Abstract
A 63-year-old female patient who had undergone cholecystectomy for inflammatory myofibroblastic tumor (IMT) in the gallbladder was referred to our hospital. The patient's disease relapsed, involving the pancreas, and was diagnosed as inoperable IMT 13 months after the cholecystectomy. The patient failed to respond to steroid and non-steroidal anti-inflammatory drug therapy, but subsequently exhibited a good response to vinorelbine and methotrexate combination chemotherapy. Little information is currently available on the efficacy of chemotherapy for adult-onset IMT. The present case suggests that chemotherapy with vinorelbine and methotrexate is a viable therapeutic option for adult patients with unresectable IMT.
Keywords: vinorelbine, methotrexate, metastatic pancreatic tumor, unresectable inflammatory myofibroblastic tumor
Introduction
Inflammatory myofibroblastic tumor (IMT) is a rare disease of unknown etiology (1–3). This tumor was previously described as an inflammatory pseudotumor, inflammatory myofibroblastoma, lymphoplasmacytic histiocytoma and fibrous pseudotumor until 1994, when myofibroblastic tumor was established as a distinct low-grade malignancy by the World Health Organization (1–3). IMT is considered to be a neoplasm of intermediate biological potential, which may recur and metastasizes infrequently (1–4). Histologically, IMT is characterized by myofibroblastic spindle cells mixed with a hyalinized stroma and various degrees of inflammatory infiltrates (1–3). IMT occurs principally in children and young adults (4–10) and may affect any site in the body, although it is most commonly found in the lungs (4–6).
It is well established that total surgical excision is the most effective therapeutic option for surgically accessible IMT (2). However, the treatment options for relapsed and/or inoperable IMT are extremely limited due to the lack of reports regarding effective treatments. In particular, little evidence has been reported in the literature regarding the efficacy of chemotherapy for IMT (7–14). We encountered a case of inoperable IMT with pancreatic involvement following cholecystectomy for IMT originating from the gallblabber. The patient failed to respond to non-steroidal anti-inflammatory drugs (NSAIDs) and steroid therapy, but subsequently showed a good response to vinorelbine (VNB) and methotrexate (MTX) combination chemotherapy. We herein describe the clinical course and present a review of the literature on the effectiveness of chemotherapy for advanced and unresectable IMT.
Case report
A 63-year-old woman with no significant past medical history was admitted to a local hospital with the complaint of abdominal fullness and pain. A tumor was detected in the gallbladder, and the patient underwent surgical resection. Distant metastasis or invasion into adjacent organs were not observed based on radiographic findings prior to the operation and intraoperative inspection. The pathological diagnosis was IMT (Fig. 1). Thirteen months following resection, tumors in the pancreatic head and tail (Fig. 2A) were identified on whole-body computed tomography (CT) during postoperative follow-up. The patient was subsequently referred to the Department of Comprehensive Cancer Therapy, Shinshu University School of Medicine (Matsumoto, Japan) for further examination. Positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET) revealed abnormal uptake by both tumors. Endoscopic examination revealed a submucosal tumor in the duodenum (Fig. 2B). Endoscopic ultrasound-guided fine-needle aspiration in the pancreatic head tumor was performed and the histological diagnosis was recurrence of IMT (Fig. 3). The histological findings and molecular analysis of specimens collected from the pancreas and gallbladder revealed no pathological evidence of malignancy. In addition, there was no presence of anaplastic lymphoma kinase (ALK) protein by immunohistochemical staining or gene rearrangement by fluorescence in situ hybridization. Treatment with NSAIDs and methylprednisolone (500 mg/dayx3 day, intravenously) followed by 1 mg/kg of prednisolone for 1 month was continued, but the tumors were not reduced in size. Subsequently, treatment with methotrexate (MTX; 30 mg/m2 on day 1) plus vinorelbine (VNB; 20 mg/m2 on days 1 and 7) per 3 weeks chemotherapy was administered. The chemotherapy was continued without any severe toxicities. The dosage was repeated six times and partial response was achieved as determined by CT and endoscopic examination (Fig. 2C and D).
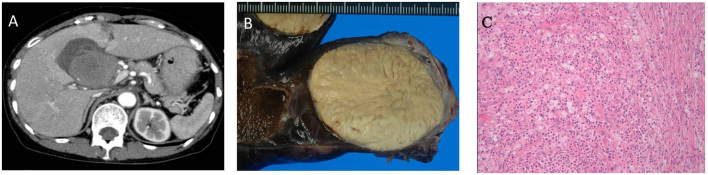
Figure 1.
