Abstract
Ellagic acid has been proven to have anticancer, antimutation, antimicrobial and antiviral functions. The present study investigated whether treatment with ellagic acid was able to prevent tetrachloride (CCl4)-induced cirrhosis through the inhibition of reactive oxygen species (ROS) formation and angiogenesis. CCl4 diluted in olive oil at a final concentration of 10% was used to induce a cirrhosis model. A total of 40 mice were random allocated into four groups, as follows: Control, cirrhosis model, 7.5 mg/kg ellagic acid and 15 mg/kg ellagic acid groups. In the control group, mice were given normal saline. The results indicated that ellagic acid exerted a protective effect, evidently preventing CCl4-induced cirrhosis. In addition, treatment with ellagic acid significantly inhibited collagen I and inducible nitric oxide synthase protein expression levels in CCl4-induced cirrhosis mice. Oxidative stress and ROS formation were also significantly reduced by ellagic acid treatment. The protein expression levels of vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2), and the caspase-3 activity were significantly inhibited by treatment with ellagic acid. In conclusions, these results suggest that ellagic acid exerted protective effects against CCl4-induced cirrhosis through the inhibition of ROS formation and angiogenesis.
Keywords: ellagic acid, tetrachloride-induced cirrhosis
Introduction
Liver cirrhosis is a chronic liver disease characterized by diffuse liver fibrosis, and formation of pseudolobules and regenerative nodules (1). Clinically, cirrhosis affects multiple systems, with manifestations including liver function lesion and portal hypertension (2). At advanced stages, it has severe complications, such as hemorrhage of the digestive tract, hepatic encephalopathy and secondary infection (3). The main pathogenesis factor of liver cirrhosis is viral hepatitis, while it may also be caused by cholestasis, circulatory disturbance, and virulence drugs, metabolic disorders, dystrophia, immunologic derangement and other unclear causes (4).
Oxidative damage is associated with numerous chronic diseases. Mice with liver cirrhosis have been demonstrated to present with evident imbalance of oxidization and oxidation resistance (5). Under normal conditions, the liver oxidative system is dynamically balanced with the antioxidant system (6). Factors causing liver injuries lead to lipid peroxidation of hepatic tissues through activation of oxygen molecules (7). The synergistic effects of oxygen molecules increase the free radical production and lipid peroxidation, thus damaging the liver. Subsequently, deterioration of the inflammation reaction is observed, causing liver injury (8,9). Such pathological changes result in difficulty in the treatment of liver cirrhosis patients (10).
Ellagic acid (Fig. 1) has various biological activities, including antioxidant, anti-mutation and inhibitory effects on human immunodeficiency virus (11). In addition, ellagic acid is an effective coagulant and has favorable inhibition effects on various bacteria and viruses (12). Treatment with ellagic acid has been observed to protect wound surface from the invasion of bacteria and from infection, inhibiting ulceration (13). Furthermore, it has been demonstrated that ellagic acid has depressurization and sedation functions (14). However, whether ellagic acid prevent cirrhosis has not yet been reported. Therefore, the present study aimed to examine whether the protective effects of ellagic acid prevented tetrachloride (CCl4)-induced cirrhosis and to assess the potential mechanisms.
Figure 1.
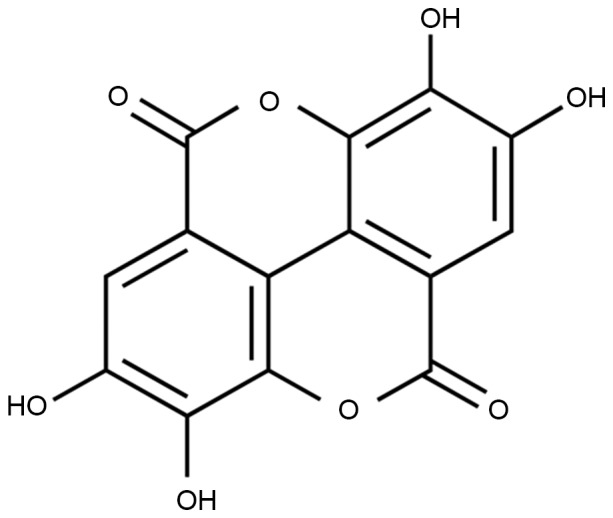
Chemical structure of ellagic acid.
