Abstract
Hashimoto's thyroiditis (HT) is typically associated with insulin resistance. The aim of the present study was to investigate the role of regulatory B cells (Bregs) in insulin resistance in patients with HT. A total of 52 female patients with type I HT and 35 matched healthy volunteers were enrolled. Demographic and laboratorial data were collected. A 75 g oral glucose tolerance test was performed on each subject. Flow cytometry was performed to evaluate the levels of CD19+CD24hiCD38hi Bregs in peripheral blood. Patients with HT exhibited significantly higher postprandial insulin levels (P<0.01), but normal glucose levels. The level of CD19+CD24hiCD38hi Bregs in patients with HT decreased significantly (P=0.0002) compared with the controls. Pearson's linear correlation model revealed a significant, negative association between anti-thyroid peroxidase antibodies (TPOAb) and homeostasis model assessment of β cell (r=−0.313, P=0.014). The same correlation model revealed a significant, negative association between TPOAb and the disposition index (DI; r=−0.305, P=0.017), and between anti-thyroglobulin antibodies and DI (r=−0.321, P=0.013). Patients with a decreased ratio of CD19+CD24hiCD38hi Bregs to CD19+ lymphocytes exhibited higher levels of total cholesterol and low-density lipoprotein cholesterol. A decrease in the ratio of CD19+CD24hiCD38hi Bregs to lymphocytes was a significant independent risk factor for hyperinsulinemia (odds ratio=1.372, P=0.035). A decrease in peripheral blood CD19+CD24hiCD38hi Bregs is associated with insulin resistance in HT patients, and was an independent risk factor for postprandial hyperinsulinemia. The present study provided a novel insight into the development of effective therapeutic strategies targeting immune mechanisms associated with HT.
Keywords: Hashimoto's thyroiditis, insulin resistance, islet function, regulatory B cell
Introduction
Hashimoto's thyroiditis (HT) is a genetic autoimmune disorder, characterized by the destruction of thyroid cells by cell- and antibody-mediated immune responses. HT accounts for 7.3–20.5% of all thyroid diseases, with a marked female predominance (female-to-male ratio of 7:1) (1–3). Patients with HT, specifically those with clinical hypothyroidism, are particularly susceptible to developing insulin resistance and metabolic syndromes (2,4–7). A previous study by the current authors (8) revealed that patients with type I HT, that is HT patients with normal thyroid function, exhibited normal levels of fasting and postprandial plasma glucose. However, the early phase and total insulin secretion was significantly increased in the patients, indicating postprandial insulin resistance. These findings are consistent with previous results from Maratou et al (3). However, to the best of our knowledge, studies on the underlying correlative mechanisms between HT and insulin resistance remain inconclusive.
Regulatory B cells (Bregs), a newly identified B cell subset, have been demonstrated to have a role in the pathogenesis of autoimmune diseases (9). Bregs serve an essential role in the induction of immune suppression, primarily by the inhibition of other immune cells through cytokine secretion and antigen presentation. Bregs are considered to be comprised of two major subpopulations: CD19+CD24hiCD38hi and CD19+CD24+CD27+, both of which are capable of producing interleukin (IL)-10 upon stimulation (10). IL-10 is an essential anti-inflammatory cytokine, which inhibits T cell activation and inflammatory reaction, thus suppressing the immune response (11). Previous evidence indicated that Bregs deficiency may be associated with the pathogenesis of HT in mice (12). However, minimal work has been conducted to investigate the association between Bregs in the immune response and the occurrence of HT. Immune-mediated injury serves an important role in the pathogenesis of insulin resistance and diabetes mellitus (DM) (13,14). A previous study indicated that patients with HT had significantly increased peripheral blood CD19+CD24+CD27+ Bregs, and the increased percentage of CD19+CD24+CD27+ Bregs was correlated with fasting insulin secretion and fasting insulin resistance in patients with HT (8). However, postprandial secretion of insulin did not demonstrate a marked association with changes in the CD19+CD24+CD27+ Breg population (8). The results indicate potential contributions from other immune factors, which act during the later phases of insulin sensitivity. Thus, further studies are required to investigate additional subsets of Bregs in HT patients with insulin resistance.
In order to eliminate the effect of thyroid hormones on insulin signaling and glucose regulation in the present study, only patients with type I HT who had normal thyroid function and were not on hormone treatment were included. In addition, as HT occurs predominantly in women, only female patients were enrolled.
