offer in the U.S. This efficiency in data transfer is a key feature of Pain Buddy that will provide accessibility of this technology to most patients and families.
Fig. 7.
Pain Buddy infrastructure and data flow.
Because the goal of the pilot study was to gather formative evaluation data, actual symptom trigger alerts were not communicated to participants’ healthcare provider. Results presented below will indicate the number of diary entries that would trigger an alert to a healthcare provider when this alert system is activated.
4.1.3. Cognitive and behavioral skills training
A goal of Pain Buddy is to empower children with strategies to complement pharmacological approaches to pain and symptom management. Accordingly, Pain Buddy is programmed to teach children the following non-pharmacological pain management strategies: guided imagery, progressive muscle relaxation, diaphragmatic breathing, mindfulness, and distraction (Fig. 3). In total, Pain Buddy provides 12 tutorials to teach children these skills via a skills training menu featuring a dedicated animated avatar, with both speech and animation components.
4.1.4. Three-dimensional (3-D) avatar
In order to engage children even further and allow for increased personalization of the program, Pain Buddy provides children with a number of avatars who will guide them through the entire program (i.e. the child’s personal “pain buddy”). In order to develop avatars for the program, a wide range of characters were selected and presented as two-dimensional drawings to 60 children who were undergoing treatment at a cancer center of a major children’s hospital. Children were stratified by age and gender, and they provided quantitative ratings and rankings of the characters. The top rated characters for each age group (8–10, 11–14, 15–18 years of age) were selected for both boys and girls. This resulted in three avatars: a panda bear, a penguin, and a polar bear (Figs. 3 and 4). The three avatars selected were modeled as three-dimensional characters in Maya v.2014 and 2015 for Mac from Autodesk. The body animation of the avatars was achieved by adding predefined biped skeletons to the avatars in Maya and exporting to iClone v.5 for Windows from Reallusion. The animation of the avatars’ face (i.e., facial expression and lip synchronization for speech) involved the recently created FacePlus software from Mixamo, which allowed for the use of USB cameras to map students’ faces as they talked and acted, in order to transcribe and record the facial animation onto the avatar’s face. Mixamo Face-Plus was successfully integrated with Unity 3D v.4 in both Windows and Mac platforms indistinctly. The actors, who were students in the Department of Drama at UC Irvine, also provided the audio for the avatars’ voices for the diary and skills training components of the program. All facial animations from FacePlus and body animations from Iclone were post-processed and further improved in Maya for integration. Maya was also a key platform to model the three dimensional avatars’ accessories that can be found in the Pain Buddy store. The game scenes were developed using Illustrator CC from Adobe (Mac version) and integrated with the avatars, accessories, and multiples algorithms using a well-known videogame development platform, Unity 3D. The Unity 3D platform allows for a fully three-dimensional environment, including a scene-shifting design where the options of the program move the “camera” view to a different part of the room for various components of the program (e.g., diary, skills training page, Pain Buddy store, etc.). Due to incompatibility issues between FacePlus and Unity 3D v5, we used both Unity 3D versions 4 and 5 to assemble the Pain Buddy application.
4.2. Phase II – formative evaluation of Pain Buddy
4.2.1. Demographic information
Twelve children ages 8–18 (12.33±3.42; 58% male) participated in this study. Children were primarily Hispanic–White (75%) and diagnosed with leukemia (58%) or tumors of the central nervous system (25%), and were undergoing cancer treatment.
4.2.2. Diary completion
The mean number of diaries completed by participants was 19.58 (SD=4.64; range=13–27). As discussed above, participants were asked to complete symptom ratings within a 10-day period. However, due to technological issues during the data upload process in this pilot study, we were unable to ensure that our date and time data were completely accurate. As such, we are unable to provide participant adherence rate. Of note, tablets were not required to be immediately returned to study staff at the 10-day mark, and therefore, participants were able to complete more diary entries past the 10-day period if they chose.
4.2.3. Content and usability
Preliminary findings indicate that children were highly satisfied with Pain Buddy (Median=10.00 [25th percentile=7.50, 75th percentile=10.00], interquartile range (IQR)=2.50; Z=2.20, p=0.024), and perceived the program to be useful (7.50 [6.50, 10.00], IQR=3.50; Z=2.04, p=0.041). Children reported hopefulness that Pain Buddy would reduce their pain (8.00 [7.00, 9.25], IQR=2.25; Z=2.21, p=0.027), and contribute to improvement in their symptoms (8.00 [5.75, 8.50], IQR=2.75; Z=2.06, p=0.039). Children also indicated they would be highly confident to recommend Pain Buddy to a friend undergoing cancer treatment (9.00 [8.00, 10.00], IQR=2.00; Z=2.20, p=0.026). The design of the 3-D Avatar was perceived to be attractive (9.00 [8.00, 10.00], IQR=2.00; Z=2.39, p=0.017), and children enjoyed the Pain Buddy “store” (8.00 [7.00, 10.00], IQR=3.00; Z=2.21, p=0.027). Children found the skills training to be useful (9.00 [8.00, 10.00], IQR=2.00; Z=2.38, p=0.017); belly breathing and distraction techniques were most frequently rated as the preferred skill.
