Abstract
Antitumor necrosis factor (TNF) agents have been widely used for the treatment of spondyloarthritis (SpA) and ankylosing spondylitis (AS). However, these agents may increase the risk of infection due to suppressing the immune response. The present meta-analysis was performed to systematically investigate the risk of overall infection, serious infection and tuberculosis in patients with SpA and AS treated with anti-TNF agents. Medline, Embase and the Cochrane library were searched for randomized controlled trials (RCTs) published between January 1998 and December 2015 about infection in patients with SpA receiving anti-TNF therapy. Data were pooled to obtain relative risks (RRs) along with their 95% confidence intervals (CIs). A total of 25 RCTs investigating SpA, including 12 investigating AS specifically, were eligible for the meta-analysis. Similar risks of overall infection were reported in patients with SpA (RR, 1.03; 95% CI, 0.92–1.15) and AS (RR, 1.06; 95% CI, 0.91–1.24) treated with anti-TNF agents. The RR of serious infection for patients with SpA or AS receiving anti-TNF therapy compared with a placebo was 1.27 (95% CI, 0.67–2.38) and 1.57 (95% CI, 0.63–3.91), respectively. In addition, 4 RCTs with outcomes of tuberculosis in patients with SpA receiving anti-TNF agents were identified, all in infliximab-treated patients (RR, 2.52; 95% CI, 0.53–12.09). However, due to the limited number of RCTs, this finding should be interpreted with caution. The present meta-analysis did not find any significantly increased risk of infection associated with anti-TNF therapy in patients with SpA or AS. However, due to short duration of follow-up in the RCTs and the rarity of serious infections and tuberculosis, patients treated with anti-TNF agents still should be closely monitored in clinical practice.
Keywords: antitumor necrosis factor agents, spondyloarthritis, ankylosing spondylitis, infection, meta-analysis
Introduction
Spondyloarthritis (SpA) is a group of chronic inflammatory rheumatic diseases with pathophysiological, clinical, radiological and genetic features of inflammatory back pain with or without peripheral arthritis, combined with certain features of extra-articular manifestations. Diseases in this category include ankylosing spondylitis (AS), reactive arthritis, psoriatic arthritis, arthropathy of inflammatory bowel disease, undifferentiated SpA and juvenile chronic arthritis (1–3). As SpA diseases progress they can develop into AS, which is the most severe and common subtype of SpA. The primary clinical symptoms of AS include pain, joint stiffness and loss of spinal mobility, which can result in severe impairment of function and a decrease in the patient's quality of life (4,5).
The primary treatments for patients with SpA include general drug treatments accompanied with physiotherapy. However, short-term corticosteroids, conventional non-steroidal anti-inflammatory drugs and disease modifying anti-rheumatic drugs, including methotrexate and sulfasalazine, have not proved to be particularly effective for the treatment of SpA while causing a high incidence of side effects (6–8). Tumor necrosis factor (TNF)-α, a pleiotropic cytokine that is produced during the inflammatory response, serves an important role in the pathogenesis of numerous chronic inflammatory and rheumatic diseases (9). The therapies used for the treatment of SpA have undergone a drastic revision since the development of anti-TNF agents, which inhibit and prevent TNF-α from promoting inflammation, and therefore are beneficial for alleviating the symptoms of SpA (10).
While anti-TNF biologics improve the function and quality of life of patients with SpA, suppressing the immune system makes patients more susceptible to infections. This is the most adverse effect of anti-TNF agent therapy, and has been demonstrated to be significantly higher in patients with SpA, particularly AS, treated with anti-TNF agents compared with those receiving non-biological treatments (11). Serious infection, whilst rare, is severe and thus the inhibitory effects of anti-TNF therapy on the immune response are important. Considering that anti-TNF agents usually require long-term application in patients with SpA and AS (10,12), a meta-analysis was performed to investigate whether the risk of serious infection, including tuberculosis, is increased in patients with SpA or AS treated with anti-TNF agents. In addition, the overall infection rate in patients with SpA or AS was investigated.
Materials and methods
Data sources and search strategy
The design of the present meta-analysis was prepared in accordance with the preferred reporting items for systematic reviews and meta-analyses statement (13). Medline (http://www.ncbi.nlm.nih.gov), Embase (http://www.embase.com) and the Cochrane Library (http://www.cochranelibrary.com) were searched for publications from January 1998 to December 2015 with the following terms: ‘Spondyloarthritis’, ‘ankylosing spondylitis’, ‘psoriatic arthritis’ or ‘reactive arthritis’ combined with ‘biologics’, ‘anti-TNF agent’ or the names of specific biologic agents, including ‘etanercept’, ‘infliximab’, ‘adalimumab’, ‘certolizumab pegol’, ‘golimumab’, and combined with ‘adverse reaction’ and ‘infection’. Searches were restricted to English language publications and studies in humans. The search was supplemented by manual searches of the proceedings of the American College of Rheumatology (http://www.rheumatology.org) and the European League Against Rheumatism (http://www.eular.org). Meanwhile, to identify all relevant articles, the reference lists from associated reviews and meta-analyses were also searched manually.
Study selection
Randomized controlled trials (RCTs) comparing anti-TNF agents with placebos (or other medications), alone or in combination, in patients with SpA were identified. The inclusion and exclusion criteria were as described below. i) Study design: The study must be an RCT; for trials with a cross-over design, a double-blind period followed by an open-label period was eligible for inclusion, while studies with a Jadad score <3 were excluded. ii) Participants: The enrolled patients must fulfill the assessment of SpA criteria for peripheral SpA and SpA in general (14), and patients who were suffering from chronic infections at the beginning of experiments were excluded. iii) Intervention: Studies comparing treatment with infliximab, etanercept, adalimumab, golimumab and certolizumab pegol, either alone or in combination with other medications, against a control group were included; trials using a single infusion or injection of an anti-TNF agent were excluded. Additionally, the duration of the placebo-controlled phases across trials were limited to short term. iv) Endpoints: All included articles must demonstrate the outcomes associated with infection. Studies were independently screened and selected by two investigators, and discrepancies were resolved through discussion.
Data extraction and quality assessment
Using a preformed form, data were extracted by two independent investigators with any discrepancies resolved by discussion. In addition to general information, including the first author, publication year, study design, underlying disease, interventions, study duration, sample size and other specific circumstances, the present study focused on extracting outcomes of the events, including the occurrence of overall infection, serious infection and tuberculosis. For the studies that included a double-blind period and an open-labeled period, only the result of the double-blind period was extracted. Notably, in one trial of etanercept with administration of 25 mg twice a week or 50 mg once a week, the decision was made to present the combined results as these regimens were demonstrated to be equivalent in terms of benefit and safety (15). Selected studies were critically appraised for quality based on the Jadad scale (16).