Materials and methods
Patients
The study population consisted of 61 female patients with type 1 HT in The Fifth People's Hospital of Shanghai, Fudan University (Shanghai, China) between March and November 2013. A total of 38 age-matched healthy volunteers were recruited as controls in the same period. The characteristics of the patients and healthy volunteers are presented in Table I. The patients were diagnosed with type 1 HT if they met the following criteria (15): Normal thyroid function; typical clinical manifestations with elevated serum anti-thyroid peroxidase antibodies (TPOAb) and/or anti-thyroglobulin antibodies (TGAb) (≥60 U/ml), or no evident clinical manifestations but had increased serum TPOAb and TGAb concentrations (≥60 U/ml) in two successive visits, at least one week apart. Patients were excluded if they fulfilled any of the following criteria: i) <18 or >60 years of age; ii) had a previously confirmed diagnosis of type 1 or type 2 DM; iii) recently taken anti-thyroid medication or thyroid hormone replacement therapy; iv) diagnosed with hyperthyroidism or hypothyroidism; v) cardiovascular and cerebrovascular diseases; vi) serious liver and kidney dysfunction; vii) malignancies; viii) severe mental disorders or poor communication capacity; ix) currently taking corticosteroids; or x) pregnant or breast-feeding women. DM and impaired glucose regulation (IGR) were diagnosed based on the World Health Organization guidelines, 1999 (16). A total of 9 (14.1%) patients with HT and 3 healthy individuals (7.8%) from the control group were diagnosed with DM via oral glucose tolerance test (OGTT) during the present study. These individuals were excluded from the study. Therefore, 52 females with type I HT and 35 matched healthy volunteers were ultimately enrolled. The present study was approved by the Institutional Review Board of Fudan University, and written informed consent was prospectively obtained from all study participants.
Table I.
Demographic characteristics and laboratory measurements of patients with HT and control groups.
| Characteristics and measurements | HT group (n=52) | Control group (n=35) | P-value |
|---|---|---|---|
| Age, years | 42.2±14.7 | 40.6±16.9 | NS |
| Waist circumference, cm | 76.4±9.4 | 77.1±8.7 | NS |
| Hip circumference, cm | 94.6±7.3 | 93.5±4.1 | NS |
| Waist-hip ratio | 0.9±0.3 | 0.9±0.3 | NS |
| Systolic blood pressure, mmHg | 122.5±8.8 | 119.7±22.9 | NS |
| Diastolic blood pressure, mmHg | 74.5±5.3 | 76.2±5.4 | NS |
| Body mass index, kg/m2 | 22.9±3.7 | 22.9±3.2 | NS |
| TCH, mmol/l | 4.8±1.2 | 4.5±0.9 | NS |
| TG, mmol/l | 1.4±0.8 | 1.1±0.8 | NS |
| HLD-C, mmol/l | 1.5±0.4 | 1.3±0.6 | NS |
| LDL-C, mmol/l | 3.3±1.3 | 3.0±0.7 | NS |
| FPG, mmol/l | 5.6±2.0 | 4.7±0.6 | NS |
| Fasting c-peptide, ng/ml | 1.7±1.8 | 1.2±0.7 | NS |
| Fasting insulin, mU/l | 12.4±16.8 | 11.6±14.1 | NS |
| 30 min plasma glucose, mmol/l | 8.6±2.1 | 7.7±1.7 | NS |
| 30 min c-peptide, ng/ml | 6.8±2.9 | 5.7±1.8 | 0.039 |
| 30 min insulin, mU/l | 80.8±21.6 | 47.9±19.3 | <0.001 |
| 120 min plasma glucose, mmol/l | 7.6±3.8 | 7.4±2.8 | NS |
| 120 min c-peptide, ng/ml | 6.1±4.1 | 5.7±2.8 | NS |
| 120 min insulin, mU/l | 71.2±3. | 28.9±36.0 | <0.001 |
| GADA, % | 0.0 | 0.0 | NS |
| IAA, % | 0.0 | 0.0 | NS |
| ICA, % | 0.0 | 0.0 | NS |
| IGR, % | 39.1 | 30.8 | NS |
| HOMA-IR | 2.5±1.9 | 1.8±1.0 | NS |
| HOMA-β | 132.4±95.7 | 187.1±153.6 | NS |
| ΔI30/ΔG30 | 20.3±18.5 | 17.3±13.2 | NS |
| Matsuda Index | 6.1±4.8 | 6.9±3.2 | NS |
| InsAUC30/GluAUC30 | 45.3±31.2 | 32.2±14.1 | 0.015 |
| InsAUC120/GluAUC120 | 61.9±38.5 | 45.1±22.2 | 0.047 |
| DI | 70.6±39.4 | 108.6±78.6 | 0.002 |
| DI30 | 216.9±161.4 | 196.3±89.1 | NS |
| DI120 | 284.5±127.6 | 260.0±107.8 | NS |
Data is presented as mean ± standard deviation. FPG, fasting plasma glucose; DI, disposition index; GADA, glutamic acid decarboxylase antibodies; HDL-C, high-density lipoprotein cholesterol; HOMA-β, homeostasis model assessment of β cell function; HOMA-IR, homeostasis model assessment of insulin resistance; HT, Hashimoto's thyroiditis; IAA, insulin autoantibodies; ICA, islet cell antibodies; IGR, impaired glucose regulation; InsAUC; area under curve of blood insulin level; GluAUC, area under curve of plasma glucose level; LDL-C, low-density lipoprotein cholesterol; NS, not significant; TCH, total cholesterol; TG, triglyceride; ΔI30/ΔG30, insulin/glucose ratio responses over the first 30 min during the oral glucose tolerance test.