4.2.4. Symptom assessments
4.2.4.1. MSAS 7–12
Three participants completed the MSAS 7–12 over the course of the study, yielding a total of 56 sets of symptom ratings. Appetite disturbance was the most frequently endorsed symptom (n=38), followed by itchiness (n=10), pain (n=7), fatigue (n=4), and vomiting (n=3). Sadness, worry, and sleep disturbances were each endorsed across the 10-day period. For those who endorsed experiencing at least one of these symptoms (n=26), the average composite score across these 8 symptoms was 0.57 (SD=0.52; range=0.13–2.22; highest possible=10). Of the responses where participants endorsed experiencing physical symptoms (n=17), the average physical symptoms subscale score was 1.15 (SD=0.77; range=0.41–3.18; highest possible=10). Of the responses where psychological symptoms were endorsed (n=5), the average psychological symptoms subscale score was 2.66 (SD=2.09; range=0.73 to 5.91; highest possible=10). Of those responses indicating experiences of distress (n=9), the average general distress index score, measuring average frequency or distress associated with each symptom, was 1.54 (SD=0.88; range=0.66–3.32; highest possible=10).
4.2.4.2. MSAS 10–18
Nine participants completed the MSAS 10–18, yielding a total of 179 sets of symptom ratings. Pain was the most frequently endorsed symptom (n=19), followed by lack of energy (n=12), nausea (n=10), itchiness (n=9), coughing (n=8), feeling nervous (n=8), sleep difficulties (n=8), and sweating (n=8). Symptoms that were not endorsed included difficulties with urination, breathing, and swallowing, along with swelling of arms and legs and feelings of not looking like oneself. Of the responses where participants endorsed experiencing physical symptoms (n=39), the average physical symptoms subscale score was 0.79 (SD=0.77; range=0.11–4.55; highest possible=10). Of the responses where psychological symptoms were endorsed (n=21), the average psychological symptoms subscale score was 0.74 (SD=0.60; range=0.17–2.67; highest possible=10). Of those responses indicating experiences of distress (n=34), the average general distress index score, measuring average frequency or distress associated with each symptom, was 0.88 (SD=0.67; range=0.25–3.00; highest possible=10).
4.2.4.3. Triggering a symptom alert
Of the 235 total MSAS symptom rating entries completed, 9 (3.83%) resulted in triggering a symptom alert that would notify a health care provider that a child was experiencing symptoms that warranted intervention. Table 1 lists the algorithm of symptom ratings that could trigger a symptom alert. Of the nine alerts that would have been triggered in this study, eight were due to pain symptoms (e.g., pain that was frequent, severe, and bothersome), and one was due to drowsiness. These alerts would have come from three different participants, and 6 of the 8 pain triggers would have been from the diary entries of one participant.
4.2.4.4. APPT
Participants who indicated they experienced pain on the MSAS in a given response set (i.e., diary entry) completed the APPT (n =25). When asked to mark the areas on which participants experienced pain, patients on average marked 3.52 areas (SD=3.72; range=0–14; highest possible=43). The front left upper arm was the body area most frequently indicated as being in pain (n=15; 56%), followed by the left forearm supinated (n=10; 40%), and right front lower leg (n=6; 24%). Four individuals (16%) indicated the following to be areas of pain: left front lower leg, front right top of foot, front left top of foot, back upper left arm and left elbow, back left lower leg, and back right lower leg.
On a scale of 0–100, with higher ratings indicating higher levels of pain, the median level of participant-rated current pain was 31.00 (IQR=53; range=0–77), median pain since last entry at 35.00 (IQR=43; range=0–70), median worst pain since last entry at 73.00 (IQR=39.50; range=0–100), and median least pain since last entry at 6.00 (IQR=46; range=0–52). The median total number of pain words endorsed was 2.00 (IQR=2.50; range=0–6; highest possible=67). With regard to pain-related words in specific domains, a median of 0 sensory words (IQR=1.50; range=0–6; highest possible=37), 0 affective words (IQR=0; range=0–3; highest possible=11), 0 evaluative words (IQR=0; range=0–2; highest possible=8), and 0 temporal words (IQR=2.00; range=0–2; highest possible=11) were endorsed. When asked about the level of impairment associated with their pain, between 8% and 16% of participants reported impairment in the domains of school activities, social activities, extracurricular activities, chores, and sleep.
