Abstract
Cell therapy is a promising approach for cardiac repair. The aim of the present study was to determine the feasibility of using biotinylated insulin-like growth factor 1 (IGF-1) with biotinylated self-assembling peptides (tethered IGF-1) combined with bone marrow stem cells (BMSCs) transplantation for the treatment of heart failure. Tethered IGF-1 was synthesized and its effect on H9c2 cells was analyzed. Reverse transcription-quantitative polymerase chain reaction and western blot assays demonstrated that tethered IGF-1 did not significantly affect the expression and phosphorylation of AKT, whereas it significantly increased the expression of cardiac troponin T (P<0.01). A rabbit myocardial infarction model was constructed and rabbits were divided into four groups: Control group (no treatment), group 1 (G1; BMSC transplantation), group 2 (G2; BMSCs + non-biotinylated IGF-1) and group 3 (G3; BMSCs + tethered IGF-1). At 4 weeks after modeling, cardiac tissues were obtained for analysis. In the control group, myocardial fibers were disordered, a large number of inflammatory cells infiltrated the cardiac tissues, and apoptosis occurred in ~50% of cells. However, in G1, G2 and G3, muscle cells were well ordered, and a lesser degree of myocardial degeneration and inflammatory cell infiltration was observed. Compared with the control group, the apoptosis rates of myocardial cells in G1-G3 were significantly decreased (P<0.01). Furthermore, compared with G1 and G2, tissue morphology was improved in G3and the number of apoptotic myocardial cells was significantly decreased (P<0.01). These results suggest that treatment with tethered IGF-1 + BMSCs significantly suppresses cell apoptosis and induces the expression of cardiac maturation proteins. These findings provide a novel insight into how the delivery of tethered IGF-1 with BMSCs could potentially enhance the prognosis of patients with heart failure treatment.
Keywords: self-assembling peptides, insulin-like growth factor 1, bone marrow stem cell, cardiac failure
Introduction
Every year, millions of people suffer from myocardial infarction (MI) (1). MI leads to a deficiency of myocardial cell regeneration, and the loss of myocardial function and myocardial cells, which ultimately leads to heart failure (2). Bone marrow stem cell (BMSC) transplantation has become a promising technique in the treatment of cardiac failure, due to the potential of BMSCs to promote myocardial regeneration and differentiation (3). A previous study using a rabbit MI model revealed that BMSC transplantation significantly reduced the infarct size by recovering the number of myocardial cells (4). However, experimental and clinical studies have reported that low levels of bone marrow-derived cardiomyocytes were present outside the infarction area (5), which was not sufficient for myocardial repair (6). Thus, the improvement of BMSC differentiation ability is of great importance for improving the efficacy of this treatment.
Insulin-like growth factor 1 (IGF-1) is an important regulator of vessel homeostasis and glucose and lipid metabolism (7). The transcription of IGF-1 directed by the P1 promoter of MI patients is reported to be lower than that of healthy individuals (8). IGF-1 deficiency is associated with myocardial disease mortality (9), and it has been demonstrated that growth factors such as IGF-1 promote the protective potential of transplanted BMSCs (10). However, IGF-1 is a small protein that easily diffuses through tissues, which allows it to transfer signals over large distances and restricts its retention within a tissue for prolonged periods (11). Self-assembling peptides are oligopeptides composed of alternating hydrophilic and hydrophobic amino acids (12). These short peptides, typically 8–16 amino acids long, are in solution at low pH and osmolarity but rapidly form fibers of 5–10 nm and assemble into a 3-dimensional scaffolds at physiological pH and osmolarity (12). They support attachment, growth and differentiation of a number of types of mammalian primary cells (13). A combination of IGF-1 with botinylated self-assembling peptides has been reported to improve the specificity and efficiency of IGF-1 delivery (14).
In the present study, a rabbit model of MI was established and a composition comprising biotinylated IGF-1 with biotinylated self-assembling peptides (tethered IGF-1) was synthesized. H9c2cells were incubated with tethered IGF-1 or non-biotinylated IGF-1 to analyze the biological effect of modified IGF-1 on cells. In vivo, MI model rabbits were injected with BMSCs, BMSCs + non-biotinylated IGF-1, or BMSCs + tethered IGF-1. The findings of the present study suggest that the delivery of tethered IGF-1 combined with BMSCs promotes cell therapy in an MI model.