Data collection
Demographic data associated with each participant was recorded, including age, weight, height, waist and hip circumferences, waist-to-hip ratio and systolic (SBP) and diastolic blood pressures (DBP). Hypertension was defined as systolic blood pressure >140 mmHg and/or a diastolic blood pressure >90 mmHg for three consecutive measurements. Body mass index (BMI) was calculated as kg/m2. Data from laboratory tests were also collected: Plasma glucose levels were measured using glucose assay kit (YZB/GER3922-2013, Roche Diagnostics GmbH, Mannheim, Germany) and a Roche biochemical analyzer cobas c 702 (16K5-05, Roche Diagnostics GmbH); serum lipid content, including total cholesterol (TCH), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), was measured using an enzymatic colorimetric assay (7600 Clinical Analyzer; Hitachi Ltd., Tokyo, Japan); serum levels of C-peptide and insulin, in addition to thyroid function, were measured using C peptide (YZB/GER5035-2014), insulin (20142404356), total triiodothyronin (YZB/GER5304-2014), total thyroxine (YZB/GER5606-2014), free triiodothyronin (YZB/GER6164-2014), free thyroxine (20142404445), thyroid stimulating hormone (YZB/GER5303-2014), TPOAb (YZB/GER5162-2014) and TGAb (YZB/GER1859-2014) kits (all Roche Diagnostics GmbH) and a Roche analyzer cobas 8000 (15L8-02, Roche Diagnostics GmbH).
Detection of peripheral blood lymphocyte subsets using flow cytometry
EDTA-anticoagulated whole blood samples (100 µl) were collected, aliquoted into two tubes, and incubated with the fluorescently-labeled monoclonal antibodies (1:20) anti-human CD19-phycoerythrin (PE)-CyTM5 (cat. no. HIB19), anti-human CD24-PE (cat. no. ML5) or anti-human CD38-fluorescein isothionate FITC (cat. no. HIT2) (BD Biosciences, Franklin Lakes, NJ, USA) for 20 min in the dark at room temperature. Isotypes of these antibodies (1:20) including CD19-immunoglobulin (Ig)G1-PE-CyTM5 (cat. no. MOPC21), CD24-IgG2a-PE (cat. no. G155-178) and CD38-IgG1-FITC (cat. no. MOPC21) (BD Biosciences) were used as controls. All antibodies were incubated in the dark at room temperature for 20 min. In order to lyse erythrocytes the stained samples were treated with Simultest solution A (Genewiz, Inc., South Planfield, NJ, USA) for 15 min at room temperature and then washed with phosphate buffered saline (PBS) twice. Next they were treated with Simultest solution B (Genewiz, Inc.) and washed with PBS twice prior to flow cytometric analysis. A FACSCalibur flow cytometer with CellQuest 5.0 software (BD Biosciences) was used to analyze the levels of CD19+CD24higCD38hig subpopulations.
Definition of variables
A 75 g oral glucose tolerance test (OGTT) was performed on each patient and the plasma insulin and glucose levels were measured at 0, 30 and 120 min. The levels of plasma glucose, serum lipid, C-peptide and insulin were measured, and thyroid function was assessed. The insulin level in the blood at time points 0, 30, and 120 min were designated as Ins0, Ins30 and Ins120, respectively. In addition, the plasma glucose levels at these time points were designated as Glu0, Glu30, and Glu120, and the mean glucose and insulin levels during OGTT were designated as Glumean and Insmean, respectively. The area under the curve (AUC) for insulin and glucose (InsAUC and GluAUC) was determined using the insulin secretion curve adjusted for glucose. The islet function and sensitivity index included the following: i) A homeostasis model assessment of β cell function (HOMA-β)=[20 × Ins0 (µIU/ml)]/[Glu0 (mmol/l)-3.5]; ii) the early insulin secretion index: insulin/glucose ratio responses over the first 30 min during the OGTT (ΔI30/ΔG30) =[(Ins30-Ins0) (µIU/ml)]/[(Glu30-Glu0) (mmol/l)]; iii) AUC for early-phase insulin secretion (corrected for glucose): InsAUC30/GluAUC30=[(Ins0+Ins30) (pmol/l)]/[(Glu0 + Glu30) (mmol/l)]; iv) AUC for total insulin secretion: InsAUC120/GluAUC120=[(Ins0 + 4 × I ns30 + 3 × Ins120) (pmol/l)]/[(Glu0 + 4 × Glu30 + 3 × Glu120) (mmol/l)]; v) the HOMA of insulin resistance (HOMA-IR)=Ins0 (µIU/ml) × Glu0 (mmol/l)/22.5; vi) the Matsuda insulin sensitivity index (ISIM)=10,000/[(Glu0 (mg/dl) × Ins0 (uIU/ml) × GluMean (mg/dl) × InsMean (uIU/ml)]0.5; vii) the disposition index (DI)=HOMA-β/HOMA-IR, representing an adjusted insulin sensitivity according to HOMA-IR; and viii) the early-phase disposition index (DI30) and the total disposition index (DI120) during the OGTT, which were calculated using the formula DI30=InsAUC30/GluAUC30 × ISIM and DI120=InsAUC120/GluAUC120 × ISIM, respectively.