4.2.5. Coping interventions used
Participants who indicated they experienced pain on the MSAS in a given response set (i.e., diary entry) completed the Coping Interventions Questionnaire (n=25). Distraction techniques were the most frequently used intervention (n=10; 40%), followed by positive self-talk (n=4; 16%) and heat packs (n=4; 16%), medication use (n=3; 12%), accessing social support (n=3; 12%) and deep breathing techniques (n=3; 12%), and followed by relaxation exercises (n=2; 8%). With regard to medication use, responses indicated that three different children used medication: one child took one dose of ibuprofen, one child took one dose of naproxen, and one child took one dose of an unspecified medication. Average perceived effectiveness of medication was 64.67 (SD=19.86; range=49–87). Imagery techniques and massage were not identified as interventions used to cope with pain. Participants who indicated they used an intervention technique were asked the degree to which they perceived it to be helpful. With regard to non-pharmacological techniques, positive self-talk was rated as the most helpful (69.25±35.90; range=32–100, n=4), followed by relaxation exercises (48.00±8.49; range=42–54, n=2), distraction (42.50±20.43; range=0–65, n=10), heat packs (39.00±15.58; range=17–51, n=4), breathing exercises (34.33±33.01; range=0–66, n=3), and social support (27.67±27.50; range=0–55, n=3). The average number of times that these intervention were used ranged from 1 to 4 times, with social support being the most frequently used (4.00± 2.00; range=2–6), followed by distraction (3.40±2.00; range=1–6) and breathing exercises (3.33±2.31; range=2–6).
5. Discussion
Interventions that bridge the gap between knowledge and practice of pain management in children are needed, particularly in those undergoing cancer treatment, who experience high rates of pain due to illness and treatment procedures [2,3,5,6,9]. By merging mobile technologies with medicine, we can begin to bridge this gap in a manner that engages children and families in health care, provides needed data to healthcare providers, and equips children and families with tools to manage cancer-related pain in the home setting [26–36]. The m-Health program, Pain Buddy, described in this paper provides an avenue for addressing inadequate pain and symptom management in pediatric cancer patients.
By working with a multidisciplinary taskforce of a variety of specialties involved in quality of life interventions in cancer patients, and with technological development, we developed an m-Health program for children undergoing cancer treatment that was well-received, engaging, and provided a wealth of data regarding the experience of children being treated in an outpatient setting. As discussed above, the target device for our mobile application was a Google Nexus 7 with a display size of 7-inches and a screen resolution of 1920 × 1200 pixel. However, the Pain Buddy application has been developed to support multiple platforms. For instance, the application has already been tested in several models of smartphones, such as Samsung Galaxy S5 and Google Nexus 5, with excellent results. However, for participants’ convenience, we offered small tablets that were easy to handle but had larger displays than smartphones. As there is a new trend of marge tablets and smartphones (phablets), such that approximately 90% of teenagers use mobile devices to “go online” daily [55] per recent research by the Pew Research Center, we might consider improving our device selection in future studies to further promote accessibility of Pain Buddy to children and adolescents.
This study provides a descriptive picture of symptom prevalence and severity in children being treated for cancer that informs both treatment and prevention efforts. In the current study, overall symptoms were relatively frequently reported, but symptom severity was low. In fact, of the total number of diaries completed by children, 4% would have triggered an alert that would notify health care providers that a child was experiencing symptoms severe enough to warrant intervention.
In terms of the nature of symptoms reported by children, appetite disturbance and pain were the most common symptoms endorsed by children. Additional common symptoms were fatigue, nausea, itchiness, and sleep disturbance. Children reported higher levels of physical symptoms compared to psychological symptoms, suggesting that overall, physical health was more impacted that emotional well-being. Overall symptom distress scores were low, indicating that even though children reported experiencing symptoms, the symptoms did not appear to significantly impact quality of life for most children. It may also be that children were more likely to complete diaries when symptom severity was low, thereby impacting symptom data obtained.
Pain was the primary symptom that triggered the Pain Buddy alert algorithm, suggesting pain was the symptom that was most troublesome when experienced. Results from this study indicate that pain is of concern for children receiving outpatient cancer treatment. Despite eight reports of clinically significant pain, only three doses of analgesics were reported. In addition, children reported using some non-pharmacological pain management strategies such as self-talk, heat, and social support. The low frequency of use of these strategies suggests an avenue by which Pain Buddy can address the gap in pain management in children with cancer. Of particular note, the most frequent strategies were not cognitive-behavioral strategies that can be effective for pain management. By providing coping skills training in use of distraction, imagery, diaphragmatic breathing, mindfulness, and progressive muscle relaxation, Pain Buddy can equip children with a wide range of strategies with which to manage pain and other symptoms. Further, Pain Buddy provides opportunities for skills rehearsal, thereby increasing self-efficacy in coping with pain.