Materials and methods
Animal model of cirrhosis
All study protocols were approved by the Animal Ethics Committee of The Affiliated Hospital of Guizhou Medical University (Guiyang, China). A total of 40 specific-pathogen-free C57BL/6 male mice (weight, 20±2 g; age, 6–7 weeks) were acquired by the Experimental Animal Center of Guizhou Medical University and maintained on standard chow in an 25.1±1°C atmosphere with a 12-h dark/light cycle. Next, 40 mice were random allocated into four groups as follows: Control, CCl4-induced cirrhosis model, 7.5 mg/kg ellagic acid and 15 mg/kg ellagic acid groups. In the control group, mice were given normal saline (100 µl; 11 weeks; via oral gavage, once daily for two days). CCl4 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was diluted into olive oil at a final concentration of 10%, and then this was used to induce a cirrhosis model by administration three times a week for 6 weeks, at a dose of 2 ml/kg each time (intraperitoneal injection). CCl4-injected mice were divided into a further three groups (n=10 each) after 6 weeks of CCl4 administration, including the cirrhosis model, 7.5 mg/kg ellagic acid and 15 mg/kg ellagic acid groups for 5 weeks.
Serum biochemistry analysis
Whole blood samples were collected from the eye socket using 2–3% chloral hydrate and centrifuged at 1,000 × g for 20 min at 4°C to collect serum. Serum levels of alanine aminotransferase (ALT; C009-2), aspartate aminotransferase (AST; C010-2) and albumin (ALB; A028-1; all Nanjing Jiancheng Biology Engineering Institute) were read at a wavelength of 560 nm using a spectrophotometer (GE Healthcare Life Sciences, Chicago, IL, USA).
Hepatic histopathology
Following treatment with ellagic acid, mice were sacrificed via decollation after anesthetization with 30 mg/kg pentobarbital sodium (Sinopharm Chemical Reagent Co., Ltd.). Following treatment with ellagic acid, liver tissue samples were acquired using 10% chloral hydrate and fixed with 4% formaldehyde. Next, the tissue samples were dehydrated in a graded ethanol series, cleared in xylene and embedded in paraffin. The tissues were then sectioned into 4-µm samples and stained hematoxylin and eosin (H&E). Images were captured using a microscope (TCS SP2; Leica Microsystems GmbH, Wetzlar, Germany).
Analysis of collagen I content of tissues
Total RNA from liver tissue samples was isolated using TRI reagent (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer's protocol. Next, 2 µg total RNA was subjected to reverse transcription using a c-DNA synthesis kit (Revert Aid First Strand c-DNA synthesis kit; Fermentas; Thermo Fisher Scientific, Inc., Waltham, MA, USA). cDNA was then amplified by polymerase chain reaction (PCR) using specific primers of collagen I: Forward, 5′-CAGAGTGGAAGAGCGATTA-3′, and reverse, 5′-CAAGGACAGTGTAGGTGAA-3′. Amplification was performed in a DNA Thermal Cycler (Applied Biosystems; Thermo Fisher Scientific, Inc.) at 95°C for 10 min, followed by 40 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 40 sec.
Reactive oxygen species (ROS) formation
Liver tissue (50 mg) sections were collected and homogenized for 10 sec at 12,000 × g for 10 min at 4°C. Total protein in the samples was measured using a BCA assay reagent (Beyotime Institute of Biotechnology, Nanjing, China). Subsequently, 30 µg protein was used to measure ROS formation (S0033; Beyotime Institute of Biotechnology) Cells were incubated with DCFH-DA for 20 min at 37°C, and washed with PBS. ROS formation was measured at a wavelength of 488 nm and emission wavelength of 525 nm using a spectrophotometer (GE Healthcare Life Sciences, Chicago, IL, USA).
Oxidative stress assay
Whole blood samples were collected from the eye socket using 10% chloral hydrate and centrifuged at 1,000 × g for 20 min at 4°C to collect serum. After cooling to room temperature, 150 µl serum was used to detect the activities of glutathione peroxidase (GSH-Px; A005), glutathione (GSH; A006-2), superoxide dismutase (SOD; A001-3) and malondialdehyde (MDA; A003-1; all Nanjing Jiancheng Biology Engineering Institute) using ELISA kits and a spectrophotometer (GE Healthcare Life Sciences) according to the manufacturer's instructions.