Statistical analysis
Extracted data were analyzed using the Mantel-Haenszel method with Review Manager software (version 5.2, The Cochrane Collaboration, Copenhagen, Denmark). The Q test and I2 statistics were used to evaluate the heterogeneity of the RCTs in accordance with the Cochrane Handbook (17). An I2 value of >50% accompanied with a P-value <0.05 for the Q test was determined to indicate the presence of significant heterogeneity. When there was no significant heterogeneity, a fixed effects model was used; otherwise a random effects model was applied. Forest plots were constructed to display relative risk (RR) estimates and 95% confidence intervals (CIs). Funnel plots were assessed for evidence of asymmetry, followed by possible publication bias or other small study effects. Subgroup analysis was performed to explore potential differences by stratifying different anti-TNF agents in patients with SpA and AS. For sparse data on serious infection, due to null values in either the intervention or control arms in several trials, either a crude analysis was performed by combining study results, or the studies were excluded, according to the methodological description by Bradburn et al (18). Sensitivity analysis was also performed to inspect the robustness of the data using Stata software (version 12.0, StataCorp LP, College Station, TX, USA).
Results
Study selection and characteristics
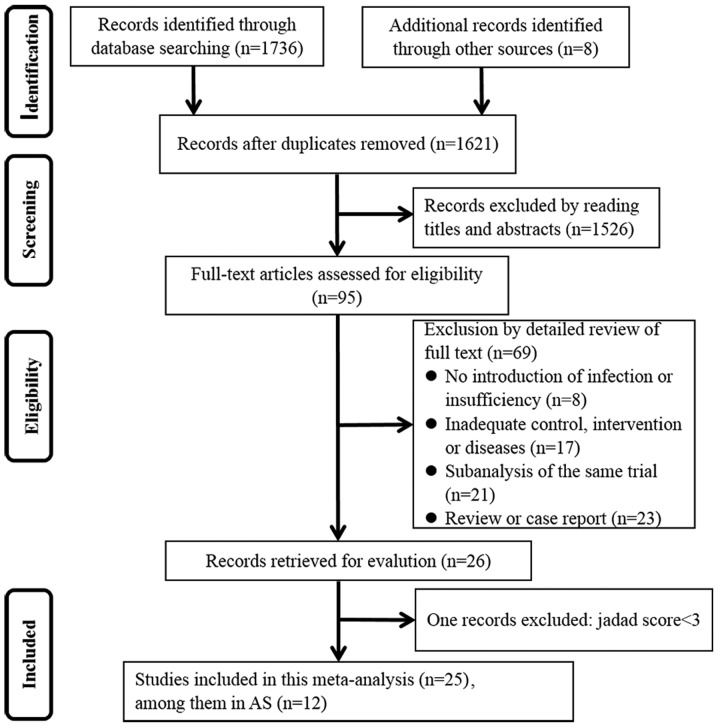
A flow diagram depicting the process of searching and selecting RCTs was presented in Fig. 1. A total of 1,621 unique RCTs were obtained after removing duplicates from the initial search that identified 1,744 studies. Of these studies, 1,526 were excluded through reading the title and abstract. Via a detailed full-text review of the remaining 95 studies, 26 articles were retrieved for evaluation. With 1 study eliminated due to a Jaded score below the cutoff level of 3 (19), ultimately 25 articles were eligible for inclusion in the analysis (2,15,20–42). Notably, 2 trials (29,42) that were not double-blind were included in the primary analysis, though the quality of their Jadad scores was not particularly high. Among the 25 articles eligible for analysis, 12 studies were investigating patients with AS.
Figure 1.
Flow diagram depicting the process of searching and selecting randomized controlled trials for the meta-analysis. AS, ankylosing spondylitis.
Table I listed the general characteristics of the 25 articles included in the present study. With regards to the anti-TNF agents utilized, 9 trials used etanercept, 7 used infliximab, 7 used adalimumab and 2 used golimumab. No RCTs investigating certolizumab pegol were eligible for analysis due to their inadequate presentation of infection outcomes (43–45). The quality of the eligible studies met the required standards, with a mean Jadad score of 5.80±1.04. The duration of placebo-controlled phases across trials ranged from 12 to 48 weeks. In addition, the dosages of the biologics used in these RCTs met US Food and Drug Administration standards.
Table I.
Characteristics of the studies included in the meta-analysis investigating the use of anti-TNF agents in patients with SpA or AS.
| Anti-TNF group | Control group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author, year | Type of disease | Duration (weeks) | Treatment | P (n) | I (n) | SI (n) | TB (n) | Treatment | P (n) | I (n) | SI (n) | TB (n) | (Refs.) |
| Dougados et al, 2014 | axSpA | 12 | ETA 50 mg Qw | 106 | 11 | 0 | 0 | PBO | 109 | 10 | 1 | 0 | (20) |
| Braun et al, 2010 | AS | 16 | ETA 50 mg Qw | 379 | 43 | 0 | 0 | SSZ | 187 | 26 | 0 | 0 | (21) |
| Dougados et al, 2010 | SpA | 12 | ETA 50 mg Qw | 12 | 5 | 1 | 0 | PBO | 12 | 1 | 0 | 0 | (22) |
| van der Heijde et al, 2006 | AS | 12 | ETA 50 mg Qw or 25 mg Biw | 305 | 68 | 2 | 0 | PBO | 51 | 12 | 0 | 0 | (15) |