Statistical analysis
All data were analyzed using the SPSS software package (version 17.0; SPSS, Inc., Chicago, IL, USA). Continuous data are expressed as mean ± standard deviation when normally distributed, or median and quartiles (25th percentile, 75th percentile) when not normally distributed. Categorical data are presented as absolute value and percentage. Comparisons of continuous variables between the groups were performed using Student's t-test, one-way analysis of variance, or non-parametric tests (the Mann-Whitney U test or Kruskal-Wallis H) when appropriate. The proportions were compared using the χ2 test. Correlations between data were analyzed using the Pearson linear correlation model. Multivariate logistic regression analysis was performed to assess the risk of hyperinsulinemia with reduced CD19+CD24higCD38hig Bregs, with results reported as relative risks (RR) and 95% confidence interval (CI). P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of laboratory findings between HT and control groups
The demographic characteristics of the patients with HT and healthy controls are presented in Table I. Patients with HT exhibited significantly higher insulin levels at 30 and 120 min during OGTT in comparison with their controls (P<0.001; Table I); whereas no similar trends were identified with glucose concentrations. No significant differences were observed regarding age, waist circumference, SBP, DBP, TCH, TG, HDL-C and LDL-C between HT and control groups. In addition, no significant differences were indicated in the prevalence of IGR between the two groups.
Comparison of β-cell function and insulin sensitivity between HT and control groups
Patients with HT exhibited higher values of InsAUC30/GluAUC30 and InsAUC120/GluAUC120, the indicators reflecting early-phase insulin release and total insulin release, respectively, compared with their controls (P=0.015 and 0.047, respectively; Table I). Patients in both groups had similar values for HOMA-β, HOMA-IR in addition to the Matsuda index. Patients with HT exhibited a significant decrease in DI, which indicates the fasting islet compensation function (P=0.002). However, indicators reflecting insulin function did not exhibit a relative decrease at 30 and 120 min during OGTT (DI 30 and DI 120, P>0.05). These results suggested that HT patients exhibited an increase in postprandial insulin secretion, but a relative lack in fasting insulin compensation.
Comparison of the percentages of CD19+CD24hiCD38hi Bregs subpopulations between HT and control groups
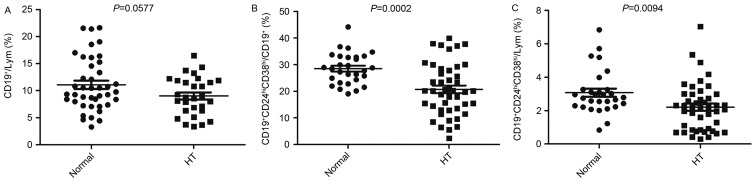
The percentages of peripheral blood CD19+ B lymphocytes were markedly decreased in the HT group when compared with the control group, although this decrease was not significant (P=0.0577; Fig. 1A). The percentages of CD19+CD24hiCD38hi Bregs cells from the CD19+ B cells and all the lymphocytes in HT patients decreased significantly when compared with the controls (P=0.0002 and 0.0094, respectively; Fig. 1B and C).
Figure 1.
Percentage peripheral blood CD19+CD24hiCD38hi Bregs decreased in patients with HT. The peripheral blood level of (A) CD19+ B Lym, (B) CD19+CD24hiCD38hi/CD19+ and (C) CD19+CD24hiCD38hi/Lym cells. Bregs, regulatory B cells; HT, Hashimoto's thyroiditis; Lym, lymphocytes; CD19+CD24hiCD38hi/CD19+, ratio of CD19+CD24hiCD38hi Bregs to CD19+ Lym; CD19+CD24hiCD38hi/Lym, ratio of CD19+CD24hiCD38hi Bregs to Lym; Normal, control.