Western blot analysis
Following treatment with ellagic acid, liver tissue samples were acquired and homogenized for 10 sec at 12,000 × g for 10 min at 4°C. Total protein was measured using a BCA assay reagent (Beyotime Institute of Biotechnology), and then 50 µg protein was subjected to 10–12% SDS-PAGE and electrotransferred onto a polyvinylidene difluoride membrane (BD Biosciences, San Jose, CA, USA). The membrane was blocked in 5% non-fat milk in phosphate-buffered saline (PBS; pH 7.4) for 2 h, and subsequently incubated overnight at 4°C with the following primary antibodies: Anti-inducible nitric oxide synthase (iNOS; 1:400; sc-649; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-vascular endothelial growth factor (VEGF; 1:500; sc-13083; Santa Cruz Biotechnology, Inc.), anti-VEGF receptor 2 (VEGFR2; 1:3,000; ab11939; Abcam, Cambridge, UK) and β-actin (1:500; sc-7210; Santa Cruz Biotechnology, Inc.). Next, the membranes were washed with PBS and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG secondary antibody (1:5,000; 7074; Cell Signaling Technology, Inc., Danvers, MA, USA) at room temperature for 2 h. Protein expression in the samples was detected by Amersham ECL Prime western blotting detection reagent (GE Healthcare Life Sciences, Piscataway, NJ, USA) and analyzed using AlphaEase FC (FluorChem FC2) software (Cell Biosciences Inc., Santa Clara, CA, USA).
Determination of caspase-3 activity
Following treatment with ellagic acid, liver tissue samples were acquired and homogenized via centrifugation at 12,000 × g for 10 min at 4°C. Total protein was measured using BCA assay reagent (Beyotime Institute of Biotechnology), and 5 µg protein was incubated with Ac-DEVD-pNA-caspase-3 activity kit (C1115; Beyotime Institute of Biotechnology) for 30 min at room temperature. The absorbance values were assessed at 405 nm using a spectrophotometer (GE Healthcare Life Sciences) according to the manufacturer's protocol.
Statistical analysis
Data are expressed as the mean ± standard error of the mean. Statistically significant differences between the groups were determined using the one-way analysis of variance test, followed by the post-hoc Tukey's test. P<0.05 was considered to indicate statistically significant differences.
Results
Protective effects of ellagic acid against CCl4-induced cirrhosis
At the beginning of the experiment, the protective effects of ellagic acid against CCl4-induced cirrhosis were determined. As shown in Fig. 2 (P=0.0018, 0.0073 and 0.0089), the serum levels of ALT, AST and ALB in the cirrhosis model mice were effectively increased when compared with those in the control group. Following treatment with ellagic acid for 5 weeks, the serum levels of ALT, AST and ALB were effectively reduced in the cirrhosis mice (Fig. 2; P=0.0056, 0.0093 and 0.0072). These data suggest that ellagic acid reduced ALT, AST and ALB serum levels and thus may serve as an adjuvant for the treatment of cirrhosis.
Figure 2.
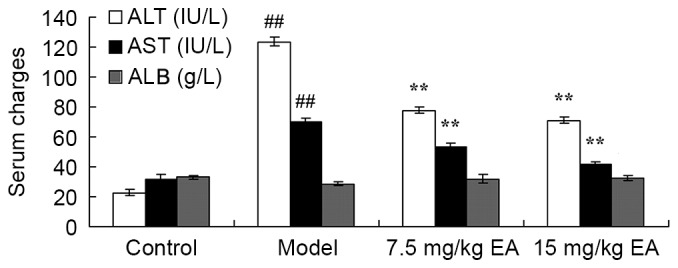
Serum levels of ALT, ALB and AST in cirrhosis mice showing the protective effects of EA against CCl4-induced cirrhosis. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Hepatic histopathological changes following ellagic acid treatment
H&E staining of liver tissue samples was used to analyze the effect of ellagic acid in the cirrhosis mice. As shown in Fig. 3, CCl4-induced cirrhosis was markedly observed in mice of the model group, compared with the control mice. However, ellagic acid treatment was found to effectively inhibit the cirrhosis degree compared with the model mice (Fig. 3). These data suggest that ellagic acid remitted hepatic histopathological changes and thus may be a novel therapeutic option for the treatment of cirrhosis.
Figure 3.
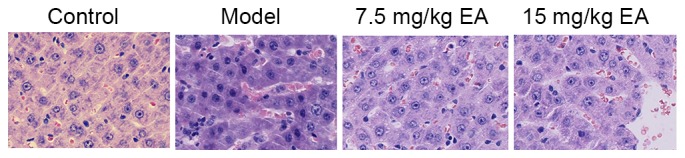
Hematoxylin and eosin staining of liver tissue samples from all groups, showing the protective effect of EA treatment against cirrhosis in mice (magnification, ×10). EA, ellagic acid.