| Mease et al, 2004 | PsA | 24 | ETA 25 mg Biw | 101 | 33 | 0 | 0 | PBO | 104 | 39 | 1 | 0 | (23) |
| Calin et al, 2004 | AS | 12 | ETA 25 mg Biw | 45 | 0 | 0 | 0 | PBO | 39 | 0 | 0 | 0 | (24) |
| Davis et al, 2003 | AS | 24 | ETA 25 mg Biw | 138 | 44 | 2 | 0 | PBO | 139 | 43 | 1 | 0 | (25) |
| Brandt et al, 2003 | AS | 24 | ETA 25 mg Biw | 14 | 6 | 0 | 0 | PBO | 16 | 6 | 0 | 0 | (26) |
| Gorman, 2002 | AS | 16 | ETA 25 mg Biw | 20 | 12 | 2 | 0 | PBO | 20 | 12 | 0 | 0 | (27) |
| Sieper et al, 2013 | axSpA | 24 | IFX 5 mg/kg+NPX 0, 2, 6, 12, 18, 24 w | 105 | 27 | 1 | 1 | PBO + NPX | 52 | 9 | 0 | 0 | (28) |
| Baranauskaite et al, 2011 | PsA | 16 | IFX 5 mg/kg+MTX 0, 2, 6, 14 w | 57 | 4 | 2 | 1 | MTX | 54 | 0 | 0 | 0 | (29) |
| Marzo-Ortega et al, 2005 | AS | 30 | IFX 5 mg/kg+MTX 0, 2, 6, 14, 22 w | 28 | 6 | 0 | 0 | PBO + MTX | 14 | 2 | 0 | 0 | (30) |
| van der Heijde et al, 2004 | AS | 24 | IFX 5 mg/kg 0, 2, 6, 12, 18 w | 201 | 86 | 2 | 0 | PBO | 78 | 27 | 0 | 0 | (31) |
| Antoni et al, 2004 | PsA | 16 | IFX 5 mg/kg 0, 2, 6, 14 w | 52 | 9 | 1 | 0 | PBO | 51 | 16 | 1 | 0 | (32) |
| Van Den Bosch et al, 2002 | SpA | 12 | IFX 5 mg/kg 0, 2, 6 w | 20 | 9 | 1 | 1 | PBO | 20 | 6 | 0 | 0 | (33) |
| Braun et al, 2002 | AS | 12 | IFX 5 mg/kg 0, 2, 6 w | 34 | 12 | 1 | 1 | PBO | 35 | 18 | 0 | 0 | (34) |
| Mease et al, 2015 | npSpA | 12 | ADA 40 mg Eow | 84 | 18 | 0 | 0 | PBO | 81 | 23 | 0 | 0 | (2) |
| Huang et al, 2013 | AS | 12 | ADA 40 mg Eow | 229 | 25 | 1 | 0 | PBO | 115 | 12 | 0 | 0 | (35) |
| Sieper et al, 2012 | axSpA | 12 | ADA 40 mg Eow | 91 | 28 | 0 | 0 | PBO | 94 | 28 | 0 | 0 | (36) |
| Paramarta et al, 2012 | SpA | 12 | ADA 40 mg Eow | 20 | 4 | 0 | 0 | PBO | 20 | 8 | 1 | 0 | (37) |
| Horneff et al, 2012 | Jo-AS | 12 | ADA 40 mg Eow | 17 | 6 | 2 | 0 | PBO | 15 | 6 | 1 | 0 | (38) |
| van der Heijde et al, 2006 | AS | 24 | ADA 40 mg Eow | 208 | 66 | 0 | 0 | PBO | 107 | 23 | 1 | 0 | (39) |
| Mease et al, 2005 | PsA | 24 | ADA 40 mg Eow | 151 | 36 | 1 | 0 | PBO | 162 | 41 | 2 | 0 | (40) |
| Sieper et al, 2015 | axSpA | 16 | GLM 50 mg Q4w | 97 | 0 | 0 | 0 | PBO | 100 | 0 | 0 | 0 | (41) |
| Mok et al, 2015 | axSpA | 48 | GLM 50 mg Q4w | 20 | 6 | 0 | 0 | PAM | 10 | 1 | 0 | 0 | (42) |
ETA, etanercept; IFX, infliximab; ADA, adalimumab; GLM, golimumab; PBO, placebo; MTX, methotrexate; NPX, naproxen; SSZ, sulfasalazine; PAM, pamidronate; P, participant; I, infection; SI, serious infection; TB, tuberculosis; n, number; w, week; Qw, once weekly; Biw, twice weekly; Eow, every other week; Q4w, every 4 weeks; SpA, spondyloarthritis; AS, ankylosing spondylitis; axSpA, axial SpA; PsA, psoriatic arthritis; npSpA, nonpsoriatic peripheral SpA; Jo-AS, juvenile onset AS.
Overall infection rate of patients with SpA or AS receiving anti-TNF agents vs. a placebo
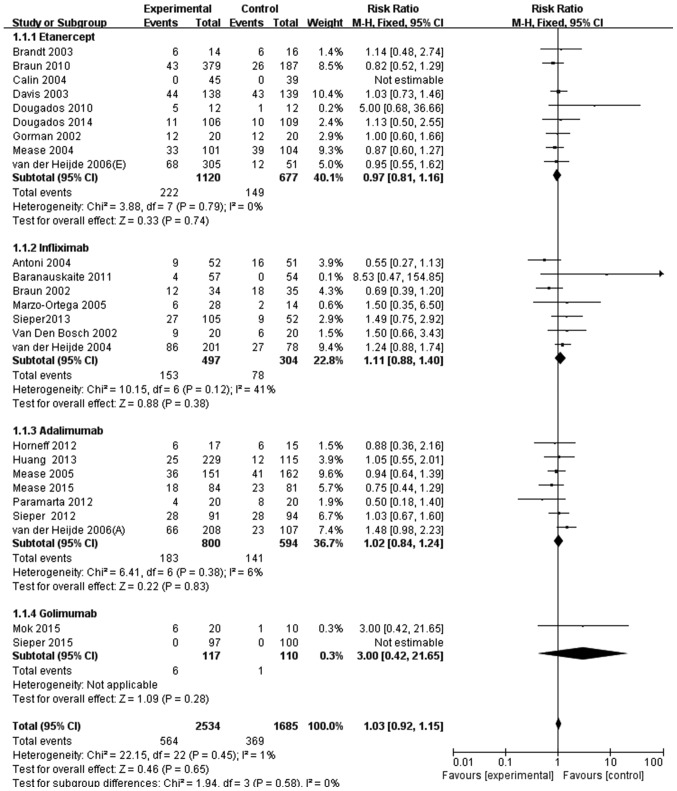
In the 25 studies identified, 564/2,534 patients with SpA (22.3%) who received anti-TNF agents and 369/1,685 patients with SpA (21.9%) who received a placebo experienced an infection. Thus, no significant difference was identified in the infection rate between patients with SpA treated with anti-TNF agents compared with those who received a placebo (RR, 1.03; 95% CI, 0.92–1.15; Fig. 2). The risk of overall infection was then stratified by the type of anti-TNF agent. No significant differences were observed in the infection rate between patients with SpA treated with a specific anti-TNF agent compared with a placebo. The individual results were as follows: Etanercept (RR, 0.97; 95% CI, 0.81–1.16); infliximab (RR, 1.11; 95% CI, 0.88–1.40); adalimumab (RR, 1.02; 95% CI, 0.84–1.24); and golimumab (RR, 3.00; 95% CI, 0.42–21.65) (Fig. 2).
Figure 2.
Meta-analysis of overall infection risk in patients with spondyloarthritis treated with anti-TNF agents. M-H, Mantel-Haenszel method; CI, confidence interval.