Association between thyroid antibodies and β-cell function or insulin sensitivity
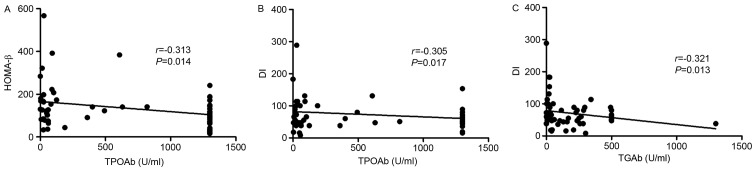
Pearson linear correlation model revealed a significant negative association between TPOAb and HOMA-β (r=−0.313, P=0.014) and between TPOAb and DI (r=−0.305, P=0.017), suggesting fasting insulin secretion and a β-cell function decrease as TPOAb titer increased (Fig. 2A and B). A significant negative association was also identified between TGAb and DI (r=−0.321, P=0.013; Fig. 2C), indicating that a high TGAb titer was closely associated with a decrease in islet β-cell function.
Figure 2.
Correlation between thyroid antibodies and β-cell function or insulin sensitivity demonstrated using Pearson's linear correlation model. Negative association between (A) TPOAb and HOMA-β, (B) TPOAb and DI, and (C) TGAb and DI. TPOAb, anti-thyroid peroxidase antibodies; HOMA-β, homeostasis model assessment of β cell function; TGAb, anti-thyroglobulin antibodies; DI, disposition index, representing adjusted insulin sensitivity according to insulin sensitivity (HOMA-β/homeostasis model assessment of insulin resistance).
Decrease in ratio of CD19+CD24hiCD38hi Bregs to lymphocytes (CD19+CD24hiCD38hi/L) significantly increases morbidity associated with IGR
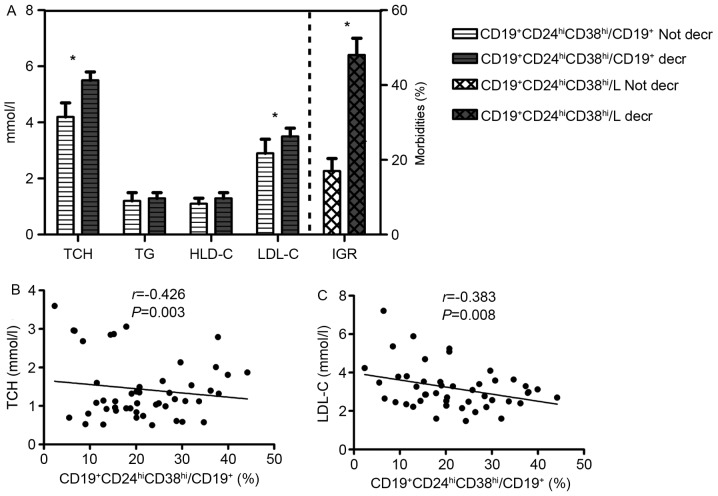
A reference range for CD19+CD24hiCD38hi/L was obtained using the interquartile range (25-75th percentile) of the percentages of CD19+CD24hiCD38hi/L in the control group. According to the reference range, all study subjects were divided into two groups: those with a lower CD19+CD24hiCD38hi/L compared with the reference range (<1.925%; decreased group), and those with a ratio of CD19+CD24hiCD38hi/L within the reference range (≥1.925%; normal group). The decrease in CD19+CD24hiCD38hi/L was demonstrated to increase the morbidities of IGR (Fig. 3).
Figure 3.
Clinical implication of CD19+CD24hiCD38hi Bregs in patients with HT. (A) The decrease in ratio of CD19+CD24hiCD38hi Bregs to lymphocytes (CD19+CD24hiCD38hi/L) significantly increases the morbidity associated with DM; HT patients with decreased CD19+CD24hiCD38hi/L (n=20) had significantly higher levels of TCH and LDL-C, compared with those without (n=64). The decrease in the ratio of CD19+CD24hiCD38hi Bregs to CD19+ lymphocytes (CD19+CD24hiCD38hi/CD19+) was associated with high (B) TCH and (C) LDL-C levels. *P<0.05 vs. Not decr. Data is presented as mean ± standard deviation. Bregs, regulatory B cells; HT, Hashimoto's thyroiditis; CD19+CD24hiCD38hi/L, ratio of CD19+CD24hiCD38hi Bregs to lymphocytes; DM, diabetes mellitus; HDL-C, high-density lipoprotein cholesterol; IGR, impaired glucose regulation; LDL-C, low-density lipoprotein cholesterol; TCH, total cholesterol; TG, triglyceride.
