Summary
Adipose tissue development is associated with modifications involving extracellular matrix remodelling, and metalloproteinases play a significant role in this process. Reduced circulating sexual hormones cause impacts on the size, morphology and functions of the adipose tissue, increasing susceptibility to diseases. This study investigated whether exercise training may be an alternative strategy to combat the effects promoted by estrogen decay through modulation in gene expression patterns in the extracellular matrix (ECM) of visceral adipose tissue of ovariectomized rats. Nulliparous rats (n = 40) were randomly distributed into four groups (n = 10/group): sham sedentary (Sh‐S), sham resistance training (Sh‐Rt), ovariectomized sedentary (Ovx‐S) and ovariectomized resistance training (Ovx‐Rt). The Sh‐S animals did not have any type of training. The body mass and food intake, ECM gene expression, gelatinase MMP‐2 activity and adipocyte area were measured. A lack of estrogen promoted an increase in body mass, food intake and the visceral, parametrial and subcutaneous adipocyte areas. The ovariectomy upregulated the expression of MMP‐2, MMP‐9, TGF‐β, CTGF, VEGF‐A and MMP‐2 activity. On the other hand, resistance training decreased the body mass, food intake and the adipocyte area of the three fat depots analysed; upregulated TIMP‐1, VEGF‐A and MMP‐2 gene expression; downregulated MMP‐9, TGF‐β and CTGF gene expression; and decreased the MMP‐2 activity. We speculate that resistance training on a vertical ladder could play an important role in maintaining and remodelling ECM by modulation in the ECM gene expression and MMP‐2 activity, avoiding its destabilization which is impaired by the lack of estrogen.
Keywords: exercise, extracellular matrix, menopause, MMP, estrogen, rats
Introduction
The development of adipose tissue is associated with vast modifications involving angiogenesis, adipogenesis and extracellular matrix (ECM) remodelling. Pathological adipose tissue expansion by rapid and massive enlargement of existing adipocytes limits the angiogenesis, which contributes to inadequate vascularization, thus leading to low tissue oxygenation and inflammation development, which are clearly associated with metabolic disturbances (Mesch et al. 2006; Pasquali & Vicennati 2008; Berg et al. 2014; Law et al. 2014; Song et al. 2016).
During adipose tissue expansion, metalloproteinases (MMPs) play a significant role in adipogenesis, angiogenesis and ECM proliferation (Song et al. 2016). Sexual hormones also participate in MMP regulation, and estrogen seems to act directly on adipose tissue due to the presence of sex hormone receptors in this tissue (Berg et al. 2014; Law et al. 2014). In this context, in postmenopausal females, the reduced circulating sexual hormones result in the development of increased intra‐abdominal adiposity, which makes these women more susceptible to diseases associated with the metabolic syndrome (Mesch et al. 2006). Considering this, Pasquali and Vicennati (2008) demonstrated that an imbalance of sexual hormones causes a profound impact on the size, morphology and functions of the adipose tissue, which can promote fat redistribution of the peripheral sites to the abdominal region, mainly in the visceral fat depot. Thus, a lack of estrogen is partly responsible for the reduction in lean mass, an increase in fat mass (mainly visceral fat), insulin resistance, dyslipidaemia (Jiang et al. 2008; Orsatti et al. 2008; Pallottini et al. 2008; Riant et al. 2009) and also destabilization of ECM. MMPs cooperate in this process (Mariman & Wang 2010). Therefore, the high flexibility of ECM, as well as the adequate balance between the ECM deposition and degradation, ensures that the healthy expansion of adipose tissue occurs (Trayhurn 2013; Martinez‐Santibanez et al. 2015; Choe et al. 2016). On the other hand, the excessive increase in adipose tissue leads to hypoxia of the tissue that induces ECM fibrosis with a consequent reduction in the tissue plasticity, which triggers the adipose tissue metabolic dysfunction (Trayhurn 2013).
Currently, there is great interest in investigating strategies to prevent or attenuate the effects of accumulating adipose tissue and low‐intensity chronic inflammation associated with obesity in the postmenopausal period (Arslan et al. 2010; Gregor & Hotamisligil 2011). Hormone replacement (HR) has shown beneficial effects in terms of preventing oxidative stress and adipocyte inflammation (Stubbins et al. 2012; Rodrigues et al. 2017). However, HR is related to the increased incidence of some types of cancer (Zhang et al. 2007), which limits its use. Therefore, evidence has shown that resistance training (on a vertical ladder) can be an efficient tool to reduce the fatty tissue depots (Pighon et al. 2009; Speretta et al. 2012) by decreasing the expression of related genes in adipose tissue lipogenesis and fat percentage in the heart and liver, which were increased in ovariectomized rats (Leite et al. 2009; Stotzer et al. 2015). Furthermore, there are very few studies associating exercise of any nature with the ECM of obese adipose tissue in ovariectomized rats.
Thus, as the physiological decrease in estrogen concentration predisposes to obesity and also alters the protective function of adipose tissue ECM, the aim of this experimental investigation was to analyse whether resistance training on a vertical ladder may be an alternative strategy to combat physiological changes due to the decay of this hormone, controlling the increase in body fat mass and the destabilization of adipose tissue ECM, mainly by the change in ECM gene expression patterns, specifically in the visceral adipose tissue of the ovariectomized rats.
Methods
Animals
For this research, nulliparous female Sprague‐Dawley rats (from the breeding colony of the State University of São Paulo/UNESP, Araraquara, Brazil) weighing approximately 220 ± 12 g were housed under a constant temperature (22 ± 2°C), humidity of 45%±15% and 12‐h light/dark cycle with light from 1:00 AM to 01:00 PM (reverse light cycle). They were kept in collective cages (four rats per cage) and had free access to water and food. The rats (n = 40) were randomly distributed into four groups (n = 10/group): sham sedentary (Sh‐S), sham resistance training (Sh‐Rt), ovariectomized sedentary (Ovx‐S) and ovariectomized resistance training (Ovx‐Rt). The animals in the sedentary group did not have any type of training.
Ethical approval statement
The animal protocol was approved by the Ethics Committee for Animal Experimentation from the Federal University of São Carlos/UFSCar (Protocol No. 008/2010). All experimental procedures were carried out according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (National Research Council 1996).
Experimental design
Details of the experimental design are shown in Figure 1. The animals from the trained groups performed 10 weeks of high‐intensity resistance training. The training was performed during the reverse light cycle, which corresponds to the active period of the animals. Ovariectomy and sham operations were performed when the rats reached 250 g of body mass, according to the technique described by Kalu (1991). Briefly, for the surgery, the rats were anaesthetized with a mixture of ketamine and xylazine (61.5–7.6 mg/kg, intraperitoneally). The animals from the sham groups underwent a surgical procedure, but did not have their ovaries removed. On the other hand, animals from the Ovx groups had their ovaries removed. All rats submitted to the surgery procedure had 3 weeks to recover before the beginning of the exercise training interventions.