Materials and methods
Experimental animals
A total of 28 healthy male New Zealand rabbits, aged 4–5 months, weighing 2.5–3.5 kg, were supplied by the Experimental Animal Center of Nanchang University (Nanchang, China). The animals were housed under standard laboratory conditions (23±2°C temperature, 47% humidity and a 12-h light/dark cycle) with standard rodent feed and water available ad libitum. The present study was approved by the Animal Research Ethics Board of Nanchang University.
Reagents
Monoclonal antibody against β-myosin heavy chain (β-MHC; catalogue no. ab55152) was from Abcam (Cambridge, MA, USA). Monoclonal antibody anti-cardiac troponin T (cTnT; catalogue no. LI4C00702) was purchased from Shanghai Linc-Bio Science Co. Ltd. (Shanghai, China; http://lzhenyul.biogo.net/sell/typeid-39.shtml), Polyclonal antibody anti-connexin-43 (CX-43; catalogue no. RLT1044) was purchased from Ruiying Biological (Suzhou, Jiangsu, China; http://www.rlgene.com/). Polyclonal antibody anti-N-cadherin (N-cad; catalogue no. YT745) were purchased from Baiaolaibo Company (Beijing, China; http://www.bjbalb.com/). Anti-AKT (catalogue no. 4691) and anti-p-AKT (catalogue no. 13038) antibodies for western blot analysis were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). GAPDH monoclonal antibodies (catalogue no. AG019) and bicinchoninic acid assay (BCA) kits (catalogue no. P0011) were obtained from Beyotime Institute of Biotechnology (Haimen, China). Ultrapure RNA kit (catalogue no. CW0581S) and SuperRT cDNA Synthesis kit (catalogue no. CW0741S) were purchased from CW Biotech (Beijing, China). Radioimmunoprecipitation assay buffer (RIPA, catalogue no. R0010) was purchased from Solarbio Life Science (Beijing, China).
Preparation of tethered IGF-1
RAD16-II (AcN-RARADA DARARADADA-CNH2; Genscript, Piscataway, NJ, USA) was molecularly attached to biotin by two Nε-fluorenylmethoxycarbonyl-ε-aminocaproic acid groups as previously described (14). Biotinylated RAD16-II peptides were dissolved in sterile sucrose (295 mM) at 1% (wt/vol) and sonicated for 10 min. The biotin sandwich method of tethering biotinylated IGF-1 to biotinylated self-assembling peptides was used as previously described.
Isolation and culture of BMSCs
Rabbits were anesthetized with 3% sodium pentobarbital (Sigma-Aldrich; Merck KGaA) at a dose of 30 mg/kg via the ear vein. A total of 5 ml bone marrow was aspirated at the tibial plateau of the knee using a 12-mlsyringe, then mixed with 0.9% NaCl at a ratio of 1:1. The mixture was added to 5 ml lymphocyte separation solution (Litton Bionetics, Frederick, MD, USA) and gently agitated. Following centrifugation at 1,000 × g for 10 min at 4°C, the cloudy layer (lymphocytes) was harvested and washed with serum-free Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich; Merck KGaA) twice. Cells were subsequently seeded into a 50-cm2 flask at a density of 1×106 cells/ml in DMEM with 15% fetal bovine serum (FBS; Sigma-Aldrich; Merck KGaA) in a humidified environment containing 5% CO2 at 37°C for 24 h. BMSCs were expanded to passage 2 for the following experiments. Prior to further experiments, cells were stained with 0.4% trypan blue dye (catalogue no. T8070; Solarbio Life Science) to analyze the cell viability as previously described (15). BMSCs were incubated with anti-rabbit CD90 (catalogue no. SC-53456, 1:100 dilution), CD34 (catalogue no. SC-51540, 1:100 dilution) and CD45 (catalogue no. SC-70686, 1:100 dilution) antibodies (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 30 min, and the immunophenotype of BMSCs was analyzed with flow cytometry according a previous study (16). The results of this analysis revealed that the BMSCs expressed CD90 (>95%) and did not significantly express CD34 (<5%) or CD45 (<5%).