When considering the utility and future improvements of Pain Buddy, it is important to consider other applications that use patient-generated data and patient and/or provider feedback in their programs. For example, Stinson and colleagues [56] developed an iPhone application (“Pain Squad”) to improve pain management among adolescents with cancer. This application consists of an electronic diary assessing pain and functional impairment, examines interventions used for pain management, and provides a reward system for diary use. This application has demonstrated high levels of adherence and satisfaction among adolescents. Baggott and colleagues [57] also developed an electronic diary (“eDiary”) to facilitate symptom recording among adolescents and young adults with cancer, in order to promote improved communication of symptoms between patients and healthcare provider. High adherence to symptom diary completion was noted. Although not specific to the cancer population, Jacob and colleagues [58] evaluated the use of a smartphone for completion of a web-based electronic diary (“e-Diary”) for children and adolescents with sickle cell disease. Results suggested that smartphones can be an effective means for increasing pediatric patients’ recording of their symptoms, thereby facilitating helpful communication with their healthcare providers. The above sample of m-Health research highlights the utility of m-Health interventions in symptom monitoring and recording among pediatric populations, and also underscores the unique value that Pain Buddy contributes to the patient-collected data literature in several ways. First, Pain Buddy shares many of the strengths of the aforementioned programs, as it serves as an electronic symptom diary that offers built-in incentives for high completion. Second, Pain Buddy provides interactive and engaging skills training on evidence-based pain management strategies and techniques. Additionally, Pain Buddy offers real-time electronic communication to patient’s healthcare providers, which further strengthens the unique value of this tool in efficient and effective management of symptoms in the home among children with cancer.
5.1. Limitations and future directions
Pain Buddy therefore appears to hold promise for the field of pain and symptom management among children undergoing cancer treatment. However, there are several limitations to note. First, due to technological difficulties, we were unable to ascertain the exact number and range of days during which participants completed diary entries, and therefore were unable to discuss adherence rates. To improve the feasibility and usability of pain buddy, it is important to consider individual characteristics of participants that may impact adherence to this intervention. For example, children who completed more diary entries may be more motivated to engage with Pain Buddy than those who completed fewer entries, and the differing levels of motivation may impact the symptom and/or satisfaction ratings provided. Those who completed more diaries may also have received increased reminders from parents to engage with Pain Buddy, and the possible differing levels of parental involvement may also impact symptom and satisfaction ratings. We chose to compute statistics at the diary-entry level in order to maximize the sample size and use of data obtained in this initial pilot study, while recognizing the limitations that this method may produce. Future evaluations of Pain Buddy would do well to more strictly standardize the number of diary entries completed per participant, provide adherence rates, as well as perform analyses at the participant level.
Second, it is important to note that Pain Buddy does require access to an Internet connection through Wi-Fi, and this requirement may limit access to Pain Buddy for some populations. When inquiring about access to mobile Internet through Wi-Fi in our study, we found that nearly 90% of all patients asked, including low income families from diverse racial and ethnic backgrounds, reported having access to Wi-Fi. Thus, m-Health interventions may be accessible for the majority of patients in the United States. In addition, all children were provided with tablets for use in the present study, and all tablets were returned to the study team at the conclusion of the study. Although it seems that Wi-Fi access was not a significant barrier in our current study, it is nevertheless important to consider implications of mobile Internet necessity in future efforts to more globally disseminate m-Health interventions, such as Pain Buddy. In areas of the world where Internet connection is not readily available, efforts to first identify ways to provide access to the Internet for targeted patients and families, as well as modifications of the implementation of Pain Buddy (e.g., identifying alternate ways to offer timely communication with healthcare providers), may be important.
The current study focused on using Pain Buddy among children presently undergoing cancer treatment. However, this novel tool has several advantages that support its versatility and adaptability across diagnoses and populations. First, Pain Buddy is delivered on a mobile device (tablet or smartphone), allowing patients to use the application in many settings (e.g., at home, in the hospital), and therefore accessible by children experiencing various forms of cancer and who are at different stages of their disease. Second, Pain Buddy is a versatile assessment device that can assess diverse symptoms across diagnoses. Third, Pain Buddy presents evidence-based pain management strategies that address various pain presentations (e.g., sickle cell disease, arthritis). The current study has focused on pediatric cancer, as children with cancer experience pain, particularly when they are at home. Therefore, a tool such as Pain Buddy can assist with management of these symptoms. It is possible that children with particularly severe symptoms, due to their illness or resulting from treatment, may find it more difficult to attend to the material provided through Pain Buddy. Evaluating potential differences in effectiveness and utility of Pain Buddy in future studies will allow us to identify and address barriers to accessing and/or using Pain Buddy.