Figure 1.
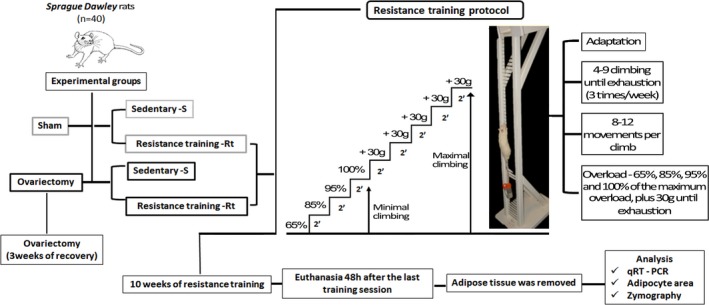
Representative figure of the experimental study design. [Colour figure can be viewed at wileyonlinelibrary.com].
Resistance training on a vertical ladder consisted of climbing sessions that were performed three times a week over 10 weeks. The rats were adapted to the protocol, which required the animals to climb a vertical ladder (1.1 m × 0.18 m, 2 cm grid, 80° incline) with weights attached to their tails and load apparatus attached to the proximal portion of the tail using a self‐adhesive foam strip. A Velcro strap was wrapped around the foam strip and fastened. Having the load apparatus attached to the tail, each rat was placed at the bottom of the ladder and familiarized with climbing. The size of the ladder induced the animals to perform 8–12 movements per climb. At the top of the ladder, the rats reached a housing chamber (20 × 20 × 20 cm), where they were allowed to rest for 120 s. This procedure was repeated until the rats would voluntarily climb the ladder for three consecutive times, without stimulus. The first training session started 3 days after this familiarization. It consisted of four to eight ladder climbs while carrying progressively heavier loads. The initial climb consisted of carrying a load that was 75% of the animal's body mass. Afterwards, a series of additional weights of 30 g were added until the load was too heavy for the animals to climb the entire length of the ladder. Failure was determined when the animals could not progress up the ladder after three successive stimuli on the tail using tweezers. The highest load successfully carried over the whole length of the ladder was considered as the rat's maximal carrying capacity for that training session. The next training sessions consisted of climbing the ladder four times with 65, 85, 95 and 100% of the rat's previous maximal carrying capacity, determined in the previous session. During subsequent ladder climbs, an additional 30 g load was added until a new maximal carrying capacity was determined. The resistance training protocol was adapted from Hornberger and Farrar (2004), according to the needs of the current research.
Body mass and food intake
Body mass and food intake were measured three times per week. The delta values were calculated using the following formula: ∆ = (initial − final)/final × 100.
Euthanasia
All animals were euthanized by decapitation, whereas the training rats were decapitated 48 h after the last session of the high‐intensity resistance training. The uterus, white adipose tissues parametrial, visceral and subcutaneous were immediately removed by procedures that were adapted for rats (Duarte et al. 2008; Sene‐Fiorese et al. 2008) using the anatomical localization of the organs, which was described by Cinti et al. (2005). The collected tissues were weighed and stored in a freezer at −80°C. We confirmed the efficiency of the ovariectomy by the uterus weight that decreased 78.3% in the Ovx‐S group (0.18 g; P = 0.0001) and decreased 98.7% in the Ovx‐Rt group (0.15 g; P = 0.0001) when compared to the Sh‐S group (0.83 g; P = 0.0001).
RNA extraction and quantitative real‐time PCR
Frozen samples of visceral adipose tissue (100 mg) were homogenized on ice in the Trizol reagent (Invitrogen Corporation, California, USA) according to the manufacturer's instructions. The purity and yield of total RNA were determined by measuring the absorbance (optical density in the NanoDrop) of aliquots at 260 and 280 nm (ratios: 260 nm/280 nm and 230/280). The RNA integrity was visualized with ethidium bromide on 2% agarose gel, by electrophoresis with a pattern of 28S and 18S ribosomal RNA. Each sample of total RNA (1 μg) was treated with DNase I (Invitrogen Corporation California, USA) to remove contaminants of genomic DNA. The cDNA was synthesized by reverse transcription using M‐MLV reverse transcriptase (Promega Corporation, Madison, WI, USA). For real‐time PCR (CFX 96 real‐time PCR – Bio‐Rad, San Francisco, USA), 20 ng of cDNA and 0.5 μM of each primer were used in 25 μl volume system mix containing SoFastTM Eva Green® (Bio‐Rad, San Francisco, USA). The primer sequences used are described in Table 1.
Table 1.
Primers sequence
| Genes | Accession | Sense primer | Antisense primer |
|---|---|---|---|
| COL‐1 | NM_053304.1 | ATCAGCCCAAACCCCAAGGAGA | CGCAGGAAGGTCAGCTGGATAG |
| COL‐3 | NM_032085.1 | TGATGGGATCCAATGAGGGAGA | GAGTCTCATGGCCTTGCGTGTTT |
| TIMP‐1 | NM_053819.1 | ATAGTGCTGGCTGTGGGGTGTG | TGATCGCTCTGGTAGCCCTTCTC |
| TIMP‐2 | NM_021989.2 | GGACACGCTTAGCATCACCCAGA | GTCCATCCAGAGGCACTCATCC |
| MMP‐2 | NM_031054.2 | CTGGGTTTACCCCCTGATGTCC | AACCGGGGTCCATTTTCTTCTTT |
| MMP‐9 | NM_031055.1 | GGATGTTTTTGATGCCATTG | CCACGTGCGGGCAATAAGAA |
| TGF‐β | NM_021578.2 | CCCCTGGAAAGGGCTCAACAC | TCCAACCCAGGTCCTTCCTAAAGTC |
| CTGF | NM_022266.2 | CAGGCTGGAGAAGCAGAGTCGT | CTGGTGCAGCCAGAAAGCTCAA |
| VEGF | NM_001110334.1 | CCACGACAGAAGGGGAGCAGAA | GGAAGATGTCCACCAGGGTCTCAA |
| RPLP0 | NM_022402.2 | AGGGTCCTGGCTTTGTCTGTGG | AGCTGCAGGAGCAGCAGTGG |
COL1 and COL3, collagen I and collagen III respectively; TGF‐β, transforming growth factor beta; CTGF, connective tissue growth factor; MMP‐2 and MMP‐9, matrix metallopeptidases 2 and 9 respectively; TIMP‐2 and TIMP‐1, tissue inhibitor of metallopeptidases 2 and 1 respectively; VEGF, vascular endothelial growth factor; RPLP0, large ribosomal protein P0.