Ellagic acid reduces collagen I gene expression in CCl4-induced cirrhosis mice
The collagen I gene expression in CCl4-induced cirrhosis model mice was detected by PCR analysis and found to be significantly increased compared with the control group (Fig. 4; P=0.0021). However, treatment with ellagic acid effectively inhibited the collagen I gene expression in cirrhosis mice (Fig. 4; P=0.0056). These data suggest that ellagic acid reduced collagen I gene expression and may thus be used to treat cirrhosis.
Figure 4.
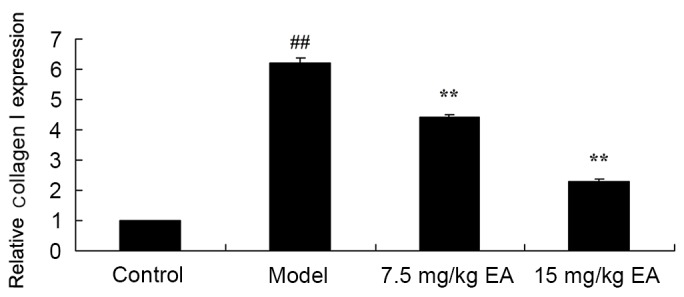
Collagen I gene expression in liver tissues of CCl4-induced cirrhosis mice treated with EA, as determined by polymerase chain reaction. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid.
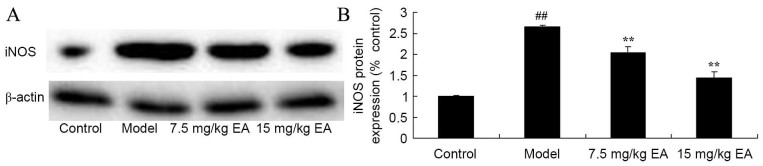
Ellagic acid inhibits iNOS protein expression in CCl4-induced cirrhosis mice
iNOS protein expression in tissue samples is shown in Fig. 5. CCl4 significantly induced iNOS protein expression in cirrhosis mice, compared with that of the control group (P=0.0023). However, administration of ellagic acid significantly suppressed the protein expression of iNOS in cirrhosis mice (Fig. 5; P=0.0081 and 0.0066). These data suggested that the protective effects of ellagic acid may prevent against CCl4-induced cirrhosis through the suppression of iNOS expression.
Figure 5.
iNOS protein expression in CCl4-induced cirrhosis mice. (A) Western blots and (B) quantified levels of iNOS protein expression are shown. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid; iNOS, inducible nitric oxide synthase.
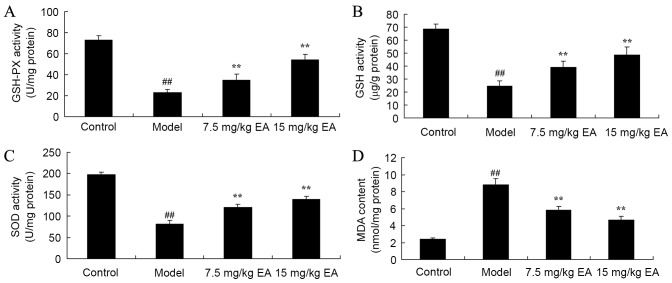
Protective effect of ellagic acid against oxidative stress in CCl4-induced cirrhosis mice
CCl4 administration to induce cirrhosis in mice was observed to inhibit the activities of GSH-PX (P=0.0022), GSH (P=0.0034) and SOD (P=0.0037), whereas it increased the MDA (P=0.0014) level in cirrhosis mice, when compared with the control group levels (Fig. 6). By contrast, treatment with ellagic acid for 5 weeks significantly reversed the GSH-PX (P=0.0082 and 0.0037), GSH (P=0.0065 and 0.0024), SOD (P=0.0056 and 0.0029) and MDA (P=0.0052 and 0.0041) activities in cirrhosis mice (Fig. 6). These data suggest that ellagic acid may have anti-oxidative effects and thus be used in the treatment of cirrhosis.
Figure 6.
Protective effects of ellagic acid against oxidative stress in CCl4-induced cirrhosis mice. The activities of (A) GSH-PX, (B) GSH, (C) SOD and (D) MDA were determined by ELISA kits. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid; GSH-Px, glutathione peroxidase; GSH, glutathione; SOD, superoxide dismutase; MDA, malondialdehyde.