6. Conclusion
The World Health Organization acknowledges that a significant proportion of cancer patients experience pain, and pain management efforts in this population are currently inadequate. In addition, due to aggressive treatment protocols, children with cancer are at high risk for the experience of treatment-related symptoms that worsen quality of life. Consistent with the growing trend and low-cost access of m-Health, Pain Buddy has the potential to address multiple gaps in the management of pain and symptoms in children undergoing outpatient cancer treatment. Pain Buddy is an innovative and interactive tool that promises to improve pain and symptom management among children undergoing cancer treatment. Current results suggest that children were highly satisfied with Pain Buddy and found this program to be useful in reducing their pain and symptoms. These results will inform future improvements to Pain Buddy, in preparation for a randomized controlled trial to assess efficacy in improving pain management and quality of life among pediatric cancer patients. Accordingly, Pain Buddy has the long-term potential of improving quality of life in tens of thousands of children undergoing treatment for cancer each year.
Acknowledgments
Source of funding
This study was supported by a Mentored Research Scholar Grant from the American Cancer Society MRSG-13-053-01-PCSM (PI: MA Fortier).
The authors would like to acknowledge the students at the California Institute for Telecommunications and Information Technology (Calit2) at UC Irvine, who were involved in the development of the Pain Buddy program: Tyler Stevens, Raquel Fallman, Tina Chau, William Cook-Spirling, Masha Yamnitski.
Footnotes
Conflicts of interest
None declared for all authors.
References
- 1.American Cancer Society. Cancer Facts and Figures. 〈 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf〉. Published 2012.
- 2.Miller E, Jacob E, Hockenberry MJ. Nausea, pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol Nurs Forum. 2011;38(5):E382–E393. doi: 10.1188/11.ONF.E382-E393. X04063486U603578 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Van Cleve L, Bossert E, Beecroft P, Adlard K, Alvarez O, Savedra MC. The Pain Experience of Children With Leukemia During the First Year After Diagnosis. Nurs Res. 2004;53(1):1–10. doi: 10.1097/00006199-200401000-00001. 〈 http://journals.lww.com/nursingresearchonline/Fulltext/2004/01000/The_Pain_Experience_of_Children_With_Leukemia.1.aspx〉. [DOI] [PubMed] [Google Scholar]
- 4.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science (80-) 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. http://dx.doi.org/10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 5.Gordon DB, Dahl JL, Miaskowski C, et al. American Pain Society Recommendations for Improving the Quality of Acute and Cancer Pain Management: American Pain Society Quality of Care Task Force. Arch Intern Med. 2005;165(14):1574–1580. doi: 10.1001/archinte.165.14.1574. http://dx.doi.org/10.1001/archinte.165.14.1574. [DOI] [PubMed] [Google Scholar]
- 6.Mercadante S. Cancer pain management in children. Palliat Med. 2004;18(7):654–662. doi: 10.1191/0269216304pm945rr. http://dx.doi.org/10.1191/0269216304pm945rr. [DOI] [PubMed] [Google Scholar]
- 7.Yeh CH, Lin CF, Tsai JL, Lai YM, Ku HC. Determinants of parental decisions on “drop out” from cancer treatment for childhood cancer patients. J Adv Nurs. 1999;30(1):193–199. doi: 10.1046/j.1365-2648.1999.01064.x. http://dx.doi.org/10.1046/j.1365-2648.1999.01064.x. [DOI] [PubMed] [Google Scholar]
- 8.Steif BL, Heiligenstein EL. Psychiatric symptoms of pediatric cancer pain. J Pain Symptom Manag. 1989;4(4):191–196. doi: 10.1016/0885-3924(89)90042-0. [DOI] [PubMed] [Google Scholar]
- 9.Hockenberry M. Symptom management research in children with cancer. J Pediatr Oncol Nurs. 2004;21(3):132–136. doi: 10.1177/1043454204264387. 〈 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15296040〉. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization in conjuction with IASP Author. Cancer Pain Relief and Palliative Care in Children. WHO Publications Center; USA: 1998. [Google Scholar]