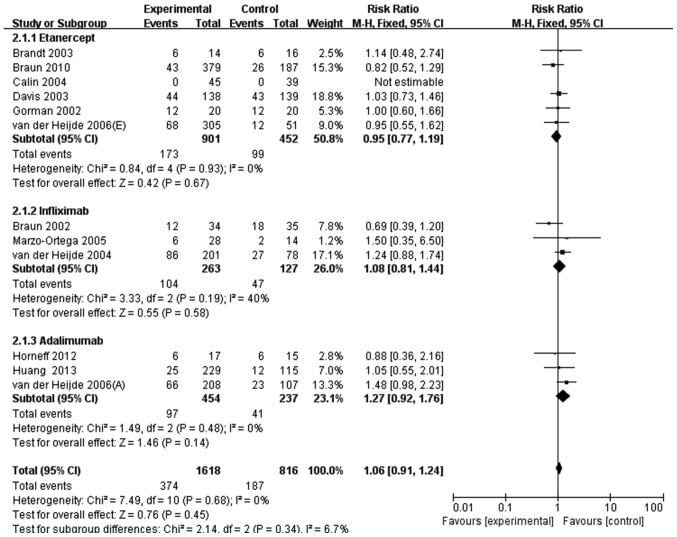
Among the RCTs evaluated, 12 investigated patients with AS. The incidence of infection in these studies was similar between the groups treated with anti-TNF agents (374/1,618 patients; 23.1%) and the control (187/816 patients; 22.9%). There was no significant difference in the overall rate of infection between these groups (RR, 1.06; 95% CI, 0.91–1.24; Fig. 3). Subgroup analysis by the type of anti-TNF agent used also revealed no significant differences, with the following individual results: Etanercept (RR, 0.95; 95% CI, 0.77–1.19); infliximab (RR, 1.08; 95% CI, 0.81–1.44); adalimumab (RR, 1.27; 95% CI, 0.92–1.24); and golimumab (RR, 3.00; 95% CI, 0.42–21.65) (Fig. 3).
Figure 3.
Meta-analysis of overall infection risk in patients with ankylosing spondylitis treated with anti-TNF agents. M-H, Mantel-Haenszel method; CI, confidence interval.
Serious infection rate of patients with SpA or AS receiving anti-TNF agents vs. a placebo
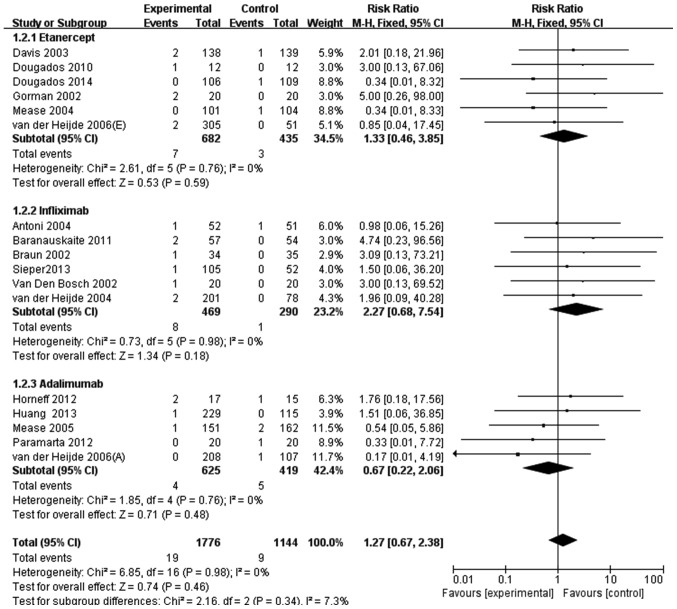
A total of 19/2,534 patients with SpA (0.75%) treated with anti-TNF agents had a serious infection compared with 9/1,685 patients (0.53%) who received a placebo. By pooling the data crudely, the relative risk of serious infection in patients with SpA treated with anti-TNF agents compared with the control group was 1.40 (95% CI, 0.64–3.10). After discarding the null studies, 17 RCTs were evaluated. Compared with patients treated with a placebo, patients receiving anti-TNF agents did not have a significantly increased risk of serious infection (RR, 1.27; 95% CI, 0.67–2.38; Fig. 4). The subgroup analysis that stratified the studies based on the anti-TNF agent used did not identify any significant heterogeneity compared with the control group either. The results were as follows: Etanercept (RR, 1.33; 95% CI, 0.46–3.85); infliximab (RR, 2.27; 95% CI, 0.68–7.54); and adalimumab (RR, 0.67; 95% CI, 0.22–2.06) (Fig. 4).
Figure 4.
Meta-analysis of serious infection risk in patients with spondyloarthritis treated with anti-TNF agents. M-H, Mantel-Haenszel method; CI, confidence interval.
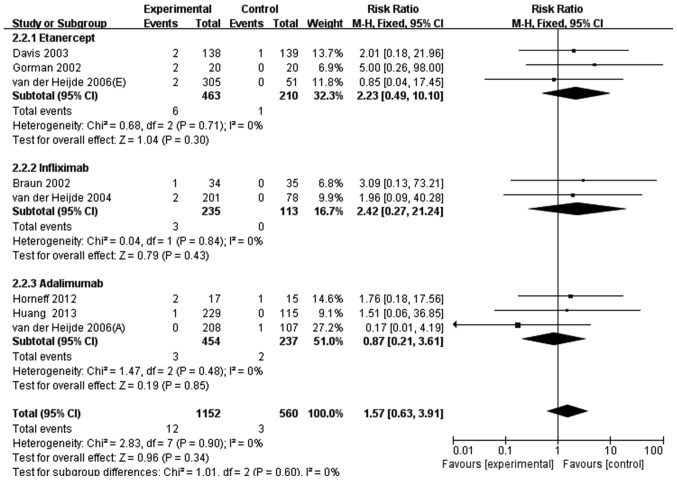
Similarly, in the 12 RCTs investigating patients with AS, 12/1,618 patients (0.74%) who received anti-TNF agent therapy experienced serious infection compared with 3/816 patients (0.37%) in the control group. This crude pooled result demonstrated that anti-TNF agents did not significantly increase the risk of serious infection in patients with AS (RR, 2.02; 95% CI, 0.57–7.13). After the 4 null RCTs abandoned, 8 studies were eligible for further analysis. The meta-analysis demonstrated that anti-TNF agents resulted in a 1.57-fold higher risk of serious infection in patient with AS compared with the control group (RR, 1.57; 95% CI, 0.63–3.91; Fig. 5). Individually, etanercept resulted in a 2.23-fold higher likelihood of serious infection (RR, 2.23; 95% CI, 0.49–10.10) and infliximab in a 2.42-fold higher likelihood (RR, 2.42; 95%, CI, 0.27–21.24), while adalimumab reduced the risk of serious infection (RR, 0.87; 95% CI, 0.21–3.61) (Fig. 5). However, none of these results were statistically significant.