Protective effect of ellagic acid against ROS formation in CCl4-induced cirrhosis mice
ROS formation was determined in order to evaluate the protective effect of ellagic acid against ROS formation in CCl4-induced cirrhosis mice. The ROS formation was significantly increased in the untreated cirrhosis mice compared with that of the control group (Fig. 7; P=0.0023). However, administration of 7.5 or 15 mg/kg ellagic acid significantly inhibited ROS formation in the cirrhosis mice (Fig. 7; P=0.0062 and 0.0046). These data indicated that ellagic acid suppressed ROS formation to inhibit oxidative stress in mice with CCl4-induced cirrhosis.
Figure 7.
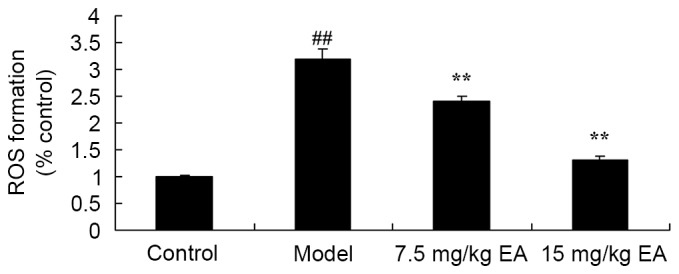
Protective effects of ellagic acid against ROS formation in CCl4-induced cirrhosis mice. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid; ROS, reactive oxygen species.
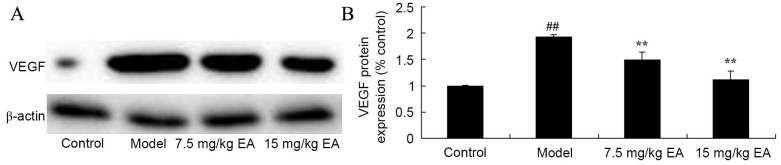
Ellagic acid treatment reduces VEGF expression in CCl4-induced cirrhosis mice
In addition, the present study examined the mechanism of ellagic acid against VEGF expression in CCl4-induced cirrhosis mice by western blot analysis. A significant decrease in VEGF protein expression was identified in the cirrhosis model mice, compared with the control group (Fig. 8; P=0.0023). However, treatment with ellagic acid significantly induced the VEGF protein expression in the cirrhosis mice (Fig. 8; P=0.0076 and 0.0037).
Figure 8.
VEGF protein expression in CCl4-induced cirrhosis mice. (A) Western blots and (B) quantified levels showed reduced VEGF protein expression following EA treatment. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid; VEGF, vascular endothelial growth factor.
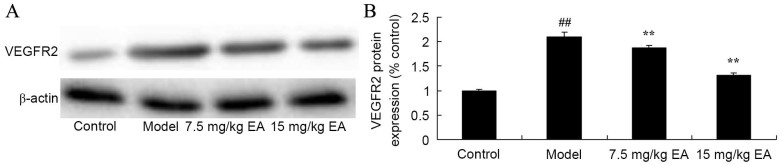
Ellagic acid treatment reduces VEGFR2 expression in CCl4-induced cirrhosis mice
The protective effect of ellagic acid against VEGFR2 expression in CCl4-induced cirrhosis mice was further investigated using western blot analysis. As shown in Fig. 9, the protein expression of VEGFR2 in cirrhosis model mice was significantly higher compared with that of the control group (P=0.0027). Treatment with 7.5 and 15 mg/kg ellagic acid significantly decreased the protein expression of VEGFR2 in the cirrhosis mice (Fig. 9; P=0.0059 and 0.0043). These data indicate that ellgaic acid may function via VEGF to exert its protective effects against cirrhosis.
Figure 9.
VEGFR2 protein expression in CCl4-induced cirrhosis mice. (A) Western blots and (B) quantified levels showed reduced VEGFR2 protein expression following EA treatment. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid; VEGFR2, vascular endothelial growth factor receptor 2.
Ellagic acid treatment reduces caspase-3 activity in CCl4-induced cirrhosis mice
The anti-apoptosis effect of ellagic acid treatment against cirrhosis in mice was examined by recording the caspase-3 activity using an ELISA kit. Compared with the control group, the caspase-3 activity was significantly enhanced in cirrhosis model mice (Fig. 10; P=0.0013). Treatment with ellagic acid, however, significantly reduced caspase-3 activity in the cirrhosis mice (Fig. 10; P=0.0041 and 0.0026).
Figure 10.