Establishment of MI animal model and grouping
Rabbits were anesthetized with 3% sodium pentobarbital at 30 mg/kg via the ear vein and fixed on a table. An incision was made at the left second intercostal space, which was then opened. The pericardium was opened, the heart was exposed and the middle of the left anterior descending coronary artery was ligated. An electrocardiogram was performed to assess whether the model had been established; an ST segment elevation of >0.2 mV for >30 min in lead II and III was considered to indicate a successful MI model. A total of 28 MI rabbits were randomly assigned to four groups (each n=7): Control group (no treatment), group 1 (G1; BMSC transplantation), group 2 (G2; transplantation + non-biotinylated IGF-1) and group 3 (G3; BMSC transplantation + tethered IGF-1). In the control group, 40 µl DMEM was injected into the free wall of the left ventricle for 4 points of implantation around the infarcted border zone with microsyringe. For groups G1, G2 and G3, 40 µl BMSCs (5×109 cells/ml), 40 µl BMSCs + 16 ng non-biotinylated IGF-1, or 40 µl BMSCs + 16 ng tethered IGF-1 were administered in the same manner, respectively. Post-operatively, all rabbits were prophylactically intramuscularly treated with dose of 200 000 UI/kg of penicillin. After 4 weeks, the rabbits were sacrificed and heart tissues were isolated immediately for further experiments.
Western blot analysis
H9c2 cells were purchased from American Type Culture Collection (Manassas, VA, USA) and cultured with DMEM containing 10% FBS in a humidified environment containing 5% CO2 at 37°C. H9c2 cells were treated with tethered IGF-1 or non-biotinylated IGF-1 for 48 h. H9c2 cells or cardiac tissues were lysed with RIPA buffer or 30 min at 4°C and subsequently centrifuged at 12,000 g for 10 min at 4°C. The supernatant liquid was collected and the quantity of proteins was detected using the BCA kit. Equal amounts of protein (~30 µg) were separated by 10% SDS-PAGE for 30–60 min, and blotted onto a nitrocellulose membrane. The membrane was blocked in 5% non-fat milk for 1 h at room temperature, and incubated overnight at 4°C with primary antibodies against cTnT, β-MHC, CX-43 or N-cad (1:100). The blot was washed three times with TBST (20 mM Tris, 140 mM NaCl, 0.2% Tween-20; pH 7.5), and then probed with the horseradish peroxidase (HRP) labeled IgG secondary antibody (catalogue no. A0208 and A0216; 1:10,000 dilution; Beyotime Institute of Biotechnology) at 37°C for 2 h. The membrane was washed with TBST three times. An enhanced chemiluminescence substrate (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to detect gene expression. The band intensities were determined using the Quantity One software version 4.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with three independent repeats.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from H9c2 cells or cardiac tissues using the Ultrapure RNA kit according to the manufacturer's protocol. Total RNA (~5 µg) was reverse transcribed into cDNA for PCR using SuperRT cDNA Synthesis kit according to the manufacturer's protocol. The PCR was performed using the UltraSYBR Mixture (cat. no. CW2602M; CWBio, Beijing, China) containing SYBR Green I stain. The primers for PCR are listed in Table I. The temperature protocol for PCR was as follows: 95°C for 5 min, 95°C for 5 sec, 60°C for 45 sec and 72°C 45 sec, repeated for 40 cycles. The relative expression of the target genes were normalized to GAPDH by using the 2−∆∆Cq method (17). Results were from three independent replicates.
Table I.
Primers used for reverse transcription-quantitative polymerase chain reaction.
| Primer | Sequence (5′→3′) |
|---|---|
| Akt | F: AGCGACGTGGCTATTGTGAAG |
| R: GCCATCATTCTTGAGGAGGAAGT | |
| cTnT | F: AGAGGACTCCAAACCCAAGC |
| R: ATTGCGAATACGCTGCTGTT | |
| β-MHC | F: TGTGCTACCCAACCCTAAGG |
| R: TGTTTCTGCCTAAGGTGCTGT | |
| N-cad | F: AGGTGGAGGAGAAGAAGACCAGGACT |
| R: GTCAGCAGCTTTAAGGCCCTCATT | |
| CX-43 | F: CTATGTGATGCGAAAGGA |
| R: AGGAAACAGTCCACCTGA | |
| GAPDH | F: AGCCACATCGCTCAGACA |
| R: TGGACTCCACGACGTACT |
F, forward; R, reverse; cTnT, cardiac troponin; β-myosin heavy chain; N-cad, N-cadherin; CX-43, connexin-43.