Figure 5.
Meta-analysis of serious infection risk in patients with ankylosing spondylitis treated with anti-TNF agents. M-H, Mantel-Haenszel method; CI, confidence interval.
Rate of tuberculosis infection in patients with SpA receiving anti-TNF agents vs. a placebo
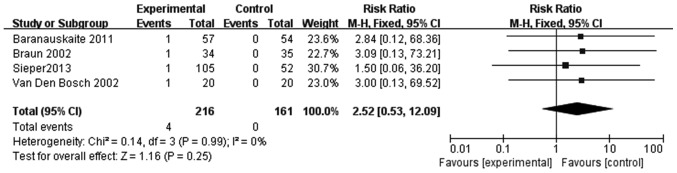
In the 25 RCTs included in the present study, only 4 studies revealed incidences of tuberculosis, which all emerged in infliximab-treated patients with SpA (Fig. 6). Thus, infliximab treatment resulted in a 2.42-fold higher likelihood of tuberculosis in patients with SpA compared with the control group (RR, 2.42; 95% CI, 0.40–14.70); however, this result was not significant (Fig. 6). In addition, due to the limited number of RCTs, this finding should be interpreted with caution.
Figure 6.
Meta-analysis of tuberculosis risk in patients with SpA treated with infliximab. M-H, Mantel-Haenszel method; CI, confidence interval.
Publication bias
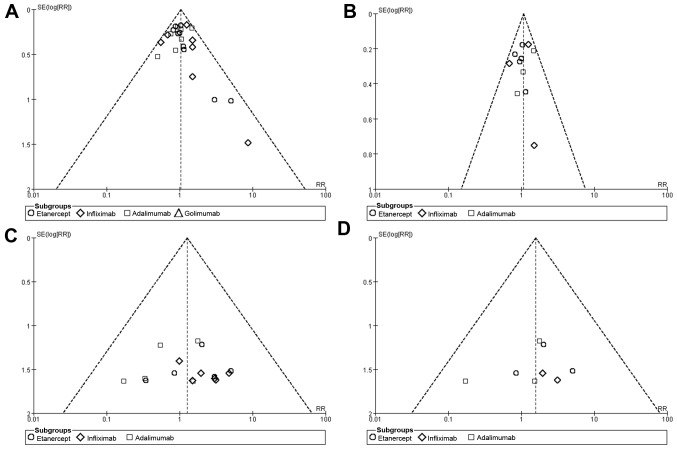
Funnel plots were produced to assess the publication bias of the included studies. The shape of funnel plots revealed no obvious asymmetry (Fig. 7), indicating that there was no publication bias for the overall and serious infection outcomes identified in patients with SpA or AS.
Figure 7.
Funnel plots for publication bias of the studies included in the present meta-analysis. Funnel plots for overall infection risk associated with anti-TNF agents in patients with (A) SpA and (B) AS. Funnel plots for serious infection risk associated with anti-TNF agents in patients with (C) SpA and (D) AS. SpA, spondyloarthritis; AS, ankylosing spondylitis; SE, standard error; RR, relative risk.
Sensitivity analysis
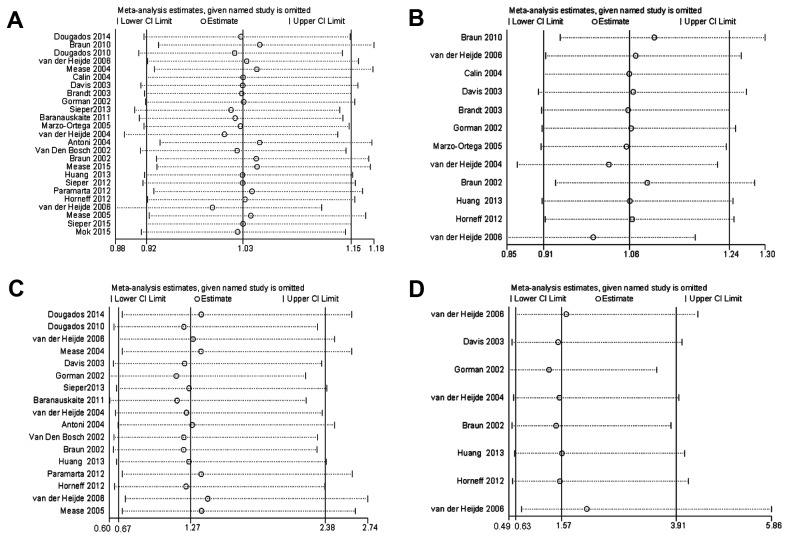
Sensitivity analysis was performed by omitting individual studies to evaluate the robustness of the data. The results revealed that the RR was not influenced meaningfully in each model (Fig. 8). Notably, the 2 studies that were not double-blinded trials did not influence the robustness of the present study.
Figure 8.
Sensitivity analysis. Sensitivity analysis was performed for overall infection associated with anti-TNF agents in patients with (A) SpA and (B) AS. Sensitivity analysis was also performed for serious infection associated with anti-TNF agents in patients with (C) SpA and (D) AS. SpA, spondyloarthritis; AS, ankylosing spondylitis; CI, confidence interval.
Discussion
Presently, there are five anti-TNF agents available for the treatment of SpA, which are divided into three categories (46,47). One category includes etanercept, a soluble receptor of the p75 TNF receptor/Fc fusion protein. Another category includes the monoclonal antibodies infliximab, adalimumab and golimumab. Infliximab is composed of a murine variable region and human constant region, while adalimumab and golimumab are human antibodies. The last category includes certolizumab pegol, a recombinant humanized antibody of the Fab region conjugated to polyethylene glycol. The use of these drugs is a double-edged sword, as suppressing the immune response improved the symptoms of patients with SpA but also increased the risk of infection (48–52). To the best of our knowledge, the present meta-analysis was the first and largest review examining the risk of infection in patients with SpA treated with anti-TNF agents in RCTs. In AS, a meta-analysis was published to assess serious infection in patients receiving TNF blockers in 2010 (53). Considering more single studies arising published after 2010 (21,35,38) and the absence of data identifying respective risk of different anti-TNF agents in the previous meta-analysis, an updated meta-analysis regarding serious infection with use of TNF inhibitors for AS was performed in the present study.