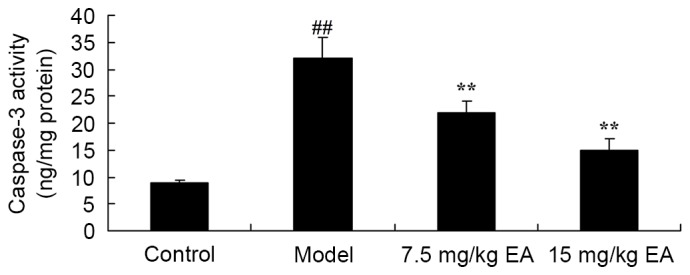
Caspase-3 activity in CCl4-induced cirrhosis mice. EA treatment significantly reduced the caspase-3 levels. ##P<0.01 vs. control group; **P<0.01 vs. model group. CCl4, tetrachloride; EA, ellagic acid.
Discussion
Acute hepatic failure based on liver cirrhosis is common (15). However, due to acute injury factors, such as quick replication of hepatitis virus and damage resulting from virulence drugs and ethyl alcohol, the liver function is worsened in a short period of time, with hemorrhage, infection, hepatic encephalopathy and high mortality rates (4). The results of the present study demonstrated that ellagic acid treatment effectively inhibited the serum levels of ALT, AST and ALB, as well as the collagen I gene expression, in cirrhosis mice.
Stress is considered as an important factor of liver injury. As far as oxidative stress is concerned, due to injuries of hyperfunction and damages of the antioxidation mechanisms, polymer reactions occur after free radicals, active oxygen and proteins, lipids and nucleic acids (10,16). It has been reported that oxidative stress is also closely associated with hepatocellular injury (17). SOD is the main protective enzyme in the anti-oxidation system, which can catalyze the disproportion reaction of the superoxide anion and prevent damages to tissues caused by the superoxide anion (18). In addition, GSH is an effective free-radical scavenger, inhibiting the launch and development of lipid peroxidation, while MDA is the end-product of cytomembrane lipid peroxidation. The levels of these indicators are abnormal under oxidative stress (10). In the current study, treatment with ellagic acid significantly suppressed iNOS protein expression, reversed the GSH-PX, GSH, SOD and MDA activities, and inhibited ROS formation in cirrhosis mice. Ural et al (12) have suggested that treatment with ellagic acid protects against malathion exposure through reducing oxidative stress and antioxidant status in Cyprinus carpio.
VEGF induces vasculogenesis and endothelial cell proliferation and has important functions in the regulation of angiogenesis (19). In recent years, VEGF has been proven to promote the formation of endothelial cells, and increase the permeability of endothelial and glomerular cells (20). Trigger point injection of VEGF promotes the formation of new vessels surrounding the sinus hepaticus in normal or liver cirrhosis mice (21). A previous study has proven that expression levels of VEGF in patients or test models are increased and can worsen liver cirrhosis (22). In liver cirrhosis mice subsequent to hepatectomy, VEGF effectively promotes liver regeneration (23,24). In the present study, ellagic acid treatment significantly induced the VEGF and VEGFR2 protein expression levels in cirrhosis mice. Similarly, Labrecque et al (25) reported that ellagic acid may be helpful for the prevention and treatment of cancer through decreasing platelet-derived growth factor and VEGF receptors.
Caspases are a group of apoptosis proteases. According to the effects of caspases in cell apoptosis, they can be divided into upstream and downstream caspases (26). Caspase-3, located on the common pathway of cell apoptosis, is the most important member of the caspase family, and the majority of factors causing apoptosis (27). Signal transduction pathways mediated by caspase-3 result in cell apoptosis, and the expression levels of caspase-3 can positively represent the apoptosis levels of the tissues (27,28). The current study findings indicated that ellagic acid significantly reduced caspase-3 activity in cirrhosis mice, thus suggesting that apoptosis was inhibited. Mishra et al (13) also reported that ellagic acid promotes apoptosis in lymphoma-bearing mice through an increase ofcaspase-3.
In conclusion, the present study demonstrated that the protective effects of ellagic acid inhibited the serum levels of ALT, AST and ALB, as well as collagen I gene expression, in cirrhosis mice. These protective effects are associated with the antioxidative mechanisms, and the inhibition of iNOS and caspase-3 expression, and induction VEGF expression in cirrhosis mice. Thus, it is suggested that ellagic acid may be a potential new drug under hypoxic conditions in CCl4-induced cirrhosis through the inhibition of ROS formation and angiogenesis.