H&E staining and TUNEL assay
The heart tissues of MI rabbits were extracted, fixed with fresh cold 4%paraformaldehyde at 4°C for 15 min, embedded in paraffin, and cut into 5-µm sections. Tissue samples were transferred onto glass slides, deparaffinized and stained with H&E. Finally, the stained sections were observed using a light microscope. Each section was evaluated by three investigators blinded to the experimental information.
A TUNEL assay was employed to visualize apoptotic cells according to the manufacturer's protocol. Briefly, sections were deparaffinized, digested with proteinase K and permeabilized with 0.5% Triton X-100. The sections were subsequently incubated with 50 µl TUNEL reaction mixture for 60 min at room temperature. Following washing with PBS, the nucleus was stained with DAPI. The slides were mounted with Neutral gum and the positively-stained cells were marked in green color detected using fluorescent microscopy (Leica Microsystems GmbH, Wetzlar, Germany). The apoptotic index was performed on at least five non-overlapping random fields.
Statistical analysis
Statistical analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). All analytical data were expressed in means + standard deviation. Comparisons between two groups were made using a Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Tethering of IGF-1 prolonged the biological effect of IGF-1
AKT (also known as protein kinase B) is a downstream target of IGF signaling (18). In order to examine whether tetheringIGF-1 interfered with the bioactivity of IGF-1, H9c2 cells were treated with tethered IGF-1 or non-biotinylated IGF-1 for 48 h, and the expression of AKT was analyzed. No significant differences in Akt mRNA levels (Fig. 1A) or AKT phosphorylation (Fig. 1B and C) were identified between tethered IGF-1andnon-biotinylatedIGF-1. These results suggest that tethered IGF-1doesnot affect the bioactivity of IGF-1.
Figure 1.
Tethered IGF-1 prolonged the biological effect of IGF-1.H9c2 cells were incubated with tethered IGF-1 or non-biotinylated IGF-1, respectively. (A) RT-qPCR results demonstrated that tethering IGF-1 did not affect Akt expression. (B) Western blotting revealed that AKT phosphorylation was not affected by the tethering of IGF-1. (C) Quantified results of western blotting. (D) RT-qPCR results demonstrated that the cTnT expression was increased following incubation with tethered IGF-1 compared with non-biotinylated IGF-1 for 14 days. (E) Western blotting revealed that cTnT protein was upregulated by tethered IGF-1 compared with non-biotinylated IGF-1. GAPDH was used as the internal control. (F) Quantified results of western blotting. **P<0.01 vs. tethered IGF-1. IGF-1, insulin-like growth factor 1; tethered IGF-1, biotinylated IGF-1 with biotinylated self-assembling peptides; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; cTnT, cardiac troponin T; p, phosphorylated.
To explore the effect of tethered IGF-1 on IGF-1 delivery, H9c2 cells were incubated with non-biotinylated IGF-1 or tethered IGF-1 for 14 days, and the expression of cTnT, a marker of cardiac maturation (19), was assessed. Compared with non-biotinylated IGF-1, treatment with tethered IGF-1 significantly increased the expression of cTnT at the mRNA (Fig. 1D; P<0.01) and protein (Fig. 1E and F; P<0.01) levels. These data suggest that tethered IGF-1 prolongsIGF-1 delivery and increases cardiac maturation and protein synthesis.
Tethered IGF-1 combined with BMSCs suppressed cardiomyocytes apoptosis in vivo
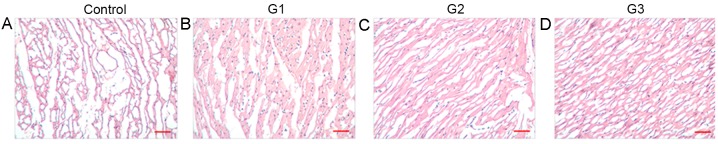
To determine the influence of tethered IGF-1 on MI in vivo, tethered IGF-1 combined with BMSCs was injected into rabbits. After 4 weeks, heart tissues were extracted and stained with H&E. In the control group, myocardial fibers were irregularly arranged, and a large number of inflammatory cells were observed to have infiltrated the tissues (Fig. 2A). However, in G1, G2 and G3, muscle cells were orderly, and the degree of myocardial degeneration and inflammatory cell infiltration was decreased (Fig. 2B-D). In addition, compared with G1 and G2, the pathological morphology observed in G3 tissues was markedly ameliorated.