- 11.Ljungman G, Gordh T, Sorensen S, Kreuger A. Pain in paediatric oncology: interviews with children, adolescents and their parents. Acta Paediatr. 1999;88(6):623–630. doi: 10.1080/08035259950169279. 〈 http://onlinelibrary.wiley.com/store/10.1111/j.1651-2227.1999.tb00011.x/asset/j.1651-2227.1999.tb00011.x.pdf?v=1&t=gnt2i9zi&s=f3a15cc245429b664ce2c28328b402efc8ca8d93〉. [DOI] [PubMed] [Google Scholar]
- 12.Fortier MA, Wahi A, Bruce C, Maurer EL, Stevenson R. Pain management at home in children with cancer: a daily diary study. Pediatr Blood Cancer. 2014;61(6):1029–1033. doi: 10.1002/pbc.24907. http://dx.doi.org/10.1002/pbc.24907. [DOI] [PubMed] [Google Scholar]
- 13.Fortier MA, Wahi A, Maurer EL, Tan ET, Sender LS, Kain ZN. Attitudes regarding analgesic use and pain expression in parents of children with cancer. J Pediatr Hematol Oncol. 2012;34(4):257–262. doi: 10.1097/MPH.0b013e318241fd07. http://dx.doi.org/10.1097/MPH.0b013e318241fd07. [DOI] [PubMed] [Google Scholar]
- 14.Fortier MA, MacLaren JE, Martin SR, Perret D, Kain ZN. Pediatric pain after ambulatory surgery: Where’s the medication? Pediatrics. 2009;124(4) doi: 10.1542/peds.2008-3529. http://dx.doi.org/10.1542/peds.2008-3529. [DOI] [PubMed] [Google Scholar]
- 15.Gorin SS, Krebs P, Badr H, et al. Meta-analysis of psychosocial interventions to reduce pain in patients with cancer. J Clin Onc. 2012 doi: 10.1200/JCO.2011.37.0437. JCO.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauman LJ, Drotar D, Leventhal JM, Perrin EC, Pless IB. A review of psychosocial interventions for children with chronic health conditions. Pediatrics. 1997;100(2 Pt 1):244–251. doi: 10.1542/peds.100.2.244. http://dx.doi.org/10.1542/peds.100.2.244. [DOI] [PubMed] [Google Scholar]
- 17.Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523–530. doi: 10.1197/jamia.M1370. 〈 http://www.sciencedirect.com/science/article/B7CPS-49WMBB3-4/2/8652d35be93665992e1ccbf1364f80fb〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates DW, Cohen M, Leape LL, Overhage JM, Shabot MM, Sheridan T. Reducing the Frequency of Errors in Medicine Using Information Technology. J Am Med Inform Assoc. 2001;8(4):299–308. doi: 10.1136/jamia.2001.0080299. 〈 http://www.jamia.org/cgi/content/abstract/8/4/299〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blumenthal D, Glaser JP. Information Technology Comes to Medicine. N Engl J Med. 2007;356(24):2527–2534. doi: 10.1056/NEJMhpr066212. http://dx.doi.org/10.1056/NEJMhpr066212. [DOI] [PubMed] [Google Scholar]
- 20.Istepanian RSH, Jovanov E, Zhang YT. Introduction to the special section on m-Health: Beyond seamless mobility and global wireless health-care connectivity. IEEE Trans Inf Technol Biomed. 2004;8(4):405–414. doi: 10.1109/titb.2004.840019. http://dx.doi.org/10.1109/TITB.2004.840019. [DOI] [PubMed] [Google Scholar]
- 21.Istepanian RSH. m-health : a decade of evolution and impact on services and global health. Br J Healthc Manag. 2014;20(7):334–337. [Google Scholar]
- 22.Knab JH, Wallace MS, Wagner RL, Tsoukatos J, Weinger MB. The Use of a Computer-Based Decision Support System Facilitates Primary Care Physicians’ Management of Chronic Pain. Anesth Analg. 2001;93(3):712–720. doi: 10.1097/00000539-200109000-00035. 〈 http://www.anesthesia-analgesia.org/cgi/content/abstract/93/3/712〉. [DOI] [PubMed] [Google Scholar]
- 23.Evans RS, Pestotnik SL, Classen DC, et al. A Computer-Assisted Management Program for Antibiotics and Other Antiinfective Agents. N Engl J Med. 1998;338(4):232–238. doi: 10.1056/NEJM199801223380406. http://dx.doi.org/10.1056/nejm199801223380406. [DOI] [PubMed] [Google Scholar]
- 24.Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of Computer-Based Clinical Decision Support Systems on Physician Performance and Patient Outcomes: A Systematic Review. JAMA. 1998;280(15):1339–1346. doi: 10.1001/jama.280.15.1339. http://dx.doi.org/10.1001/jama.280.15.1339. [DOI] [PubMed] [Google Scholar]
- 25.Pestotnik SL, Classen DC, Evans RS, Burke JP. Implementing Antibiotic Practice Guidelines through Computer-Assisted Decision Support: Clinical and Financial Outcomes. Ann Intern Med. 1996;124(10):884–890. doi: 10.7326/0003-4819-124-10-199605150-00004. http://dx.doi.org/10.1059/0003-4819-124-10-199605150-00004. [DOI] [PubMed] [Google Scholar]