References
- 1.Patin E, Kutalik Z, Guergnon J, Bibert S, Nalpas B, Jouanguy E, Munteanu M, Bousquet L, Argiro L, Halfon P, et al. Genome-wide association study identifies variants associated with progression of liver fibrosis from HCV infection. Gastroenterology. 2012;143(1244–1252):e1–e12. doi: 10.1053/j.gastro.2012.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caprai S, Vajro P, Ventura A, Sciveres M, Maggiore G. SIGENP Study Group for Autoimmune Liver Disorders in Celiac Disease: Autoimmune liver disease associated with celiac disease in childhood: A multicenter study. Clin Gastroenterol Hepatol. 2008;6:803–806. doi: 10.1016/j.cgh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Assy N, Kayal M, Mejirisky Y, Gorenberg M, Hussein O, Schlesinger S. The changes in renal function after a single dose of intravenous furosemide in patients with compensated liver cirrhosis. BMC Gastroenterol. 2006;6:39. doi: 10.1186/1471-230X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuiper EM, Hansen BE, Lesterhuis W, Robijn RJ, Thijs JC, Engels LG, Koek GH, Aparicio MN, Kerbert-Dreteler MJ, van Buuren HR. Dutch PBC study group: The long-term effect of ursodeoxycholic acid on laboratory liver parameters in biochemically non-advanced primary biliary cirrhosis. Clin Res Hepatol Gastroenterol. 2011;35:29–33. doi: 10.1016/j.gcb.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Mišík M, Hoelzl C, Wagner KH, Cavin C, Moser B, Kundi M, Simic T, Elbling L, Kager N, Ferk F, et al. Impact of paper filtered coffee on oxidative DNA-damage: Results of a clinical trial. Mutat Res. 2010;692:42–48. doi: 10.1016/j.mrfmmm.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Spahr L, Bresson-Hadni S, Amann P, Kern I, Golaz O, Frossard JL, Hadengue A. Allopurinol, oxidative stress and intestinal permeability in patients with cirrhosis: An open-label pilot study. Liver Int. 2007;27:54–60. doi: 10.1111/j.1478-3231.2006.01382.x. [DOI] [PubMed] [Google Scholar]
- 7.Simón-Talero M, Garcia-Martinez R, Torrens M, Augustin S, Gómez S, Pereira G, Guevara M, Ginés P, Soriano G, Román E, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: A randomized double-blind study. J Hepatol. 2013;59:1184–1192. doi: 10.1016/j.jhep.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Deng G, Wang J, Zhang Q, He H, Wu F, Feng T, Zhou J, Zou K, Hattori M. Hepatoprotective effects of phloridzin on hepatic fibrosis induced by carbon tetrachloride against oxidative stress-triggered damage and fibrosis in rats. Biol Pharm Bull. 2012;35:1118–1125. doi: 10.1248/bpb.b12-00057. [DOI] [PubMed] [Google Scholar]
- 9.Fang H, Liu A, Dirsch O, Dahmen U. Liver transplantation and inflammation: Is lipopolysaccharide binding protein the link? Cytokine. 2013;64:71–78. doi: 10.1016/j.cyto.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 10.Marotta F, Yoshida C, Barreto R, Naito Y, Packer L. Oxidative-inflammatory damage in cirrhosis: Effect of vitamin E and a fermented papaya preparation. J Gastroenterol Hepatol. 2007;22:697–703. doi: 10.1111/j.1440-1746.2007.04937.x. [DOI] [PubMed] [Google Scholar]
- 11.Nutan, Modi M, Goel T, Das T, Malik S, Suri S, Rawat AK, Srivastava SK, Tuli R, Malhotra S, Gupta SK. Ellagic acid & gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 protease & reverse transcriptase activity. Indian J Med Res. 2013;137:540–548. [PMC free article] [PubMed] [Google Scholar]
- 12.Ural MŞ, Yonar ME, Yonar Mişe S. Protective effect of ellagic acid on oxidative stress and antioxidant status in Cyprinus carpio during malathion exposure. Cell Mol Biol (Noisy-le-grand) 2015;61:58–63. [PubMed] [Google Scholar]