In addition, a standard curve of rat white adipose tissue cDNA was run in duplicate for every plate to produce a standard curve for quantification. All reactions were performed in duplicate. The relative amounts of RNAs were calculated using the comparative Ct method (Livak and Schmittgen (2001)). The thermal cycling programme was 95°C for 30 s, followed by 40 cycles of 95°C for 15 s, 57–61°C for 30 s and 72°C for 30 s. The specificity of the amplification products was confirmed by size estimations on a 2% agarose gel and by analysing their melting curves. The expression of all genes was normalized for the RPLPO expression.
Determination of MMP activity by gelatin zymography
Proteins with gelatinolytic activity were identified by electrophoresis in the presence of SDS in 10% polyacrylamide gels containing 1 mg/ml gelatin due to the proteins’ capacity to digest the gelatin substrate. Briefly, aliquots of 30 μg of the total protein, extracted from 50 mg of visceral adipose tissue (extraction buffer: 10 mM cacodylic acid pH 5.0; 0.15M NaCl; 1 μM ZnCl2; 20 mM CaCl2; 1.5 mM NaN3; 0.01% Triton X‐100 v/v) were loaded directly onto the gels. The total protein was measured against a standard protein sample using a BCA Protein Assay Kit (Pierce, Rockford, IL, USA). After electrophoresis, the proteins were renatured by exchanging sodium dodecyl sulphate (SDS) with 2.5% Triton X‐100 (20‐min incubation). The gels were then incubated for 20 h at 37°C in 50 mmol/l Tris–HCl, pH 8, 5 mmol/l CaCl2 and 0.02% NaN3 and stained with Coomassie blue. The gelatinolytic activity was visualized as areas of lytic activity on an otherwise blue gel. Migration of proteins was compared with that of a pertained molecular weight marker (Fermentas brand's precoloured molecular mass marker SM0671). The tissue extract was tested for the negative activity of MMP‐2 using the same technique, adding to each sample 4 μl of 450 mM EDTA (a protease inhibitor and subjected to SDS‐PAGE with gelatin as described above). At the time the gel was taken for incubation, 3.3 ml of 450 mM EDTA was added to the incubation buffer. EDTA is a zinc chelator. When added to the incubation buffer, it removes the zinc from the buffer. As MMP‐2 is zinc dependent, the buffer no longer has zinc for the activation of MMP‐2. The gels were scanned by an imaging densitometer and quantified using the Gene Tools version 3.06 software (Syngene, Cambridge, UK). All procedures were performed as previously described by Marqueti et al. (2012).
Adipocyte size and adipocyte number
A fragment (100 mg) of the visceral, parametrial and subcutaneous white adipose tissues was fixed in a 0.2 M collidine buffer, pH 7.4, containing 2% of osmium tetroxide. The samples were incubated at 37°C overnight. After this, they were washed with warmed saline as described by Hirsch and Gallian (1968) and Pfaffl et al. (2002). The mean adipocyte area of at least 400 adipocytes per animal was measured using the Image Pro plus software v7.01 (Media Cybernetics, Silver Spring, MD, USA) and expressed as μm2.
Another fragment of visceral adipose tissue was fixed for 24 h in formalin, embedded in paraffin and sectioned. A morphometric study of the adipocyte number was performed in five‐micron‐thick visceral adipose tissue sections stained with haematoxylin–eosin (HE). Slides were randomly digitized under a light microscope for HE (10 fields per animal – 20 μm, original magnification ×400). The total adipocyte number in visceral fat depot was calculated from the fat cell volume using the formula , where d is the mean diameter of at least 30 measured cells in the field and σ is the standard deviation of the diameter. To determinate fat cell weight, the fat cell density was applied (0.92 g/ml). Thus, the total fat cell number in the whole visceral depot of each animal was determined by dividing the total fat depot weight by the mean cell weight of all captured fields (Pascual‐Serrano et al. 2017).
Statistical analysis
Data are represented as mean ± standard deviation (SD). Statistical analysis for body mass, food intake, adipocyte area, adipocyte number and zymography was performed using the program STATISTICA version 7.0 for Windows (StatSoftInc., Tulsa, OK, USA). The Kolmogorov–Smirnov test was used to assess the assumption of normality for the data. The statistical significant trends were analysed by two‐way ANOVA followed by a Tukey post hoc test or Kruskal–Wallis test when appropriate. Statistical significance was accepted at a value of P < 0.05.
The relative gene analyses of the study groups were performed using the REST 2009 (Relative Expression Software Tool program Qiagen® [30]), in accordance with the literature (Leduc et al. 2011; Salis et al. 2014; Tafsiri et al. 2015). The statistical significance was accepted at a value of P < 0.05.
Results
Food intake and body mass
Table 2 shows that the food intake and body mass in the ovariectomized (Ovx‐S) rats were greater than in the intact animals (Sh‐S). The exercise training was effective in terms of decreasing the food intake and body mass of the Sh‐Rt group in comparison with the Sh‐S group (Table 2). On the other hand, the training promoted a decrease in food intake of the Ovx‐Rt group, but did not change the body mass in these animals in relation to the Ovx‐S group (Table 2).
Table 2.
Total body mass and food intake (gram/week/group)
| Sham Sedentary | Ovx Sedentary | Sham‐Rt | Ovx‐Rt | |
|---|---|---|---|---|
| Body mass initial | 290.13 ± 22.90 | 317.40 ± 15.90* | 278.87 ± 16.10* | 319.60 ± 8.11* , *** |
| Body mass final | 336.62 ± 27.30 | 392.50 ± 25.40* | 309.62 ± 18.71* | 372.40 ± 8.60* , *** |
| ∆ body mass (%) | 16.03 | 23.66 | 11.03 | 16.52 |
| Food intake initial | 19.35 ± 1.66 | 22.5 ± 2.12* | 19.21 ± 0.40 | 21.61 ± 1.28*** |
| Food intake final | 21.13 ± 0.99 | 23.37 ± 1.39* | 19.37 ± 1.69* | 19.77 ± 1.19** |
| ∆ food intake (%) | 9.22 | 3.94 | 0.87 | −8.53 |
Sh‐S, sham sedentary; Ovx‐S, ovariectomized sedentary; Sh‐Rt, sham resistance training; Ovx‐Rt, ovariectomized resistance training. ∆ represents the percentage of increase or decrease in the body mass and food intake in relation to initial week.
Different superscripts (n = 10 per group): * denotes significantly different from sham sedentary; ** denotes significantly different from ovariectomy; *** denotes significantly different from resistance training. Groups are significantly different from each other at P < 0.05.