Decreased ratio of CD19+CD24hiCD38hi Bregs to CD19+ lymphocytes (CD19+CD24hiCD38hi/CD19+) associated with high TCH and LDL-C levels
Patients were divided into two groups, those with a lower CD19+CD24hiCD38hi/CD19+ ratio (<21.145%, decreased group) and those with a normal CD19+CD24hiCD38hi/CD19+ ratio (≥21.145%; normal group), according to the reference range from healthy controls. As presented in Fig. 3, TCH and LDL-C were significantly increased in the decreased group (P<0.05), and the ratio of CD19+CD24hiCD38hi/CD19+ was significantly negatively correlated with both TCH (r=−0.426, P=0.003) and LDL-C (r=−0.383, P<0.008), suggesting that the decreased ratio of CD19+CD24hiCD38hi/CD19+ is accompanied by dyslipidemia.
A decrease in CD19+CD24hiCD38hi/L ratio is an independent risk factor of hyperinsulinemia
Hyperinsulinemia was defined as a fasting insulin ≥15 mU/l, and/or insulin levels at 2 h ≥80 mU/l during OGTT. Multivariate logistic regression analysis was performed to assess the association between a decreased percentage of Bregs subsets and hyperinsulinemia. The results indicated that decreases in both CD19+CD24hiCD38hi/CD19+ and CD19+CD24hiCD38hi/L were independent risk factors for hyperinsulinemia. After adjusting for confounding factors including TPOAb, TGAb, age and gender, there was a 1.051-fold higher risk of developing hyperinsulinemia with a decreasing CD19+CD24hiCD38hi/CD19+ ratio (95% CI: 1.003–1.102, P=0.038). Patients with a decreased CD19+CD24hiCD38hi/L ratio had a 1.44-fold higher risk of developing hyperinsulinemia (95% CI: 1.076–1.926, P=0.014; Table II). Following further adjustment for height, weight, BMI, waist and hip circumferences and blood pressure, a decreased CD19+CD24hiCD38hi/L ratio was associated with a 1.37-fold higher likelihood of developing hyperinsulinemia (OR: 1.372; 95% CI: 1.022–1.843, P=0.035, Table II), and was an independent risk factor for predicting hyperinsulinemia.
Table II.
Logistic regression analysis for predicting hyperinsulinemia.
| Models | B | SE | P-value | OR (95% CI) |
|---|---|---|---|---|
| Model I | ||||
| −Decrease in CD19+CD24hiCD38hi/CD19+ | 0.043 | 0.022 | 0.041 | 1.044 (1.0011.090) |
| −Decrease in CD19+CD24hiCD38hi/L | 0.338 | 0.122 | 0.006 | 1.402 (1.103–1.782) |
| Model II | ||||
| −Decrease in CD19+CD24hiCD38hi/CD19+ | 0.05 | 0.024 | 0.038 | 1.051 (1.003–1.102) |
| −Decrease in CD19+CD24hiCD38hi/L | 0.365 | 0.148 | 0.014 | 1.440 (1.076–1.926) |
| Model III | ||||
| −Decrease in CD19+CD24hiCD38hi/L | 0.046 | 0.024 | 0.035 | 1.372 (1.022–1.843) |
Model I, variables were introduced into the logistic regression model; Model II, adjustment for confounding factors including anti-thyroid peroxidase antibodies, anti-thyroglobulin antibodies, age and gender; Model III, further adjustment for height, weight, body mass index, waist and hip circumferences, and blood pressure. B, regression coefficient; SE, standard error; OR, odds ratio; CI, confidence interval; L, lymphocytes.
Discussion
HT is an autoimmune disease, that may be accompanied by insulin resistance and metabolic syndrome (2–6). The present study is, to the best of our knowledge, the first to provide evidence that female patients with type I HT have increased early phase and total insulin secretion, and a decrease in peripheral blood CD19+CD24hiCD38hi Bregs. This is an independent risk factor for hyperinsulinemia, and serves a critical role in development of hyperinsulinemia in those patients.
Insulin resistance is a state or condition in which the body fails to properly use insulin to regulate glucose metabolism. The accumulation of excessive insulin in the blood, described as hyperinsulinemia, with normal blood glucose levels indicates the presence of insulin resistance (17,18). Insulin resistance occurs in association with various medical conditions, including type 2 DM, metabolic syndromes, obesity, hypertension and dyslipidemia (19). The current study revealed that levels of fasting and postprandial glucose levels remained normal during OGTT in the HT group. However, patients with HT had an increase in postprandial insulin secretion, indicating the occurrence of insulin resistance in those patients. There is evidence to suggest that the fasting levels of c-peptide, insulin and TGs are markedly higher in HT patients than in the control individuals (20). A previous study by Dimitriadis et al (7) demonstrated that, despite the increase in plasma insulin levels in hypothyroidism, net glucose uptake in the forearm muscles and liver is reduced after eating, suggesting the presence of insulin resistance and a compensatory increase in insulin secretion in patients with HT. These findings are consistent with the results of the present study.