Figure 2.
Tethered IGF-1 with BMSCs ameliorated the pathological morphology of rabbit MI. Rabbit MI models were constructed and injected with various BMSC treatments: (A) Control; (B) G1; (C) G2; and (D) G3. Heart tissues were extracted after 4 weeks and subjected to hematoxylin and eosin staining. Among the MI groups, tissues from G3 rabbits were observed to have the most improved morphology. Scale bar, 100 µm. IGF-1, insulin-like growth factor 1; tethered IGF-1, biotinylated IGF-1 with biotinylated self-assembling peptides; BMSCs, bone marrow stem cells; MI, myocardial infarction; G1, group 1 (BMSC transplantation); G2, group 2 (BMSCs + non-biotinylated IGF-1); G3, group 3 (BMSCs + tethered IGF-1).
A TUNEL assay was performed on the extracted heart tissues of rabbits from each group. In the control group, apoptosis occurred in ~50% of the cells (Fig. 3Aa), and the number of apoptotic myocardial cells in groups G1-G3 (Fig. 3Ab-Ad) was revealed to be significantly lower (Fig. 3B; P<0.01). Furthermore, the TUNEL assay revealed that the apoptotic rate of myocardial cells was lowest in group G3 (Fig. 3B; P<0.01). These results suggest that tethered IGF-1 combined with BMSCs suppresses the deterioration of myocardial tissues and myocardial cell apoptosis in a rabbit model of MI.
Figure 3.
Tethered IGF-1 with BMSCs suppressed cardiomyocyte apoptosis in vivo. Rabbit MI models were injected with different BMSC treatments and after 4 weeks the heart tissues were isolated. (A) TUNEL staining in the (a) control, (b) G1, (c) G2 and (d) G3 groups indicated that tethered IGF-1 + BMSCs inhibited cell apoptosis. Scale bar, 100 µm. (B) Quantified AI. **P<0.01 vs. control group and ##P<0.01 vs. G1 and G2. IGF-1, insulin-like growth factor 1; tethered IGF-1, biotinylated IGF-1 with biotinylated self-assembling peptides; BMSCs, bone marrow stem cells; MI, myocardial infarction; AI, apoptosis index; G1, group 1 (BMSC transplantation); G2, group 2 (BMSCs + non-biotinylated IGF-1); G3, group 3 (BMSCs + tethered IGF-1).
Tethered IGF-1 with BMSCs improved the expression of specific myocardial maturation proteins
N-cad, β-MHC and CX-43 proteins are cardiac muscle specific markers, and they serve important roles in cardiomyocytes (20). To address the mechanism of tethered IGF-1 functioning, rabbits were injected with BMSCs alone or in combination with tethered or free IGF-1 and the expression levels of N-cad, β-MHC and CX-43 were analyzed using RT-qPCR and western blot analysis. The expression levels of N-cad, β-MHC and CX-43 at them RNA (Fig. 4A) and protein (Fig. 4B and C) levels were significantly increased in the G1, G2 and G3 groups compared with the control group (P<0.01). Furthermore, the levels of N-cad, β-MHC and CX-43 were significantly higher in G3 compared with G1 and G2 (P<0.01). These data suggest that tethered IGF-1 combined with BMSC transplantation increases the expression of cardiac muscle-specific markers.
Figure 4.

Tethered IGF-1 with BMSCs improved the expression of specific myocardial maturation markers. Total RNA and proteins were extracted from heart tissues. (A) Reverse transcription-quantitative polymerase chain reaction results revealed that mRNA levels of N-cad, β-MHC and CX-43 were increased by tethered IGF-1. GAPDH was used as an internal control. (B) Western blotting demonstrated that N-cad, β-MHC and CX-43 protein expression was increased by tethered IGF-1 treatment. (C) Quantified western blotting results. **P<0.01 vs. control group and ##P<0.01 vs. G1 and G2. IGF-1, insulin-like growth factor 1; tethered IGF-1, biotinylated IGF-1 with biotinylated self-assembling peptides; BMSCs, bone marrow stem cells; N-cad, N-cadherin; β-MHC, β-myosin heavy chain; CX-43, connexin-43.