The results of the present study revealed that there was no increased risk of overall infection with the short-term use of anti-TNF agents for patients with SpA or AS. Among the studies identified, the majority of reported infections were minor, particularly upper respiratory tract infections. With regard to the risk of serious infection, the crude pooled result did not find an increased risk in patients with SpA or AS treated with anti-TNF agents. For the risk of serious infection in patients with AS, the result of the present study was in accordance with the previous meta-analysis (53). However, compared with the prior meta-analysis, the present study had certain advantages. Firstly, the estimates were based on the pooling of 12 RCTs with an overall population of 2,434 participants compared with the prior study's sample size of 1,496 patients. With the large number of patients and similar results to the previous meta-analysis, it was unlikely that new individual RCTs would affect the conclusion of the present analysis. In addition, the present study brought golimumab into the meta-analysis and performed a subgroup analysis to explore the individual risk of infection from each anti-TNF agent. Furthermore, since SpA may develop into AS, in the meta-analysis, we didn't simply limit to the disease of AS and severity of infection, which could lead to a more comprehensive understanding and comparison as diseases progresses. Overall, the present study provided further support to the existing observational data, and was an updated and extended meta-analysis with detailed outcomes for infection.
On account of its seriousness and infectivity, tuberculosis was also an outcome that the present study investigated in patients with SpA treated with anti-TNF agents. In the analysis conducted in the current study, only 4 incidences of tuberculosis were detected in anti-TNF agent-treated patients with SpA, with the anti-TNF agent being infliximab in all cases. Certain previous studies have demonstrated that the incidence of tuberculosis is 3–4 times higher in patients with SpA receiving monoclonal antibody anti-TNF agents compared with those receiving etanercept (54,55), which may be associated with the different mechanisms of tuberculosis infection. Saliu et al (56) reported that adalimumab and infliximab reduced the proportion of tuberculosis-responsive cluster of differentiation (CD)69+ CD4 cells by 50–70% and suppressed antigen-induced interferon γ production in an in vitro intracellular infection model; however, etanercept had no significant effect. A Markov model performed by Wallis (57) revealed that infliximab-associated tuberculosis occurred within a very short time after taking the medicine (12–21 weeks), whereas infection onset was 3–5 times later with etanecept. In addition, the risk of latent tuberculosis recurrence after infliximab was 12.1 times higher compared with etanecept (57). These results indicated that anti-TNF agents should be carefully selected and that tuberculosis should be screened for in a timely manner in the clinical practice of patients with SpA.
The present analysis had several limitations. The study search was limited to papers published in English, which may have excluded other relevant non-English language studies and affected the results. However, all large RCTs were included, so the exclusion of several small trials should not have altered the results. In addition, no publication bias was identified for any condition. Another limitation was that serious infection was determined according to each RCTs definition, which would increase heterogeneity to a certain extent. However, the sensitivity analysis performed would have minimized the role of such heterogeneity. In addition, due to the rarity of serious infection, and the short duration of treatment and follow-up, the present study did not assess the risk of serious infection stratified by follow-up time.
In conclusion, the results of the present meta-analysis suggest that there is not a significantly increased risk of infection in patients with SpA or AS receiving anti-TNF agent therapy. However, since anti-TNF agents typically need to be used for a long period of time, even lifelong, in patients with SpA, particularly AS, more long-term follow-up studies are required to confirm the findings of the present study, in addition to exploring the potential occurrence of tuberculosis.
Acknowledgements
The present study was supported by the National Natural Science Funds (grant nos. 81472033 and 30901308), the National Science Foundation of Hubei Province (grant nos. 2013CFB233 and 2013CFB235), the Scientific and Technological Project of Wuhan City (grant no. 2014060101010045), and the Hubei Province Health and Family Planning Scientific Research Project (grant no. WJ2015Q021).
References
- 1.Dougados M, Baeten D. Spondyloarthritis. Lancet. 2011;377:2127–2137. doi: 10.1016/S0140-6736(11)60071-8. [DOI] [PubMed] [Google Scholar]
- 2.Mease P, Sieper J, Van den Bosch F, Rahman P, Karunaratne PM, Pangan AL. Randomized controlled trial of adalimumab in patients with nonpsoriatic peripheral spondyloarthritis. Arthritis Rheumatol. 2015;67:914–923. doi: 10.1002/art.39008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Braun J, Dougados M, Emery P, Fitzgerald O, Helliwell P, Kavanaugh A, Kvien TK, Landewé R, Luger T, et al. Treating spondyloarthritis, including ankylosing spondylitis and psoriatic arthritis, to target: Recommendations of an international task force. Ann Rheum Dis. 2014;73:6–16. doi: 10.1136/annrheumdis-2013-203419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. doi: 10.1016/S0140-6736(07)60635-7. [DOI] [PubMed] [Google Scholar]
- 5.Davis JC, van der Heijde D, Dougados M, Woolley JM. Reductions in health-related quality of life in patients with ankylosing spondylitis and improvements with etanercept therapy. Arthritis Rheum. 2005;53:494–501. doi: 10.1002/art.21330. [DOI] [PubMed] [Google Scholar]
- 6.Guellec D, Nocturne G, Tatar Z, Pham T, Sellam J, Cantagrel A, Saraux A. Should non-steroidal anti-inflammatory drugs be used continuously in ankylosing spondylitis? Joint Bone Spine. 2014;81:308–312. doi: 10.1016/j.jbspin.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Visser K, van der Heijde DM. Risk and management of liver toxicity during methotrexate treatment in rheumatoid and psoriatic arthritis: A systematic review of the literature. Clin Exp Rheumatol. 2009;27:1017–1025. [PubMed] [Google Scholar]
- 8.Lateef O, Shakoor N, Balk RA. Methotrexate pulmonary toxicity. Expert Opin Drug Saf. 2005;4:723–730. doi: 10.1517/14740338.4.4.723. [DOI] [PubMed] [Google Scholar]