Adipocyte area and adipocyte number
The absence of estrogen (Ovx‐S) promoted an increase in the adipocyte area in comparison with the Sh‐S group in all the adipose depots analysed – visceral (Figure 2a), parametrial (Figure 2b) and subcutaneous (Figure 2c) white adipose tissues. Concerning the resistance training, the visceral and parametrial adipocyte areas were still greater than in the Sh‐S group after exercise (Sh‐Rt) (Figure 2a and b). However, in the absence of estrogen, the Rt was effective in terms of decreasing the adipocyte size in Ovx‐Rt rats in both visceral (Figure 2a) and parametrial (Figure 2b) fat depots in relation to the Ovx‐S. On the other hand, the subcutaneous adipocyte area (Figure 2c) decreased in both training groups (Ovx‐Rt and Sh‐Rt) compared to Ovx‐S and Sh‐S.
Figure 2.
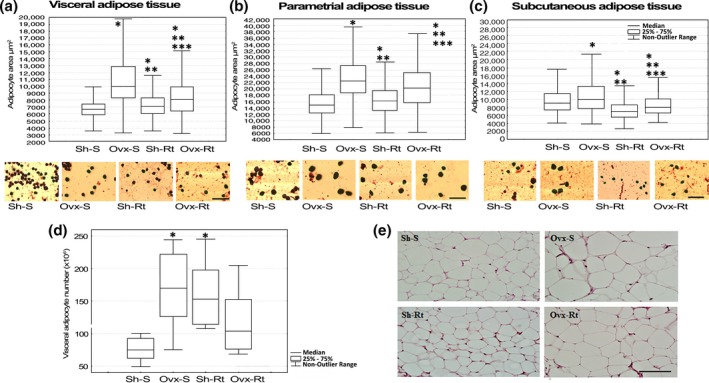
Adipocyte area and adipocyte number. Adipocyte area measured by osmium tetroxide fixation and photomicrographs of corresponding group. (a) Visceral adipose tissue; (b) parametrial adipose tissue; (c) subcutaneous adipose tissue. Representative adipocytes photomicrographs (10 μm – original magnification ×400). Results are expressed as means and standard deviation of the mean (n = 8 per group). At least 400 adipocytes of each depot fat per animal had their mean diameter measured. (d) Adipocyte number measured by haematoxylin–eosin (HE). (e) Visceral adipose tissue histology. Slides were randomly digitized under a light microscope for HE (10 fields per animal – 20 μm, original magnification ×400). Different superscripts: * denotes significantly different from sham sedentary; ** denotes significantly different from ovariectomy; *** denotes significantly different from resistance training. Groups are significantly different from each other at P < 0.05. Abbreviations: Sh‐S, sham sedentary; Ovx‐S, ovariectomized sedentary; Sh‐Rt, sham resistance training; Ovx‐Rt, ovariectomized resistance training. [Colour figure can be viewed at wileyonlinelibrary.com].
The analysis of the number of adipocytes in the visceral fat depot (Figure 2d and e) shows that in the absence of estrogen (Ovx‐S), there are more adipocytes than in intact group (Sh‐S). The number of adipocytes in ovariectomized rats (Ovx‐Rt) was not significantly affected by resistance training compared to the Sh‐S and Ovx‐S groups (Figure 2d and e). However, it is noteworthy that resistance training promotes higher median values in the number of adipocytes in Ovx animals. Moreover, resistance training promoted an increase in the number of adipocytes in the intact trained group (Sh‐Rt) when compared to sedentary group (Sh‐S) (Figure 2d and e).
Visceral fat extracellular matrix (ECM) gene expressions
When comparing the Sh‐S group with the Ovx‐S group, we observed that the MMP‐2 (Figure 3e), MMP‐9 (Figure 3f), TGF‐β (Figure 3g), CTGF (Figure 3h) and VEGF (Figure 3i) extracellular matrix gene expressions in visceral adipose tissue were upregulated for the ovariectomized animals in relation to the intact rats. The resistance training for the sham (Sh‐Rt) group upregulated the TIMP‐1 (Figure 3c), MMP‐2 (Figure 3e), MMP‐9 (Figure 3f), CTGF (Figure 3h) and VEGF (Figure 3i) extracellular matrix gene expression in the visceral adipose tissue compared to the sedentary group (Sh‐S). On the other hand, the ECM gene expressions of TIMP‐1 (Figure 3c), MMP‐2 (Figure 3e), TGF‐β (Figure 3g), CTGF (Figure 3h) and VEGF (Figure 3i) were upregulated in the Ovx‐Rt group in relation to the Sh‐S group.
Figure 3.
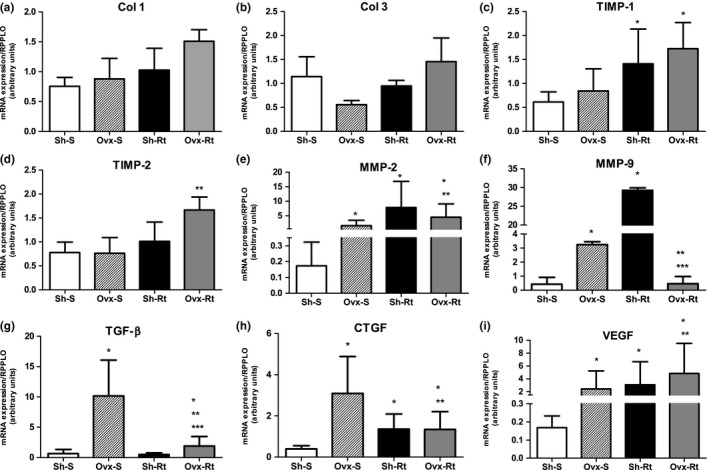
Extracellular matrix (ECM) mRNA expression in visceral adipose tissue. Results are expressed as means and standard deviation of the mean (n = 8 per group). Different superscript: * denotes significantly different from sham sedentary; ** denotes significantly different from ovariectomy; *** denotes significantly different from resistance training. Groups are significantly different from each other at P < 0.05. Abbreviations: Sh‐S, sham sedentary; Ovx‐S, ovariectomized sedentary; Sh‐Rt, sham resistance training; Ovx‐Rt, ovariectomized resistance training; COL1 and COL3, collagen I and collagen III respectively; TGF‐β, transforming growth factor beta; CTGF, connective tissue growth factor; MMP‐2 and MMP‐9, matrix metallopeptidases 2 and 9 respectively; TIMP‐2 and TIMP‐1, tissue inhibitor of metallopeptidases 2 and 1 respectively; VEGF, vascular endothelial growth factor.