Discussion
IGF-1 exerts potent proliferative, angiogenic and anti-apoptotic effects in target tissues via different signaling pathways (21), such as the Ras-Raf-mitogen active protein kinase, phosphoinositide 3-kinase/AKT pathway (22). IGF-1 is a potent cardiomyocyte growth and differentiation factor (23). The overexpression of IGF-1 induces cell proliferation and growth, and a deficiency of IGF-1 in mice leads to cell apoptosis following MI (24). To improve the efficiency of IGF-1 delivery, a composition comprising biotinylated IGF-1with self-assembling peptides was synthesized according to a previously described method (14) and the levels of AKT were analyzed. In the present study, it was demonstrated that transfection with tethered IGF-1 did not affect the mRNA level or phosphorylation of AKT, which indicated that modified IGF-1 does not affect AKT bioactivity. Following 14 days of transfection with tethered IGF-1, the expression of cTnT was significantly increased compared with that in cells incubated with non-biotinylated IGF-1, suggesting that tethered IGF-1 prolongs the delivery of IGF-1 and promotes cardiomyocyte cell maturation. These findings indicate that this may be a promising approach for the delivery of different factors to tissues for prolonged periods.
Stem cells represent a promising approach for the treatment of heart failure (25). BMSCs are an attractive source of cells for heart disease cell therapy due to their ability to differentiate into cardiomyocytes and vascular endothelial cells (26). It has been reported that BMSCs are able to generate myocardium and improve the outcome of MI (27). However, it has also been reported that only a small fraction of BMSCs are involved in generating cardiac tissues following the injection of a large number of BMSCs (28). It is therefore critical to ensure that BMSCs differentiate into the desired tissues. Growth factors are able to initiate signal transduction to regulate cell differentiation and induce stem cells to mobilize to regions of tissue damage (29). Differentiation of BMSCs into cardiomyocytes maybe induced by treating them with IGF-1, or basic fibroblast growth factor (30). In the present study, pretreatment of BMSCs with IGF-1 was demonstrated to improve myocardial morphology, suppress cell apoptosis and increase the expression of N-cad, β-MHC and CX-43, similar to the results of Zhang et al (10). It was also revealed that this effect was most evident when tethered IGF-1 was combined with BMSCs, providing new evidence to suggest that tethered IGF-1 combined with BMSC transplantation is a promising approach for cell therapy in MI.
In summary, the transfection of tethered IGF-1 allows IGF-1 to be released slowly, and prolongs the action time of IGF-1 in vitro. In vivo, tethered IGF-1 combined with BMSCs is able to suppress cell apoptosis and promote the expression of cardiomyocyte specific proteins, which may improve the repair and regeneration of damaged cardiac tissues. The results of the present study indicate that IGF-1 combined with BMSCs represents a promising cell therapy for the treatment of MI.
Acknowledgements
The present study was supported by the Natural Science Youth Fund of Jiangxi Province (grant no. 20132BAB215039).