- 9.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell LJ, Zochling J, Boonen A, Singh JA, Veras MM, Ghogomu Tanjong E, Jandu Benkhalti M, Tugwell P, Wells GA. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst Rev. 2015;18:CD005468. doi: 10.1002/14651858.CD005468.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampaio-Barros PD, van der Horst-Bruinsma IE. Adverse effects of TNF inhibitors in SpA: Are they different from RA? Best Pract Res Clin Rheumatol. 2014;28:747–763. doi: 10.1016/j.berh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Poddubnyy D, Fedorova A, Listing J, Haibel H, Baraliakos X, Braun J, Sieper J. Physical function and spinal mobility remain stable despite radiographic spinal progression in patients with ankylosing spondylitis treated with TNF-α inhibitors for Up to 10 Years. J Rheumatol. 2016;43:2142–2148. doi: 10.3899/jrheum.160594. [DOI] [PubMed] [Google Scholar]
- 13.Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39:91–92. doi: 10.1016/j.jcms.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Rudwaleit M, van der Heijde D, Landewé R, Akkoc N, Brandt J, Chou CT, Dougados M, Huang F, Gu J, Kirazli Y, et al. The assessment of SpondyloArthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D, Da Silva JC, Dougados M, Geher P, van der Horst-Bruinsma I, Juanola X, Olivieri I, Raeman F, Settas L, Sieper J, et al. Etanercept 50 mg once weekly is as effective as 25 mg twice weekly in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:1572–1577. doi: 10.1136/ard.2006.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Naunyn-Schmiedebergs Archiv für experimentelle Pathologie und Pharmakologie. 2011;5:S38. [Google Scholar]
- 18.Bradburn MJ, Deeks JJ, Berlin JA, Localio Russell A. Much ado about nothing: A comparison of the performance of meta-analytical methods with rare events. Stat Med. 2007;26:53–77. doi: 10.1002/sim.2528. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Zhang Y, Zhao L, Li ZH, Shi ZJ. The efficacy and safety of infliximab used in patients with ankylosing spondylitis after unilateral total hip arthroplasty. Hip Int. 2013;23:406–410. doi: 10.5301/hipint.5000033. [DOI] [PubMed] [Google Scholar]
- 20.Dougados M, van der Heijde D, Sieper J, Braun J, Maksymowych WP, Citera G, Miceli-Richard C, Wei JC, Pedersen R, Bonin R, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: A multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66:2091–2102. doi: 10.1002/art.38721. [DOI] [PubMed] [Google Scholar]
- 21.Braun J, van der Horst-Bruinsma IE, Huang F, Burgos-Vargas R, Vlahos B, Koenig AS, Freundlich B. Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: A randomized, double-blind trial. Arthritis Rheum. 2011;63:1543–1551. doi: 10.1002/art.30223. [DOI] [PubMed] [Google Scholar]
- 22.Dougados M, Combe B, Braun J, Landewé R, Sibilia J, Cantagrel A, Feydy A, van der Heijde D, Leblanc V, Logeart I. A randomised, multicentre, double-blind, placebo-controlled trial of etanercept in adults with refractory heel enthesitis in spondyloarthritis: The HEEL trial. Ann Rheum Dis. 2010;69:1430–1435. doi: 10.1136/ard.2009.121533. [DOI] [PubMed] [Google Scholar]
- 23.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Tsuji W. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004;50:2264–2272. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 24.Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, Mola EM, Salvarani C, Sanmartí R, Sany J, et al. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis. 2004;63:1594–1600. doi: 10.1136/ard.2004.020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis JC, Van Der Heijde D, Braun J, Dougados M, Cush J, Clegg DO, Kivitz A, Fleischmann R, Inman R, Tsuji W. Enbrel Ankylosing Spondylitis Study Group: Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: A randomized, controlled trial. Arthritis Rheum. 2003;48:3230–3236. doi: 10.1002/art.11325. [DOI] [PubMed] [Google Scholar]
- 26.Brandt J, Khariouzov A, Listing J, Haibel H, Sörensen H, Grassnickel L, Rudwaleit M, Sieper J, Braun J. Six-month results of a double-blind, placebo-controlled trial of etanercept treatment in patients with active ankylosing spondylitis. Arthritis Rheum. 2003;48:1667–1675. doi: 10.1002/art.11017. [DOI] [PubMed] [Google Scholar]
- 27.Gorman JD, Sack KE, Davis JC., Jr Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349–1356. doi: 10.1056/NEJMoa012664. [DOI] [PubMed] [Google Scholar]
- 28.Sieper J, Lenaerts J, Wollenhaupt J, Rudwaleit M, Mazurov VI, Myasoutova L, Park S, Song Y, Yao R, Chitkara D, et al. Efficacy and safety of infliximab plus naproxen versus naproxen alone in patients with early, active axial spondyloarthritis: Results from the double-blind, placebo-controlled INFAST study, Part 1. Ann Rheum Dis. 2014;73:101–107. doi: 10.1136/annrheumdis-2012-203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baranauskaite A, Raffayová H, Kungurov NV, Kubanova A, Venalis A, Helmle L, Srinivasan S, Nasonov E, Vastesaeger N. RESPOND investigators: Infliximab plus methotrexate is superior to methotrexate alone in the treatment of psoriatic arthritis in methotrexate-naive patients: The RESPOND study. Ann Rheum Dis. 2012;71:541–548. doi: 10.1136/ard.2011.152223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marzo-Ortega H, McGonagle D, Jarrett S, Haugeberg G, Hensor E, O'connor P, Tan AL, Conaghan PG, Greenstein A, Emery P. Infliximab in combination with methotrexate in active ankylosing spondylitis: A clinical and imaging study. Ann Rheum Dis. 2005;64:1568–1575. doi: 10.1136/ard.2004.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Heijde D, Dijkmans B, Geusens P, Sieper J, DeWoody K, Williamson P, Braun J. Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group: Efficacy and safety of infliximab in patients with ankylosing spondylitis: Results of a randomized, placebo-controlled trial (ASSERT) Arthritis Rheum. 2005;52:582–591. doi: 10.1002/art.20852. [DOI] [PubMed] [Google Scholar]
- 32.Antoni CE, Kavanaugh A, Kirkham B, Tutuncu Z, Burmester GR, Schneider U, Furst DE, Molitor J, Keystone E, Gladman D, et al. Sustained benefits of infliximab therapy for dermatologic and articular manifestations of psoriatic arthritis: Results from the infliximab multinational psoriatic arthritis controlled trial (IMPACT) Arthritis Rheum. 2005;52:1227–1236. doi: 10.1002/art.20967. [DOI] [PubMed] [Google Scholar]
- 33.Van Den Bosch F, Kruithof E, Baeten D, Herssens A, de Keyser F, Mielants H, Veys EM. Randomized double-blind comparison of chimeric monoclonal antibody to tumor necrosis factor alpha (infliximab) versus placebo in active spondylarthropathy. Arthritis Rheum. 2002;46:755–765. doi: 10.1002/art.511. [DOI] [PubMed] [Google Scholar]