In the Ovx‐Rt group, the TIMP‐2 (Figure 3d), MMP‐2 (Figure 3e) and VEGF (Figure 3i) ECM gene expressions were upregulated and the MMP‐9 (Figure 3f), TGF‐β (Figure 3g) and CTGF (Figure 3h) gene expressions were downregulated in comparison with Ovx‐S in the visceral depot. Comparing the two trained groups (Sh‐Rt vs. Ovx‐Rt), the resistance training promoted downregulation only in the MMP‐9 ECM gene expression (Figure 3f) and upregulation in the TGF‐β (Figure 3g) in the Ovx‐Rt group.
Zymography
Both ovariectomy (Ovx‐S) and exercise training (Sh‐Rt) increased the gelatinase MMP‐2 activity in visceral fat depots in relation to the Sh‐S group (Figure 4). On the other hand, physical exercise decreased the MMP‐2 activity in the Ovx‐Rt group in relation to the S‐Sh and Ovx‐S groups (Figure 4). The MMP‐2 activity was lower in the Ovx‐Rt group than in the Sh‐S group (Figure 4).
Figure 4.
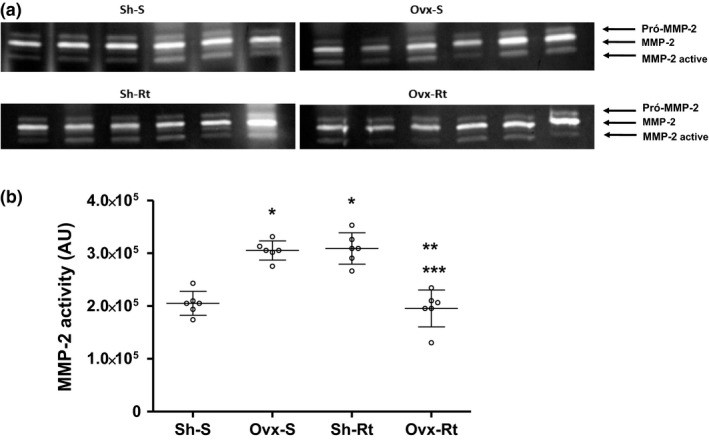
MMP‐2 activity in visceral adipose tissue. (a) Illustrative image of zymography assay of MMP‐2 activity. (b) Representative values of MMP‐2 activity in adipose tissue. Results are expressed as means and standard deviation of the mean (n = 8 per group). Different superscripts: * denotes significantly different from sham sedentary; ** denotes significantly different from ovariectomy; *** denotes significantly different from resistance training. Groups are significantly different from each other at P < 0.05. Abbreviations: Sh‐S, sham sedentary; Ovx‐S, ovariectomized sedentary; Sh‐Rt, sham resistance training; Ovx‐Rt, ovariectomized resistance training; AU, arbitrary unit.
Discussion
It is known that the physiological interaction between adipose tissue cells and a variety of ECM proteins promotes the regulation of adipose tissue function. This includes the modulation of adipogenesis which is associated with ECM remodelling during tissue expansion. Thus, the modulation of angiogenesis and ECM remodelling promote differentiation of de novo adipocytes, preventing the formation of hypertrophic adipocytes by providing additional capacity to store extra lipids. However, considering obesity, chronic excessive energy storage in the adipose tissue initiates pathological ECM remodelling and the absence of estrogen, which are characteristic of menopause and may be an important obesity‐triggering factor (Clegg 2012; Lizcano & Guzmán 2014; Sweeney et al. 2016). Complementing these findings, Leite et al. (2009) observed an increase in the liver, heart and muscle lipid contents in ovariectomized rats. In fact, in the current study, the absence of estrogen, due to ovariectomy, increased body mass and food consumption. In accordance with Rogers et al. (2009), the ovariectomy promoted a reduction in oxygen consumption associated with a reduction in voluntary physical activity contributing to the increase in adipose mass. Taking this into account, we observed an increase in the adipocyte area of the visceral, parametrial and subcutaneous fat depots, being that the visceral fat depot also develops hyperplasia. It is important to emphasize that for adipocytes to increase in size and number, the ECM should be malleable and that the lack of flexibility of the ECM could cause inflammation of the adipose tissue (Sun et al. 2013).
During obesity‐mediated fat mass development, MMPs are involved in controlling the proteolysis and adipogenesis (Christiaens & Lijnen 2006; Berg et al. 2014). Studies demonstrate that the gelatinase subgroup (MMP‐2 and MMP‐9) is secreted by adipose tissue and their activity is modulated during adipose tissue expansion/regression (Bouloumié et al. 2001; Lu et al. 2011), as observed in our study. There is an increase in the MMP‐2 and ‐9 mRNA gene expression and MMP‐2 activity, associated with an increase in the adipocyte area of the visceral adipose tissue of Ovx rats, in agreement with the high plasma levels of MMP‐2 observed in obese patients by other researchers (Maquoi et al. 2002; Miksztowicz et al. 2012). On the other hand, Miksztowicz et al. (2014) suggest that adipose tissue is not the main cause for circulating MMPs because there was a decrease in the activity of MMP‐2 in the visceral adipose tissue in rats with insulin resistance induced by a sucrose‐rich diet. Moreover, this did not reflect the changes in the plasma activity of this enzyme. The TIMPs (family members of tissue inhibitors of matrix metalloproteinase), under physiological conditions, act molecularly as a brake against MMP‐dependent ECM degradation (Murphy 2011). TIMPs and MMPs are regulated differently while obesity progresses, which could explain the fact that there is a difference between the TIMPs mRNA gene expression and MMP‐2 mRNA in the Ovx group in the current study. In fact, Chavey et al. (2003) demonstrated that changes in the MMP gene expression in obesity lead to a rise in proteolytic activities. Thus, the release and activation of MMPs create a proteolytic environment where molecules can be degraded by the increase in the MMP‐2 activity.
In addition, when adipose tissue mass increases, structural remodelling also occurs, modifying growth factors that are involved in tissue biology. MMP‐2 and ‐9 can release transforming growth factor beta (TGF‐β) that changes cell migration and regulates the ECM protein expression, mainly collagens and fibronectin, suppressing the activity and expression of MMP‐2 (Divoux & Clément 2011). TGF‐β is the main profibrotic factor and also upregulates the connective tissue growth factor (CTGF) in adipocytes, which inhibits adipogenesis (Buechler et al. 2015). These proteins modulate cell function by interacting with cell surface receptors, proteases, hormones and structural matrix proteins. In obesity, the level of expression of these proteins is modified in white adipose tissue (Divoux & Clément 2011), as observed in our study. Thus, the lack of estrogen leads to ECM instability, once the upregulation of ECM cell structure and components occurs, suggesting that estrogen should play an important role in increasing the adipose mass both in estrogen‐deprived rodents and in postmenopausal women (Disanzo & You 2014).