Glossary
Abbreviations
- IGF-1
insulin-like growth factor 1
- tethered IGF-1
biotinylated IGF-1 with biotinylated self-assembling peptides
- BMSC
bone marrow stem cell
- MI
myocardial infarction
- H&E
hematoxylin and eosin
- β-MHC
β-myosin heavy chain
- cTnT
cardiac troponin T
- CX-43
connexin-43
- N-cad
N-cadherin
References
- 1.Tao L, Bei Y, Zhang H, Xiao J, Li X. Exercise for the heart: Signaling pathways. Oncotarget. 2015;6:20773–20784. doi: 10.18632/oncotarget.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le HH, El-Khatib C, Mombled M, Guitarian F, Al-Gobari M, Fall M, Janiaud P, Marchant I, Cucherat M, Bejan-Angoulvant T, Gueyffier F. Impact of aldosterone antagonists on sudden cardiac death prevention in heart failure and post-myocardial infarction patients: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2016;11:e0145958. doi: 10.1371/journal.pone.0145958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 4.Ji LL, Long XF, Tian H, Liu YF. Effect of transplantation of bone marrow stem cells on myocardial infarction size in a rabbit model. World J Emerg Med. 2013;4:304–310. doi: 10.5847/wjem.j.issn.1920-8642.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 6.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 7.Dai Z, Wu F, Yeung EW, Li Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Horm IGF Res. 2010;20:275–281. doi: 10.1016/j.ghir.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Palmen M, Daemen MJ, Bronsaer R, Dassen WR, Zandbergen HR, Kockx M, Smits JF, van der Zee R, Doevendans PA. Cardiac remodeling after myocardial infarction is impaired in IGF-1 deficient mice. Cardiovasc Res. 2001;50:516–524. doi: 10.1016/S0008-6363(01)00237-1. [DOI] [PubMed] [Google Scholar]
- 9.Koudstaal S, Bastings MM, Feyen DA, Waring CD, van Slochteren FJ, Dankers PY, Torella D, Sluijter JP, Nadal-Ginard B, Doevendans PA, et al. Sustained delivery of insulin-like growth factor-1/hepatocyte growth factor stimulates endogenous cardiac repair in the chronic infarcted pig heart. J Cardiovasc Transl Res. 2014;7:232–241. doi: 10.1007/s12265-013-9518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang GW, Gu TX, Guan XY, Sun XJ, Qi X, Li XY, Wang XB, Lv F, Yu L, Jiang DQ, Tang R. HGF and IGF-1 promote protective effects of allogeneic BMSC transplantation in rabbit model of acute myocardial infarction. Cell Prolif. 2015;48:661–670. doi: 10.1111/cpr.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306:247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 13.Holmes TC, de Lacalle S, Su X, Liu G, Rich A, Zhang S. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci USA. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strober W. Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix. 2001;3 doi: 10.1002/0471142735.ima03bs21. Appendix 3B. [DOI] [PubMed] [Google Scholar]
- 16.Guo JQ, Gao X, Lin ZJ, Wu WZ, Huang LH, Dong HY, Chen J, Lu J, Fu YF, Wang J, et al. BMSCs reduce rat granulosa cell apoptosis induced by cisplatin and perimenopause. BMC Cell Biol. 2013;14:18. doi: 10.1186/1471-2121-14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 19.Duan Y, Liu Z, O'Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res. 2011;4:605–615. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen XP, Lu SJ, Huang K, Zhang W, Liu ZW, Zhong JH. Effects of Ang II perfusion on transmural heterogeneous of Cx43 in acute myocardial ischemia reperfusion. Asian Pac J Trop Med. 2016;9:96–99. doi: 10.1016/j.apjtm.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Haleagrahara N, Chakravarthi S, Mathews L. Insulin like growth factor-1 (IGF-1) causes overproduction of IL-8, an angiogenic cytokine and stimulates neovascularization in isoproterenol-induced myocardial infarction in rats. Int J Mol Sci. 2011;12:8562–8574. doi: 10.3390/ijms12128562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu M, Wang H, Xu Y, Yu D, Li D, Liu X, Du W. Insulin-like growth factor-1 (IGF-1) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol Int. 2015;39:910–922. doi: 10.1002/cbin.10466. [DOI] [PubMed] [Google Scholar]
- 23.Noguchi S. The biological function of insulin-like growth factor-I in myogenesis and its therapeutic effect on muscular dystrophy. Acta Myol. 2005;24:115–118. [PubMed] [Google Scholar]
- 24.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, et al. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 25.Ko IK, Kim BS. Mesenchymal stem cells for treatment of myocardial infarction. Int J Stem Cells. 2008;1:49–54. doi: 10.15283/ijsc.2008.1.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2016;12:CD007888. doi: 10.1002/14651858.CD007888.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 28.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 29.Busilacchi A, Gigante A, Mattioli-Belmonte M, Manzotti S, Muzzarelli RA. Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr Polym. 2013;98:665–676. doi: 10.1016/j.carbpol.2013.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Cui H, Yi Q, Feng J, Yang L, Tang L. Mechano growth factor E peptide regulates migration and differentiation of bone marrow mesenchymal stem cells. J Mol Endocrinol. 2014;52:111–120. doi: 10.1530/JME-13-0157. [DOI] [PubMed] [Google Scholar]