(A) Abdominal computed tomography findings at initial presentation in the present case. A gallbladder tumor was detected prior to the operation. (B) Macroscopically, the resected tumor was a multinodular tan mass with a fleshy surface on cross-section. (C) The histological findings on hematoxylin and eosin staining showed proliferation of myofibroblastic spindle cells with lymphoplasmacytic inflammatory infiltrates. These findings were consistent with the diagnosis of inflammatory myofibroblastic tumor.
Figure 2.
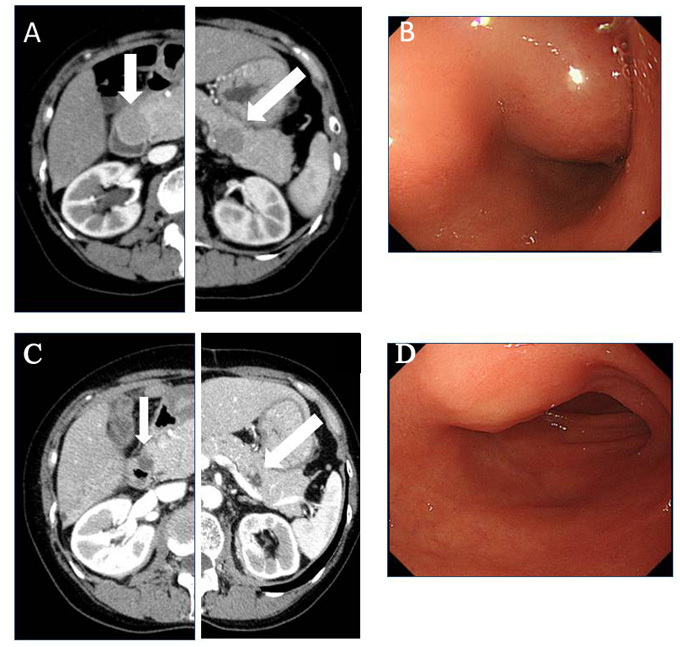
(A) Abdominal computed tomography (CT) showed pancreatic head and tail tumors and (B) endoscopic examination revealed a submucosal tumor in the duodenum prior to chemotherapy. The (C) abdominal CT and (D) endoscopic findings after 6 cycles of chemotherapy revealed that the pancreatic tumors in the head and tail of the pancreas had shrunk following vinorelbine and methotrexate chemotherapy.
Figure 3.
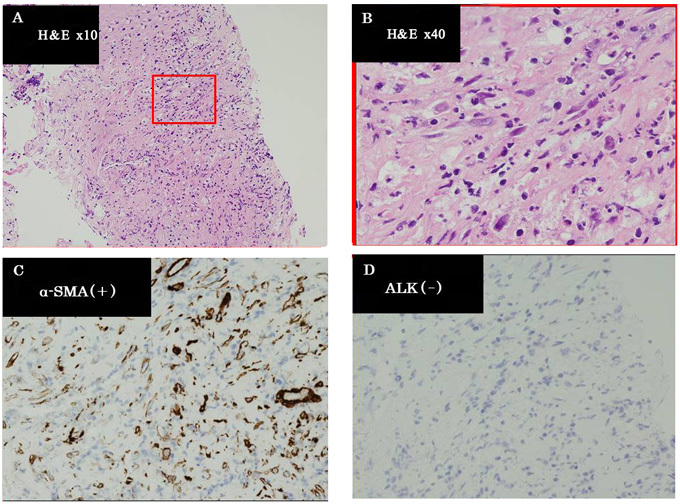
(A and B) The histological findings of specimens collected from the pancreatic tumor revealed a relapsed inflammatory myofibroblastic tumor [hematoxylin and eosin (H&E) staining]. Immunohistochemical examination showed (C) positive staining for α-smooth muscle actin (SMA) and (D) negative staining for anaplastic lymphoma kinase (ALK).
Discussion
We herein describe a case of abdominal IMT that developed in an elderly female and responded well to VNB and MTX chemotherapy. IMT is usually encountered in children and adolescents, mostly occurring between 2 and 16 years of age and mainly involving the lungs (1–3). Kovach et al (4) summarized 44 cases of IMT in their institutes and reported a mean age of 44 years, ranging from 9 to 88 years. In addition, the tumor location included several extrapulmonary lesions in addition to the lung (4). The patient reported herein had abdominal IMT and was aged 63 years. The association between age of onset and the involved location of IMT remains unclear. However, it has been suggested that recurrence was more common if the lesions were extrapulmonary (4).