Therefore, the pathological expansion of adipose tissue is linked to an inappropriate oxygen supply and a decrease in the microvascular density (Gomes‐Gatto et al. 2016) that causes important changes in the functioning of ECM enzymes, mainly those involved in collagen synthesis. Considering this, Elias et al. (2012) found that mice that have an enhanced expression of vascular endothelial growth factor (VEGF) in adipose tissue are protected against hypoxia and obesity, as well as increased glucose tolerance and insulin sensitivity, even when fed on a high‐fat diet. Maybe the increase in the VEGF mRNA expression in visceral adipose tissue, observed in our study, was an attempt to protect tissue against the hypoxia and fibrosis of ECM as the absence of estrogen promoted an increase in the adipocyte area and inhibition of adipogenesis, observed by an increase in CTGF mRNA gene expression accompanied by a decrease in the number of adipocytes. Consequently, the VEGF overexpression seems to play an important role in the control of energy metabolism and adipose tissue function (Elias et al. 2013).
Thus, research on physical activity was conducted with the aim of increasing total energy expenditure during and after doing exercise, controlling the increase in body mass and menopause symptoms (Orsatti et al. 2008; Prestes et al. 2009). Likewise, regular exercise has been shown to regulate proangiogenic pathways by inducing hypoxia‐inducible factor‐1α (HIF‐1α), which induces transcription of VEGF (Sakurai et al. 2010; Zachwieja et al. 2015). In fact, we observed that resistance training upregulated VEGF mRNA, even in the absence of estrogen. Moreover, we observed a decrease in body mass, food intake and a significant decrease in the adipocyte area of fat depots, reinforcing the decrease in lipid content in the adipose tissue depots observed previously by our research group (Domingos et al. 2012).
It is important to emphasize that during the growth of white adipose tissue, the vascular network needs to be continuously remodelled. Adipose tissue expansion can be sustained by neovascularization for adipocyte hyperplasia and remodelling of existing capillaries for adipocyte hypertrophy. In fact, we observed that resistance training stimulates adipocyte (Christiaens & Lijnen 2010) hyperplasia. Surprisingly, even having a lack of estrogen, resistance training promoted remodelling of the ECM structure as most of the animals showed an increase in the number of adipocytes accompanied by a decrease in the adipocyte area. Thus, these results suggest that during ECM remodelling, when the adipocyte area was increased or decreased, a rise in the VEGF and MMP‐2 gene expression occurred, which could explain our results.
Normally, MMPs are expressed in lower levels, but are quickly induced at times of active tissue remodelling. Along these lines, interestingly, resistance training modulates the ECM gene expression and MMP‐2 activity in visceral adipose tissue differently when there is an absence of estrogen. Bauters et al. (2015) used precursor cells with gene silencing, genetic deficiency or overexpression of MMP‐2 and showed the role of MMP‐2 in vitro preadipocyte differentiation and demonstrated in vivo de novo formed fat pads. Furthermore, sex hormones also participated in MMP regulation and played an important role in adipocyte differentiation (Maquoi et al. 2002), supporting our data. Considering this, in postmenopausal women, hormone replacement therapy promoted an increase in MMP‐2 activity, culminating in breaking ECM homeostasis, accelerating the process of vascular pathologies (Berg et al. 2014). In turn, resistance training improved the amount of animal fat mass with the absence of estrogen and also improved the stability of ECM visceral fat. In fact, in our study, a lack of estrogen, as well as resistance training, stimulated the synthesis of MMP‐2 mRNA which should be translocated to the proactive form of the MMP protein (pro‐MMP‐2). The pro‐MMP‐2, now present in the cytoplasm, is exposed to activation, as well as inhibition by TIMP. TIMP‐2 binds preferentially to pro‐MMP2 and is related to its activation (Curry & Osteen 2001, 2003). Thus, we note that TIMP‐2 was upregulated and therefore should have inhibited the activation of MMP‐2 that remained in its non‐active form. Together with the downregulation on the TGF and CTGF mRNA gene expression, which worsened when there was a lack of estrogen, these results show that the ECM stability improved favouring adipocyte hyperplasia. Taking this into consideration, according to Rodrigues et al. (2014), hyperplasia is associated with better metabolic regulation as it allows for better vascularization. Unlike hypertrophy, which results in the accumulation of secondary products of lipid metabolism, it promotes low‐intensity inflammation, insulin resistance and lipolysis (Rodrigues et al. 2014).
Maquoi et al. (2002) reported MMP‐2 mRNA upregulation throughout the preadipocyte differentiation process, with a maximal expression between day 12 and day 19 in mature adipocytes. Concerning MMP‐9 mRNA, the researchers described downregulation on the first day of the differentiation process and a subsequent increase in the mature adipocyte (Maquoi et al. 2002). It is likely that the remodelling of ECM occurred later in Ovx rats compared to intact animals, which showed upregulated MMP‐9 mRNA.
The results obtained are very important because the blockade of TGF‐β signalling protects against obesity, insulin resistance and fatty liver (Buechler et al. 2015). Considering this, Elias et al. (2012) studied transgenic mice with VEGF overexpression, and both brown and white adipose tissues showed that VEGF overexpression does not lead to an increase in adipose mass, supporting our data. Finally, the regulation of MMPs and their corresponding inhibitors suggest important roles of these factors in adipose tissue pathophysiology (Law et al. 2014), and resistance training appears to be important in terms of controlling these factors mainly in the absence of estrogen. Thus, all these important findings show that the resistance training protocol can be used to protect the visceral adipose tissue ECM integrity, as it reverses the effects promoted by the lack of estrogen.
Conclusion
Our study provides evidence that a lack of estrogen changes the gene expression of a variety of ECM proteins culminating in the ECM destabilization and impairing the interaction between ECM proteins and adipocytes. In addition, resistance training on a vertical ladder, surprisingly, modulates the expression of genes related to structural proteins and ECM growth factors, as well as MMP‐2 activity. Therefore, it can be concluded that resistance training may play an important role in maintaining and remodelling ECM, previously altered by a lack of estrogen, promoting an improvement in homeostasis of visceral adipose tissue, which is essential for maintaining metabolically healthy tissue.
Conflict of interests
The authors declare that they have no conflict of interest.
Funding source
This work was supported by a grant from the Brazilian Agency Coordenação de Aperfeiçoamentode Pessoal de Nível Superior‐CAPES (23038.027825/2009‐96) and the Fundação de Amparo à Pesquisa do Estado de São Paulo‐FAPESP (2011/50713‐8).
Acknowledgements
We would like to thank Prof. Sérgio Eduardo de Andrade Perez (PhD) who provided us with the laboratory to perform the physical training of rats.