- 26.Franklin VL, Greene A, Waller A, Greene SA, Pagliari C. Patients’ engagement with “Sweet Talk” - a text messaging support system for young people with diabetes. J Med Internet Res. 2008:10. doi: 10.2196/jmir.962. http://dx.doi.org/10.2196/jmir.962. [DOI] [PMC free article] [PubMed]
- 27.Miloh T, Annunziato R, Arnon R, et al. Improved adherence and outcomes for pediatric liver transplant recipients by using text messaging. Pediatrics. 2009;124:e844–e850. doi: 10.1542/peds.2009-0415. http://dx.doi.org/10.1542/peds.2009-0415. [DOI] [PubMed] [Google Scholar]
- 28.Franklin VL, Waller A, Pagliari C, Greene SA. A randomized controlled trial of Sweet Talk, a text-messaging system to support young people with diabetes. Diabet Med. 2006;23(12):1332–1338. doi: 10.1111/j.1464-5491.2006.01989.x. http://dx.doi.org/10.1111/j.1464-5491.2006.01989.x. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro JR, Bauer S, Hamer RM, Kordy H, Ward D, Bulik CM. Use of Text Messaging for Monitoring Sugar-sweetened Beverages, Physical Activity, and Screen Time in Children: A Pilot Study. J Nutr Educ Behav. 2008;40(6):385–391. doi: 10.1016/j.jneb.2007.09.014. 〈 http://www.sciencedirect.com/science/article/B82X5-4TTHS6d-F/2/fec2569c27ce3619336886e79be64a63〉. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neville R, Greene A, McLeod J, Tracey A, Surie J. Mobile phone text messaging can help young people manage asthma. BMJ. 2002;325:600. doi: 10.1136/bmj.325.7364.600/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stone AA, Shiffman SS, Schwartz JE, Broderick JE, Hufford MR. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182–199. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 32.Palermo TM, Valenzuela D, Stork PP. A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain. 2004;107(3):213–219. doi: 10.1016/j.pain.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Gaertner J, Elsner F, Pollmann-Dahmen K, Radbruch L, Sabatowski R. Electronic pain diary: a randomized crossover study. J Pain Symptom Manag. 2004;28(3):259–267. doi: 10.1016/j.jpainsymman.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 34.Stinson J, Petroz GC, Tait G, et al. e-Ouch: Usability testing of an electronic chronic pain diary for adolescents with arthritis. Clin J Pain. 2006;22:295–305. doi: 10.1097/01.ajp.0000173371.54579.31. [DOI] [PubMed] [Google Scholar]
- 35.Marceau LD, Link C, Jamison RN, Carolan S. Electronic diaries as a tool to improve pain management: is there any evidence? Pain Med. 2007;8(Suppl 3):S101–S109. doi: 10.1111/j.1526-4637.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 36.Lewandowski AS, Palermo TM, Kirchner HL, Drotar D. Comparing diary and retrospective reports of pain and activity restriction in children and adolescents with chronic pain conditions. Clin J Pain. 2009;25(4):299–306. doi: 10.1097/AJP.0b013e3181965578. http://dx.doi.org/10.1097/AJP.0b013e3181965578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hendershot E, Murphy C, Doyle S, Van-Clieaf J, Lowry J, Honeyford L. Outpatient chemotherapy administration: decreasing wait times for patients and families. J Pediatr Oncol Nurs. 2005;22(1):31–37. doi: 10.1177/1043454204272539. [DOI] [PubMed] [Google Scholar]
- 38.Ferrell BR, Grant M, Borneman T, Juarez G, ter Veer A. Family caregiving in cancer pain management. J Palliat Med. 1999;2:185–195. doi: 10.1089/jpm.1999.2.185. [DOI] [PubMed] [Google Scholar]
- 39.Howard RF. Current status of pain management in children. JAMA. 2003;290(18):2464–2469. doi: 10.1001/jama.290.18.2464. [DOI] [PubMed] [Google Scholar]
- 40.Chung WW, Martinez A, Gago-Masague S, Fortier MA. Int Soc Res Internet Interv; Pain Buddy: An Interactive, Mobile Intervention for Pain and Symptom Management in Children with Cancer; Seattle. 2016; 2016. [Google Scholar]
- 41.Gago-Masague S, Zhang A, Martinez A, Fortier MA. Pain Buddy - Using Virtual Characters to Improve Home-Based Therapy for Children Suffering from Cancer Maui, Medicine 2.0: Social Media, Mobile Apps 2.0 in Health. Medicine and Biomedical Research. 2014 [Google Scholar]