Although our patient had an ALK-negative tumor, it has been demonstrated that ~50% of IMTs harbor ALK gene rearrangement (2,15). Coffin et al (2) analyzed 59 IMTs and reported that ALK-negative IMTs had a more aggressive clinical course with a high risk of distant metastasis. In addition, the absence of ALK expression was associated with older age (2). Thus, clinical manifestations, including age, location and ALK negativity in the present case were consistent with those reported in the literature.
Surgical resection has been considered the preferred treatment for IMT, and may also be used to confirm this diagnosis (2,16). However, IMT is considered to be a neoplasm of intermediate biological potential, which may recur and metastasize, although infrequently (1–4). In unresectable cases, radiotherapy and chemotherapy, including steroids and NSAIDs, were applied (7–14,17,18). Indeed, several clinical reports indicated the usefulness of steroids and NSAIDs (11,13,17,18), although both failed to shrink the tumor in the present case. Various cytotoxic agents or regimens, including MTX, VNB, vincristine, cyclophosphamide, doxorubicin, 5-fluorouracil, cisplatin, carboplatin, paclitaxel, ifosfamide and etoposide, have been used. However, due to the rarity of IMT, the availability of data regarding the efficacy of cytotoxic chemotherapeutic agents is limited. Previously reported treatment regimens, consisting of cytotoxic chemotherapy alone or in combination with NSAIDs, that were found to be effective for IMT are summarized in Table I. However, none of the chemotherapeutic regimens had been evaluated in a case series. In addition, the majority of data were obtained from pediatric populations. Thus, there is still a lack of definitive clinical evidence, particularly for adults. As Favini et al (10) reported a case of IMT that responded to VNB plus MTX, this chemotherapy regimen was used in the present case. Chemotherapy was performed without any severe toxicities and the patient responded well. Although the efficacy of this chemotherapy was evaluated in aggressive fibromatosis in cases series (19,20), our experience, including the case reported by Favini et al (10), may provide a treatment choice for patients with unresectable IMT. With regard to other optimal regimens, Kubo et al (12) described the case of an adult IMT patient (aged 26 years) who responded to carboplatin+paclitaxel chemotherapy. As this combination is the most commonly used chemotherapy regimen in various malignancies, carboplatin+paclitaxel may be an optimal choice for adult and inoperable IMT. An optimal agent or combination chemotherapy has not yet been determined for unresectable IMT. Further clinical experience and studies are required to determine the usefulness of chemotherapy for inoperable IMT.
Table I.
Previous case reports successfully treated with cytotoxic chemotherapy.
| Case | Age, years | Location | Agent/regimen | Response | NSAIDs | Surgery | (Refs.) |
|---|---|---|---|---|---|---|---|
| 1 | 7 | Abdomen | Vincristine+etoposide→cisplatin, adriamycin, methotrexate | PR | + | + | (8) |
| 2 | 10 | Abdomen | Methotrexate+vinblastine, adriamycin, ifosfamide | PR | + | (9) | |
| 3 | 12 | Conjuctiva | Methotrexate+vinorelbine | PR | (10) | ||
| 4 | 14 | Peritoneum | Cisplatin+methotrexate | CR | + | + | (11) |
| 5 | 26 | Mediastinum | Carboplatin+paclitaxel | CR | (12) | ||
| 6 | 64 | Frontal bone | Methotrexate | PR | + | (13) | |
| 7 | 64 | Abdomen | Doxorubicin+ifosfamide | PR | (14) |
PR, partial response; CR, complete response; NSAIDs, non-steroidal anti-inflammatory drugs.
In summary, our observations in the present case suggest that intermediate-dose chemotherapy using vinorelbine and methotrexate is a feasible therapeutic option in adult patients with unresectable IMT.