References
- Arslan N., Erdur B. & Aydin A. (2010) Hormones and cytokines in childhood obesity. Indian Pediatr. 47, 829–839. [DOI] [PubMed] [Google Scholar]
- Bauters D., Scroyen I., Van Hul M., Lijnen H.R. (2015) Gelatinase A (MMP‐2) promotes murine adipogenesis. Biochem. Biophys. Acta. 1850, 1449–1456. [DOI] [PubMed] [Google Scholar]
- Berg G., Schreier L. & Miksztowicz V. (2014) Circulating and adipose tissue matrix metalloproteinases in cardiometabolic risk environments: pathophysiological aspects. Horm. Mol. Biol. Clin. Investig. 17, 79–87. [DOI] [PubMed] [Google Scholar]
- Bouloumié A., Sengenès C., Portolan G., Galitzky J., Lafontan M. (2001) Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes 50, 2080–2086. [DOI] [PubMed] [Google Scholar]
- Buechler C., Krautbauer S. & Eisinger K. (2015) Adipose tissue fibrosis. World J. Diabetes. 6, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavey C. et al (2003) Matrix Metalloproteinases Are Differentially Expressed in Adipose Tissue during Obesity and Modulate Adipocyte Differentiation. J. Biol. Chem. 278, 11888–11896. [DOI] [PubMed] [Google Scholar]
- Choe S.S., Huh J.Y., Hwang I.J., Kim J.I., Kim J.B. (2016) Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 7, p. 30 Available at: http://journal.frontiersin.org/Article/10.3389/fendo.2016.00030/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens V. & Lijnen H.R. (2006) Role of the fibrinolytic and matrix metalloproteinase systems in development of adipose tissue. Arch. Physiol. Biochem. 112, 254–259. [DOI] [PubMed] [Google Scholar]
- Christiaens V. & Lijnen H.R. (2010) Angiogenesis and development of adipose tissue. Mol. Cell. Endocrinol. 318, 2–9. [DOI] [PubMed] [Google Scholar]
- Cinti S. et al (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355. [DOI] [PubMed] [Google Scholar]
- Clegg D.J. (2012) Minireview: the year in review of estrogen regulation of metabolism. Mol. Endocrinol. 26, 1957–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry T.E. & Osteen K.G. (2001) Cyclic changes in the matrix metalloproteinase system in the ovary and uterus. Biol. Reprod. 64, 1285–1296. [DOI] [PubMed] [Google Scholar]
- Curry T.E. & Osteen K.G. (2003) The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr. Rev. 24, 428–465. [DOI] [PubMed] [Google Scholar]
- Disanzo B.L. & You T. (2014) Effects of exercise training on indicators of adipose tissue angiogenesis and hypoxia in obese rats. Metabolism 63, 452–455. [DOI] [PubMed] [Google Scholar]
- Divoux A. & Clément K. (2011) Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes. Rev. 12, e494–e503. [DOI] [PubMed] [Google Scholar]
- Domingos M.M. et al (2012) Resistance training restores the gene expression of molecules related to fat oxidation and lipogenesis in the liver of ovariectomized rats. Eur. J. Appl. Physiol. 112, 1437–1444. [DOI] [PubMed] [Google Scholar]
- Duarte F.O. et al (2008) Caloric restriction and refeeding promoted different metabolic effects in fat depots and impaired dyslipidemic profile in rats. Nutrition 24, 177–186. [DOI] [PubMed] [Google Scholar]
- Elias I. et al (2012) Adipose tissue overexpression of vascular endothelial growth factor protects against diet‐induced obesity and insulin resistance. Diabetes 61, 1801–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias I., Franckhauser S. & Bosch F. (2013) New insights into adipose tissue VEGF‐A actions in the control of obesity and insulin resistance. Adipocyte. 2, 109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Valle Gomes‐Gatto C., Duarte F.O., Stotzer U.S., Rodrigues M.F.C., de Andrade Perez S.E., de Selistre‐Araujo H.S. (2016) Estrogen deficiency in ovariectomized rats: can resistance training re‐establish angiogenesis in visceral adipose tissue? Clinics (Sao Paulo). 71, 528–536.Available at: http://www.clinics.org.br/article.php?id=1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M.F. & Hotamisligil G.S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445. [DOI] [PubMed] [Google Scholar]
- Hirsch J. & Gallian E. (1968) Methods for the determination of adipose cell size in man and animals. J. Lipid Res. 9, 110–119. [PubMed] [Google Scholar]
- Hornberger T.A. & Farrar R.P. (2004) Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. 29, 16–31. [DOI] [PubMed] [Google Scholar]
- Jiang J.M.Y., Sacco S.M., Ward W.E. (2008) Ovariectomy‐induced hyperphagia does not modulate bone mineral density or bone strength in rats. J. Nutr. 138, 2106–2110. [DOI] [PubMed] [Google Scholar]
- Kalu D.N. (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 15, 175–191. [DOI] [PubMed] [Google Scholar]
- Law J., Bloor I., Budge H., Symonds M.E. (2014) The influence of sex steroids on adipose tissue growth and function. Horm. Mol. Biol. Clin. Investig. 19, 13–24. [DOI] [PubMed] [Google Scholar]
- Leduc V., Legault V., Dea D., Poirier J. (2011) Normalization of gene expression using SYBR green qPCR: a case for paraoxonase 1 and 2 in Alzheimer's disease brains. J. Neurosci. Methods 200, 14–19. [DOI] [PubMed] [Google Scholar]
- Leite R.D. et al (2009) Effects of ovariectomy and resistance training on lipid content in skeletal muscle, liver, and heart; fat depots; and lipid profile. Appl. Physiol. Nutr. Metab. 34, 1079–1086. [DOI] [PubMed] [Google Scholar]
- Livak K.J. & Schmittgen T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lizcano F. & Guzmán G. (2014) Estrogen Deficiency and the Origin of Obesity during Menopause. Biomed. Res. Int. 2014, 757461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Takai K., Weaver V.M., Werb Z. (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 3(12), p.a005058. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21917992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquoi E., Munaut C., Colige A., Collen D., Lijnen H.R. (2002) Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes 51, 1093–1101. [DOI] [PubMed] [Google Scholar]
- Mariman E.C.M. & Wang P. (2010) Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell. Mol. Life Sci. 67, 1277–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marqueti R.C., Micocci K.C., Leite R.D., de Selistre‐Araujo H.S. (2012) Nandrolone inhibits MMP‐2 in the left ventricle of rats. Int. J. Sports Med. 33, 181–185. [DOI] [PubMed] [Google Scholar]