- 34.Braun J, Brandt J, Listing J, Zink A, Alten R, Golder W, Gromnica-Ihle E, Kellner H, Krause A, Schneider M, et al. Treatment of active ankylosing spondylitis with infliximab: A randomised controlled multicentre trial. Lancet. 2002;359:1187–1193. doi: 10.1016/S0140-6736(02)08215-6. [DOI] [PubMed] [Google Scholar]
- 35.Huang F, Gu J, Zhu P, Bao C, Xu J, Xu H, Wu H, Wang G, Shi Q, Andhivarothai N, et al. Efficacy and safety of adalimumab in Chinese adults with active ankylosing spondylitis: Results of a randomised, controlled trial. Ann Rheum Dis. 2014;73:587–594. doi: 10.1136/annrheumdis-2012-202533. [DOI] [PubMed] [Google Scholar]
- 36.Sieper J, van der Heijde D, Dougados M, Mease PJ, Maksymowych WP, Brown MA, Arora V, Pangan AL. Efficacy and safety of adalimumab in patients with non-radiographic axial spondyloarthritis: Results of a randomised placebo-controlled trial (ABILITY-1) Ann Rheum Dis. 2013;72:815–822. doi: 10.1136/annrheumdis-2012-201766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paramarta JE, De Rycke L, Heijda TF, Ambarus CA, Vos K, Dinant HJ, Tak PP, Baeten DL. Efficacy and safety of adalimumab for the treatment of peripheral arthritis in spondyloarthritis patients without ankylosing spondylitis or psoriatic arthritis. Ann Rheum Dis. 2013;72:1793–1799. doi: 10.1136/annrheumdis-2012-202245. [DOI] [PubMed] [Google Scholar]
- 38.Horneff G, Fitter S, Foeldvari I, Minden K, Kuemmerle-Deschner J, Tzaribacev N, Thon A, Borte M, Ganser G, Trauzeddel R, Huppertz HI. Double-blind, placebo-controlled randomized trial with adalimumab for treatment of juvenile onset ankylosing spondylitis (JoAS): Significant short term improvement. Arthritis Res Ther. 2012;14:R230. doi: 10.1186/ar4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Heijde D, Kivitz A, Schiff MH, Sieper J, Dijkmans BA, Braun J, Dougados M, Reveille JD, Wong RL, Kupper H, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–2146. doi: 10.1002/art.21913. [DOI] [PubMed] [Google Scholar]
- 40.Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA. Adalimumab Effectiveness in Psoriatic Arthritis Trial Study Group: Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: Results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2005;52:3279–3289. doi: 10.1002/art.21306. [DOI] [PubMed] [Google Scholar]
- 41.Sieper J, van der Heijde D, Dougados M, Maksymowych WP, Scott BB, Boice JA, Berd Y, Bergman G, Curtis S, Tzontcheva A, et al. A randomized, double-blind, placebo-controlled, sixteen-week study of subcutaneous golimumab in patients with active nonradiographic axial spondyloarthritis. Arthritis Rheumatol. 2015;67:2702–2712. doi: 10.1002/art.39257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mok CC, Li OC, Chan KL, Ho LY, Hui PK. Effect of golimumab and pamidronate on clinical efficacy and MRI inflammation in axial spondyloarthritis: A 48-week open randomized trial. Scand J Rheumatol. 2015;44:480–486. doi: 10.3109/03009742.2015.1038300. [DOI] [PubMed] [Google Scholar]
- 43.Sieper J, Kivitz A, van Tubergen A, Deodhar A, Coteur G, Woltering F, Landewé R. Impact of certolizumab pegol on patient-reported outcomes in patients with axial spondyloarthritis. Arthritis Care Res (Hoboken) 2015;67:1475–1480. doi: 10.1002/acr.22594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, van der Heijde D. Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) Ann Rheum Dis. 2014;73:48–55. doi: 10.1136/annrheumdis-2013-203696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, Reveille JD, Rudwaleit M, van der Heijde D, Stach C, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73:39–47. doi: 10.1136/annrheumdis-2013-204231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thalayasingam N, Isaacs JD. Anti-TNF therapy. Best Pract Res Clin Rheumatol. 2011;25:549–567. doi: 10.1016/j.berh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Taylor PC. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr Opin Pharmacol. 2010;10:308–315. doi: 10.1016/j.coph.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Kim SY, Solomon DH. Tumor necrosis factor blockade and the risk of viral infection. Nat Rev Rheumatol. 2010;6:165–174. doi: 10.1038/nrrheum.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Solomon DH, Lunt M, Schneeweiss S. The risk of infection associated with tumor necrosis factor alpha antagonists: Making sense of epidemiologic evidence. Arthritis Rheum. 2008;58:919–928. doi: 10.1002/art.23396. [DOI] [PubMed] [Google Scholar]
- 50.Iseman MD, Fischer A. Tumor necrosis factor-alpha at the intersection of mycobacterial immunity and pathogenesis: An important new address in medicine. Clin Infect Dis. 2008;46:1741–1742. doi: 10.1086/587990. [DOI] [PubMed] [Google Scholar]
- 51.Gomez-Reino JJ, Carmona L, Descalzo Angel M. Biobadaser Group: Risk of tuberculosis in patients treated with tumor necrosis factor antagonists due to incomplete prevention of reactivation of latent infection. Arthritis Rheum. 2007;57:756–761. doi: 10.1002/art.22768. [DOI] [PubMed] [Google Scholar]
- 52.Roos JC, Ostor AJ. Anti-tumor necrosis factor alpha therapy and the risk of JC virus infection. Arthritis Rheum. 2006;54:381–382. doi: 10.1002/art.21688. [DOI] [PubMed] [Google Scholar]
- 53.Fouque-Aubert A, Jette-Paulin L, Combescure C, Basch A, Tebib J, Gossec L. Serious infections in patients with ankylosing spondylitis with and without TNF blockers: A systematic review and meta-analysis of randomised placebo-controlled trials. Ann Rheum Dis. 2010;69:1756–1761. doi: 10.1136/ard.2008.098822. [DOI] [PubMed] [Google Scholar]
- 54.Mariette X, Gottenberg JE, Ravaud P, Combe B. Registries in rheumatoid arthritis and autoimmune diseases: Data from the French registries. Rheumatology (Oxford) 2011;50:222–229. doi: 10.1093/rheumatology/keq368. [DOI] [PubMed] [Google Scholar]
- 55.Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A. B S R B R Control Centre Consortium, Symmons DP; BSR Biologics Register: Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: Results from the British society for rheumatology biologics register (BSRBR) Ann Rheum Dis. 2010;69:522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saliu OY, Sofer C, Stein DS, Schwander SK, Wallis RS. Tumor-necrosis-factor blockers: Differential effects on mycobacterial immunity. J Infect Dis. 2006;194:486–492. doi: 10.1086/505430. [DOI] [PubMed] [Google Scholar]
- 57.Wallis RS. Mathematical modeling of the cause of tuberculosis during tumor necrosis factor blockade. Arthritis Rheum. 2008;58:947–952. doi: 10.1002/art.23285. [DOI] [PubMed] [Google Scholar]