References
- 1.Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press; Lyon: 2002. Inflammatory myofibroblastic tumor. In: World Health Organization Classification of Tumours; pp. 91–93. [Google Scholar]
- 2.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: Comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 3.Mergan F, Jaubert F, Sauvat F, Hartmann O, Lortat-Jacob S, Révillon Y, Nihoul-Fékété C, Sarnacki S. Inflammatory myofibroblastic tumor in children: Clinical review with anaplastic lymphoma kinase, Epstein-Barr virus, and human herpesvirus 8 detection analysis. J Pediatr Surg. 2005;40:1581–1586. doi: 10.1016/j.jpedsurg.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Kovach SJ, Fischer AC, Katzman PJ, Salloum RM, Ettinghausen SE, Madeb R, Koniaris LG. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94:385–391. doi: 10.1002/jso.20516. [DOI] [PubMed] [Google Scholar]
- 5.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Tunçözgür B, Ustünsoy H, Bakir K, Uçak R, Elbeyli L. Inflammatory pseudotumor of the lung. Thorac Cardiovasc Surg. 2000;48:112–113. doi: 10.1055/s-2000-9865. [DOI] [PubMed] [Google Scholar]
- 7.Sanders BM, West KW, Gingalewski C, Engum S, Davis M, Grosfeld JL. Inflammatory pseudotumor of the alimentary tract: Clinical and surgical experience. J Pediatr Surg. 2001;36:169–173. doi: 10.1053/jpsu.2001.20045. [DOI] [PubMed] [Google Scholar]
- 8.Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, Moscow JA. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003;25:153–158. doi: 10.1097/00043426-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Bertocchini A, Lo Zupone C, Callea F, Gennari F, Serra A, Monti L, de Ville de Goyet J. Unresectable multifocal omental and peritoneal inflammatory myofibroblastic tumor in a child: Revisiting the role of adjuvant therapy. J Pediatr Surg. 2011;46:e17–e21. doi: 10.1016/j.jpedsurg.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Favini F, Resti AG, Collini P, Casanova M, Meazza C, Trecate G, Ferrari A. Inflammatory myofibroblastic tumor of the conjunctiva: Response to chemotherapy with low-dose methotrexate and vinorelbine. Pediatr Blood Cancer. 2010;54:483–485. doi: 10.1002/pbc.22342. [DOI] [PubMed] [Google Scholar]
- 11.Tao YL, Wang ZJ, Han JG, Wei P. Inflammatory myofibroblastic tumor successfully treated with chemotherapy and nonsteroidals: A case report. World J Gastroenterol. 2012;18:7100–7103. doi: 10.3748/wjg.v18.i47.7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubo N, Harada T, Anai S, Otsubo K, Yoneshima Y, Ijichi K, Koga T, Takayama K, Nakanishi Y. Carboplatin plus paclitaxel in the successful treatment of advanced inflammatory myofibroblastic tumor. Intern Med. 2012;51:2399–2401. doi: 10.2169/internalmedicine.51.7599. [DOI] [PubMed] [Google Scholar]
- 13.Kusunoki-Nakamoto F, Matsukawa T, Tanaka M, Miyagawa T, Yamamoto T, Shimizu J, Ikemura M, Shibahara J, Tsuji S. Successful treatment of an unresectable inflammatory myofibroblastic tumor of the frontal bone using a cyclooxygenase-2 inhibitor and methotrexate. Intern Med. 2013;52:623–628. doi: 10.2169/internalmedicine.52.8785. [DOI] [PubMed] [Google Scholar]
- 14.Inadomi K, Kumagai H, Takayoshi K, Ariyama H, Kusaba H, Nishie A, Yamamoto H, Takase K, Tanaka M, Sagara K, et al. Successful combination chemotherapy for metastatic inflammatory myofibroblastic tumor: A case report. Oncol Lett. 2015;10:2981–2985. doi: 10.3892/ol.2015.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, Ladanyi M, Capelletti M, Rodig SJ, Ramaiya N, et al. Crizotinib in ALK rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janik JS, Janik JP, Lovell MA, Hendrickson RJ, Bensard DD, Greffe BS. Recurrent inflammatory pseudotumors in children. J Pediatr Surg. 2003;38:1491–1495. doi: 10.1016/S0022-3468(03)00501-3. [DOI] [PubMed] [Google Scholar]
- 17.Berger A, Kim C, Hagstrom N, Ferrer F. Successful preoperative treatment of pediatric bladder inflammatory myofibroblastic tumor with anti-inflammatory therapy. Urology. 2007;70(372):e13–e15. doi: 10.1016/j.urology.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Chan PW, Omar KZ, Ramanujam TM. Successful treatment of unresectable inflammatory pseudotumor of the lung with COX-2 inhibitor. Pediatr Pulmonol. 2003;36:167–169. doi: 10.1002/ppul.10308. [DOI] [PubMed] [Google Scholar]
- 19.Azzarelli A, Gronchi A, Bertulli R, Tesoro JD, Baratti D, Pennacchioli E, Dileo P, Rasponi A, Ferrari A, Pilotti S, Casali PG. Low-dose chemotherapy with methotrexate and vinblastine for patients with advanced aggressive fibromatosis. Cancer. 2001;92:1259–1264. doi: 10.1002/1097-0142(20010901)92:5<1259::AID-CNCR1446>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 20.Meazza C, Bisogno G, Gronchi A, Fiore M, Cecchetto G, Alaggio R, Milano GM, Casanova M, Carli M, Ferrari A. Aggressive fibromatosis in children and adolescents: The Italian experience. Cancer. 2010;116:233–240. doi: 10.1002/cncr.24679. [DOI] [PubMed] [Google Scholar]