- 13.Mishra S, Vinayak M. Role of ellagic acid in regulation of apoptosis by modulating novel and atypical PKC in lymphoma bearing mice. BMC Complement Altern Med. 2015;15:281. doi: 10.1186/s12906-015-0810-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang HM, Zhao L, Li H, Xu H, Chen WW, Tao L. Research progress on the anticarcinogenic actions and mechanisms of ellagic acid. Cancer Biol Med. 2014;11:92–100. doi: 10.7497/j.issn.2095-3941.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Minicis S, Brenner DA. Oxidative stress in alcoholic liver disease: Role of NADPH oxidase complex. J Gastroenterol Hepatol. 2008;23(Suppl 1):S98–S103. doi: 10.1111/j.1440-1746.2007.05277.x. [DOI] [PubMed] [Google Scholar]
- 16.Oettl K, Stadlbauer V, Petter F, Greilberger J, Putz-Bankuti C, Hallström S, Lackner C, Stauber RE. Oxidative damage of albumin in advanced liver disease. Biochim Biophys Acta. 2008;1782:469–473. doi: 10.1016/j.bbadis.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Vidali M, Occhino G, Ivaldi A, Rigamonti C, Sartori M, Albano E. Combination of oxidative stress and steatosis is a risk factor for fibrosis in alcohol-drinking patients with chronic hepatitis C. Am J Gastroenterol. 2008;103:147–153. doi: 10.1111/j.1572-0241.2007.01596.x. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Wang Y. Effect of different methods of hypoxic exercise training on free radical oxidation and antioxidant enzyme activity in the rat brain. Biomed Rep. 2013;1:925–929. doi: 10.3892/br.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan Z, Qu K, Zhang J, Huang Q, Qu P, Xu X, Yuan P, Huang X, Shao Y, Liu C, et al. CD147 promotes liver fibrosis progression via VEGF-A/VEGFR2 signalling-mediated cross-talk between hepatocytes and sinusoidal endothelial cells. Clin Sci (Lond) 2015;129:699–710. doi: 10.1042/CS20140823. [DOI] [PubMed] [Google Scholar]
- 20.Tekkesin N, Taga Y, Sav A, Almaata I, Ibrisim D. Induction of HGF and VEGF in hepatic regeneration after hepatotoxin-induced cirrhosis in mice. Hepatogastroenterology. 2011;58:971–979. [PubMed] [Google Scholar]
- 21.Kishta SA, Abd-Alhade AA, Hamam O, Raoof EA, Abeya S. Prognostic value of TNF a mRNA and VEGF mRNA expression in patients with chronic hepatitis C genotype-4, with and without cirrhosis and hepatocellular carcinoma to predict disease outcome. J Egypt Soc Parasitol. 2010;40:515–530. [PubMed] [Google Scholar]
- 22.Jaroszewicz J, Januszkiewicz M, Flisiak R, Rogalska M, Kalinowska A, Wierzbicka I. Circulating vascular endothelial growth factor and its soluble receptors in patients with liver cirrhosis: Possible association with hepatic function impairment. Cytokine. 2008;44:14–17. doi: 10.1016/j.cyto.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Mukozu T, Nagai H, Matsui D, Kanekawa T, Sumino Y. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 2013;33:1013–1021. [PubMed] [Google Scholar]
- 24.Surapaneni KM, Vishnu Priya V, Mallika J. Effect of pioglitazone, quercetin, and hydroxy citric acid on vascular endothelial growth factor messenger RNA (VEGF mRNA) expression in experimentally induced nonalcoholic steatohepatitis (NASH) Turk J Med Sci. 2015;45:542–546. doi: 10.3906/sag-1404-136. [DOI] [PubMed] [Google Scholar]
- 25.Labrecque L, Lamy S, Chapus A, Mihoubi S, Durocher Y, Cass B, Bojanowski MW, Gingras D, Béliveau R. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis. 2005;26:821–826. doi: 10.1093/carcin/bgi024. [DOI] [PubMed] [Google Scholar]
- 26.Izawa T, Horiuchi T, Atarashi M, Kuwamura M, Yamate J. Anti-fibrotic Role of miR-214 in Thioacetamide-induced liver cirrhosis in rats. Toxicol Pathol. 2015;43:844–851. doi: 10.1177/0192623315573587. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, Sun M, Liu C, Liu P. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complement Altern Med. 2012;12:51. doi: 10.1186/1472-6882-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu XX, Wu LM, Fan JJ, Qin Y, Chen G, Wu XF, Shen Y, Sun Y, Xu Q. Cortex Dictamni extract induces apoptosis of activated hepatic stellate cells via STAT1 and attenuates liver fibrosis in mice. J Ethnopharmacol. 2011;135:173–178. doi: 10.1016/j.jep.2011.03.010. [DOI] [PubMed] [Google Scholar]