At present, few studies have investigated the mechanism of insulin resistance in HT patients. One study demonstrated that clinical or subclinical hypothyroidism is associated with insulin resistance, potentially due to impaired translocation of glucose transporter type 4 (GLUT4) on the plasma membrane (3). Chronic inflammation and autoimmunity as a result of immune dysregulation has been implicated in insulin resistance and pathogenesis of DM (21,22). In patients with type 2 DM, CD4+T, CD8+T and T helper 17 cells (Th17) reduced glucose uptake (including translocation of GLUT4 on the plasma membrane) and utilization in muscle and liver tissue. The activation and proliferation of CD4+T, CD8+T and Th17 cells were regulated by multiple factors, specifically Bregs. It has been reported that the frequency in occurrence of Bregs is decreased in patients with DM, triggering a decrease in the production of the anti-inflammatory cytokine IL-10 from those Bregs (23,24). The findings of the current study have a number of similarities with previous investigations. In the present study, the percentage of CD19+CD24hiCD38hi Bregs decreased significantly in patients with HT, and the decrease in CD19+CD24hiCD38hi/L was an independent risk factor for predicting hyperinsulinemia following adjustment for confounders including age, gender, TPOAb and TGAb, revealing the involvement of CD19+CD24hiCD38hi Bregs in patients with HT and hyperinsulinemia. One possible hypothesis may be that chronic inflammatory injury exists in muscle, liver tissues and islets of patients with type I HT, which is similar to patients with type 2 DM. The decrease in CD19+CD24hiCD38hi Bregs, which serve as immunosuppressive cells, leads to a reduced ability to suppress immune cell activation, thereby resulting in a disorder of glucose metabolism.
The present study also indicated that patients with HT and a decreased CD19+CD24hiCD38hi/L ratio exhibited significantly increased levels of serum TCH and LDL-C. Lipotoxicity serves an important role in insulin resistance and impaired islet function (25). Therefore, CD19+CD24hiCD38hi Bregs associated with insulin resistance contribute to immunosuppressive effects and also interfere with lipid metabolism. The current study only included patients with type 1 HT and normal thyroid function. These patients were also positive for TPOAb and TGAb. TPOAb and TGAb are autoantibodies for thyroid antigens. TPOAb and TGAb were demonstrated to be negatively associated with the fasting insulin secretion (HOMA-β) and fasting insulin compensation (DI). Consequently, DI gradually decreases with a concomitant increase in TPOAb and TGAb, revealing a close association between a high thyroid antibody titer and reduced fasting islet compensation function. The present study speculated that impaired fasting compensation function may be associated with the presence of thyroid antibodies in patients with type I HT. Further investigation is required to determine if TPOAb and TGAb are able to act as immune indicators of insulin resistance and islet β-cell function in patients with HT.
In the present study, no significant difference was identified in the prevalence of DM and IGR between patients with and without HT, which is consistent with previous studies (3,7,20). However, based on findings by Gierach et al (26) diabetes has been confirmed in 27.8% of patients with HT. Impaired fasting glycaemia or IGR occurs in 16.6% of HT patients, indicating that 44.4% of HT patients present with different types of glucose metabolism disorders. One of the reasons for these findings is that all patients recruited in the Gierach study were enrolled from the Department of Endocrinology. Typically, patients with HT did not require hospitalization unless they presented with serious complications caused by hypothyroidism or diabetes. Secondly, patients in the Gierach study had a higher mean age than those in the present study (59.9 vs. 42.2 years). The prevalence of diabetes increases gradually with age. Therefore, it may be the case that both of these studies overestimate the prevalence of DM and IGR in HT.
In conclusion, the results of the present study indicate significant increases in the early phase insulin secretion and total insulin secretion and a decrease in DI during OGTT in patients with HT and normal thyroid function. The decrease in peripheral blood CD19+CD24hiCD38hi Bregs involved in insulin resistance was an independent risk factor for postprandial hyperinsulinemia. The role of CD19+CD24hiCD38hi Bregs in insulin resistance contributes not only to the immunosuppressive effects, but also to the associated interference with lipid metabolism.
Acknowledgements
This study was funded by grant from the Scientific Research Plan Project of Health and Family Planning Commission of Shanghai (grant no. 201440514).