- 42.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. http://dx.doi.org/10.1037/0033-295X.84.2.191. [DOI] [PubMed] [Google Scholar]
- 43.Strecher VJ, DeVellis BM, Becker MH, Rosenstock IM. The role of self-efficacy in achieving health behavior change. Heal Educ Q. 1986;13:73–92. doi: 10.1177/109019818601300108. [DOI] [PubMed] [Google Scholar]
- 44.Kazdin AE. The separate and combined effects of covert and overt rehearsal in developing assertive behavior. Behav Res Ther. 1982;20(1):17–25. doi: 10.1016/0005-7967(82)90004-3. http://dx.doi.org/10.1016/0005-7967(82)90004-3. [DOI] [PubMed] [Google Scholar]
- 45.S-Wang L, Wu P-Y. The role of feedback and self-efficacy on web-based learning: The social cognitive perspective. Comput Educ. 2008;51(4):1589–1598. http://dx.doi.org/10.1016/j.compedu.2008.03.004. [Google Scholar]
- 46.Chinman M, Imm P, Wandersman A. Get. Outcomes 2004: Promot. Account Methods Tools Plan, Implement, Eval. 2004
- 47.Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: a revised child anxiety and depression scale. Behav Res Ther. 2000;38(8):835–855. doi: 10.1016/s0005-7967(99)00130-8. 〈 http://www.sciencedirect.com/science/article/B6V5W-4096PG0-8/2/b9319f3148e25ce762a6407c1d5a494f〉. [DOI] [PubMed] [Google Scholar]
- 48.Collins JJ, Devine TD, Dick GS, et al. The measurement of symptoms in young children with cancer: the validation of the Memorial Symptom Assessment Scale in children aged 7–12. J Pain Symptom Manag. 2002;23(1):10–16. doi: 10.1016/s0885-3924(01)00375-x. S088539240100375X [pii] [DOI] [PubMed] [Google Scholar]
- 49.Collins JJ, Byrnes ME, Dunkel IJ, et al. The Measurement of Symptoms in Children with Cancer. J Pain Symptom Manag. 2000;19(5):363–377. doi: 10.1016/s0885-3924(00)00127-5. 〈 http://www.sciencedirect.com/science/article/B6T8R-40JFY9K-B/2/4272e608a07530760583334c446fc96a〉. [DOI] [PubMed] [Google Scholar]
- 50.Savedra MC, Holzemer WL, Tesler MD, Wilkie DJ. Assessment of post-operation pain in children and adolescents using the adolescent pediatric pain tool. Nurs Res. 1993;42(1):5–9. 〈 http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8424069〉. [PubMed] [Google Scholar]
- 51.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain. 1983;17(1):33–44. doi: 10.1016/0304-3959(83)90125-2. 0304-3959(83)90125-2 [pii] [DOI] [PubMed] [Google Scholar]
- 52.Schanberg LE, Sandstrom MJ, Starr K, et al. The relationship of daily mood and stressful events to symptoms in juvenile rheumatic disease. Arthritis Care Res. 2000;13(1):33–41. doi: 10.1002/1529-0131(200002)13:1<33::aid-art6>3.0.co;2-s. http://dx.doi.org/10.1002/1529-0131(200002)13:1<33::AID-ART6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 53.Collins SK, Kuck K.Anonymous. Music therapy in the neonatal intensive care unit. Neonatal Netw. 1991;9(6):23–26. 〈 http://www.ncbi.nlm.nih.gov/htbin-post/Entrez/query?db=m&form=6&dopt=r&uid=0002000074〉. [PubMed] [Google Scholar]
- 54.Savedra M, Tesler M, Holzemer W, Ward J. Adolescent Pediatric Pain Tool (APPT): Preliminary User’s Manual. University of California; San Francisco: 1989. [Google Scholar]
- 55.Lenhart A. Washington DC, Teens, Social Media Technol Overv. Washington D.C: 2015. 〈 http://www.pewinternet.org/2015/04/09/teens-social-media-technology-2015/〉. [Google Scholar]
- 56.Stinson JN, Jibb LA, Nguyen C, et al. Development and testing of a multidimensional iPhone pain assessment application for adolescents with cancer. J Med Internet Res. 2013;15(3):e51. doi: 10.2196/jmir.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baggott C, Gibson F, Coll B, Kletter R, Zeltzer P, Miaskowski C. Initial evaluation of an electronic symptom diary for adolescents with cancer. J Med Internet Res. 2012;1(2):e23. doi: 10.2196/resprot.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacob E, Stinson J, Duran J, et al. Usability testing of a smartphone for accessing a web-based e-diary for self-monitoring of pain and symptoms in sickle cell disease. J Pediatr Hematol Oncol. 2012;34(5):326–335. doi: 10.1097/MPH.0b013e318257a13c. [DOI] [PMC free article] [PubMed] [Google Scholar]