- Martinez‐Santibanez G., Singer K., Cho K.W., DelProposto J.L., Mergian T., Lumeng C.N. (2015) Obesity‐induced remodeling of the adipose tissue elastin network is independent of the metalloelastase MMP‐12. Adipocyte. 4, 264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesch V.R. et al (2006) Metabolic syndrome throughout the menopausal transition: Influence of age and menopausal status. Climacteric. 9, 40–48. [DOI] [PubMed] [Google Scholar]
- Miksztowicz V., Siseles N., Machulsky N.F., Schreier L., Berg G. (2012) Increase in MMP‐2 activity in overweight and obese women is associated with menopausal status. Climacteric. 15, 602–606. [DOI] [PubMed] [Google Scholar]
- Miksztowicz V., Morales C., Zago V., Friedman S., Schreier L., Berg G. (2014) Effect of insulin‐resistance on circulating and adipose tissue MMP‐2 and MMP‐9 activity in rats fed a sucrose‐rich diet. Nutr. Metab. Cardiovasc. Dis. 24, 294–300. [DOI] [PubMed] [Google Scholar]
- Murphy G. (2011) Tissue inhibitors of metalloproteinases. Genome Biol. 12, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council . (1996) Guide for the Care and Use of Laboratory Animals J. Chem. Educ.. Washington, D.C.; National Academy Press, 140 pp. [Google Scholar]
- Orsatti F.L., Nahas E.A.P., Maesta N., Nahas‐Neto J., Burini R.C. (2008) Plasma hormones, muscle mass and strength in resistance‐trained postmenopausal women. Maturitas. 59, 394–404. [DOI] [PubMed] [Google Scholar]
- Pallottini V., Bulzomi P., Galluzzo P., Martini C., Marino M. (2008) Estrogen regulation of adipose tissue functions: involvement of estrogen receptor isoforms. Infect. Disord. Drug Targets 8, 52–60. [DOI] [PubMed] [Google Scholar]
- Pascual‐Serrano A. et al (2017) Grape seed proanthocyanidin supplementation reduces adipocyte size and increases adipocyte number in obese rats. Int. J. Obes. (Lond). 1–10. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28373675 [Accessed May 16, 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R. & Vicennati V. (2008) Steroids and the metabolic syndrome. J. Steroid Biochem. Mol. Biol. 109, 258–265. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W., Horgan G.W. & Dempfle L. (2002) Relative expression software tool (REST) for group‐wise comparison and statistical analysis of relative expression results in real‐time PCR. Nucleic Acids Res. 30, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pighon A. et al (2009) Resistance training attenuates fat mass regain after weight loss in ovariectomized rats. Maturitas. 64, 52–57. [DOI] [PubMed] [Google Scholar]
- Prestes J. et al (2009) Effects of ovariectomy and resistance training on MMP‐2 activity in skeletal muscle. Appl. Physiol. Nutr. Metab. 34, 700–706. [DOI] [PubMed] [Google Scholar]
- Riant E., Waget A., Cogo H., Arnal J.‐F., Burcelin R., Gourdy P. (2009) Estrogens protect against high‐fat diet‐induced insulin resistance and glucose intolerance in mice. Endocrinology 150, 2109–2117. [DOI] [PubMed] [Google Scholar]
- Rodrigues T., Matafome P. & Seiça R. (2014) A vascular piece in the puzzle of adipose tissue dysfunction: mechanisms and consequences. Arch. Physiol. Biochem. 120, 1–11. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.F.C. et al (2017) Effects of resistance training and estrogen replacement on adipose tissue inflammation in ovariectomized rats. Appl. Physiol. Nutr. Metab. 68(9), pp. 1247–1254. p.apnm‐2016‐0443. Available at: http://www.ncbi.nlm.nih.gov/pubmed/28177709 [Accessed March 23, 2017]. [DOI] [PubMed] [Google Scholar]
- Rogers N.H., Perfield J.W., Strissel K.J., Obin M.S., Greenberg A.S. (2009) Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy‐induced obesity. Endocrinology 150, 2161–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. et al (2010) Effects of exercise training on adipogenesis of stromal‐vascular fraction cells in rat epididymal white adipose tissue. Acta Physiol. 200, 325–338. [DOI] [PubMed] [Google Scholar]
- Salis O., Bedir A., Gulten S., Okuyucu A., Kulcu C., Alacam H. (2014) Cytotoxic effect of fluvastatin on MCF‐7 cells possibly through a reduction of the mRNA expression levels of SGK1 and CAV1. Cancer Biother. Radiopharm. 29, 368–375. [DOI] [PubMed] [Google Scholar]
- Sene‐Fiorese M. et al (2008) Efficiency of intermittent exercise on adiposity and fatty liver in rats fed with high‐fat diet. Obesity (Silver Spring). 16, 2217–2222. [DOI] [PubMed] [Google Scholar]
- Song Y.H. et al (2016) Adipose‐derived stem cells increase angiogenesis through matrix metalloproteinase‐dependent collagen remodeling. Integr Biol (Camb)., 8, 205–215. Available at: https://doi.org/xlink.rsc.org/?doi=c5ib00277j [Accessed June 3, 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speretta G.F.F. et al (2012) The effects of exercise modalities on adiposity in obese rats. Clinics (Sao Paulo). 67, 1469–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotzer U.S. et al (2015) Resistance Training Suppresses Intra‐abdominal Fatty Acid Synthesis in Ovariectomized Rats. Int. J. Sports Med. 36, 226–233. [DOI] [PubMed] [Google Scholar]
- Stubbins R.E., Najjar K., Holcomb V.B., Hong J., Núñez N.P. (2012) Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab. 14, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun K., Tordjman J., Clément K., Scherer P.E. (2013) Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney G. et al (2016) Adipose Tissue Remodeling: its Role in energy Metabolism and Metabolic Disorders. Front. Endocrinol 7, 303389–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafsiri E., Darbouy M., Shadmehr M.B., Zagryazhskaya A., Alizadeh J., Karimipoor M. (2015) Expression of miRNAs in non‐small‐cell lung carcinomas and their association with clinicopathological features. Tumour Biol. 36, 1603–1612. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. (2013) Hypoxia and adipose tissue function and dysfunction in obesity. Physiol. Rev. 93, 1–21. [DOI] [PubMed] [Google Scholar]
- Zachwieja N.J. et al (2015) Loss of Adipocyte VEGF Impairs Endurance Exercise Capacity in Mice. Med. Sci. Sports Exerc. 47, 2329–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.M., Manson J.E., Rexrode K.M., Cook N.R., Buring J.E., Lee I.M. (2007) Use of oral conjugated estrogen alone and risk of breast cancer. Am. J. Epidemiol. 165, 524–529. [DOI] [PubMed] [Google Scholar]