References
- 1.Hayashi N, Tamaki N, Konishi J, Yonekura Y, Senda M, Kasagi K, Yamamoto K, Iida Y, Misaki T, Endo K, et al. Sonography of Hashimoto's thyroiditis. J Clin Ultrasound. 1986;14:123–126. doi: 10.1002/jcu.1870140208. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Real JM, López-Bermejo A, Castro A, Casamitjana R, Ricart W. Thyroid function is intrinsically linked to insulin sensitivity and endothelium-dependent vasodilation in healthy euthyroid subjects. J Clin Endocrinol Metab. 2006;91:3337–3343. doi: 10.1210/jc.2006-0841. [DOI] [PubMed] [Google Scholar]
- 3.Maratou E, Hadjidakis DJ, Kollias A, Tsegka K, Peppa M, Alevizaki M, Mitrou P, Lambadiari V, Boutati E, Nikzas D, et al. Studies of insulin resistance in patients with clinical and subclinical hypothyroidism. Eur J Endocrinol. 2009;160:785–790. doi: 10.1530/EJE-08-0797. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Zhou G, Ozaki T, Nishihara E, Matsuzuka F, Bai Y, Liu Z, Taniguchi E, Miyauchi A, Kakudo K. Distinct histopathological features of Hashimoto's thyroiditis with respect to IgG4-related disease. Mod Pathol. 2012;25:1086–1097. doi: 10.1038/modpathol.2012.68. [DOI] [PubMed] [Google Scholar]
- 5.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 6.Jornayvaz FR, Lee HY, Jurczak MJ, Alves TC, Guebre-Egziabher F, Guigni BA, Zhang D, Samuel VT, Silva JE, Shulman GI. Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology. 2012;153:583–591. doi: 10.1210/en.2011-1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimitriadis G, Mitrou P, Lambadiari V, Boutati E, Maratou E, Panagiotakos DB, Koukkou E, Tzanela M, Thalassinos N, Raptis SA. Insulin action in adipose tissue and muscle in hypothyroidism. J Clin Endocrinol Metab. 2006;91:4930–4937. doi: 10.1210/jc.2006-0478. [DOI] [PubMed] [Google Scholar]
- 8.Yang M, Du C, Wang Y, Liu J. Increased CD19+CD24+CD27+ B regulatory cells are associated with insulin resistance in patients with type I Hashimoto's thyroiditis. Mol Med Rep. 2017;15:4338–4345. doi: 10.3892/mmr.2017.6507. [DOI] [PubMed] [Google Scholar]
- 9.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–132. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 11.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. B cell-deficient NOD.H-2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med. 2006;203:349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serreze DV, Chapman HD, Niens M, Dunn R, Kehry MR, Driver JP, Haller M, Wasserfall C, Atkinson MA. Loss of intra-islet CD20 expression may complicate efficacy of B-cell-directed type 1 diabetes therapies. Diabetes. 2011;60:2914–2921. doi: 10.2337/db11-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler AI, Le Page MA, Maxwell MJ, Stolp J, Guo H, Jayasimhan A, Hibbs ML, Santamaria P, Miller JF, Plebanski M, et al. The CD19 signalling molecule is elevated in NOD mice and controls type 1 diabetes development. Diabetologia. 2013;56:2659–2668. doi: 10.1007/s00125-013-3038-2. [DOI] [PubMed] [Google Scholar]
- 15.Slatosky J, Shipton B, Wahba H. Thyroiditis: Differential diagnosis and management. Am Fam Physician. 2000;61:1047–1052. 1054. [PubMed] [Google Scholar]
- 16.World Health Organization, corp-author. Definition, diagnosis and classification of Diabetes mellitus and its complication: WHO/NCD NCs. 1999:31–33. [Google Scholar]
- 17.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diabetes.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 18.Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: Similarities and differences. J Clin Hypertens (Greenwich) 2011;13:238–243. doi: 10.1111/j.1751-7176.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuel VT, Shulman GI. Mechanisms for insulin resistance: Common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matejková-Behanová M, Zamrazil V, Vondra K, Vrbíková J, Kucera P, Hill M, Andel M. Autoimmune thyroiditis in non-obese subjects with initial diagnosis of Type 2 diabetes mellitus. J Endocrinol Invest. 2002;25:779–784. doi: 10.1007/BF03345512. [DOI] [PubMed] [Google Scholar]
- 21.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 22.Donath MY, Størling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: A link between type 1 and type 2 diabetes. J Mol Med (Berl) 2003;81:455–470. doi: 10.1007/s00109-003-0450-y. [DOI] [PubMed] [Google Scholar]
- 23.Jagannathan M, McDonnell M, Liang Y, Hasturk H, Hetzel J, Rubin D, Kantarci A, Van Dyke TE, Ganley-Leal LM, Nikolajczyk BS. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci USA. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Prato S. Role of glucotoxicity and lipotoxicity in the pathophysiology of Type 2 diabetes mellitus and emerging treatment strategies. Diabet Med. 2009;26:1185–1192. doi: 10.1111/j.1464-5491.2009.02847.x. [DOI] [PubMed] [Google Scholar]
- 26.Gierach M, Gierach J, Skowrońska A, Rutkowska E, Spychalska M, Pujanek M, Junik R. Hashimoto's thyroiditis and carbohydrate metabolism disorders in patients hospitalised in the department of endocrinology and diabetology of ludwik rydygier collegium medicum in bydgoszcz between 2001 and 2010. Endokrynol Pol. 2012;63:14–17. [PubMed] [Google Scholar]