Abstract
Basic fibroblast growth factor (bFGF), a known angiogenic factor, may provide a potential strategy for the treatment of myocardial infarction (MI), but it is limited by a relatively short half-life. Dex-PCL-HEMA/PNIPAAm hydrogel provides a reservoir for the controlled release of growth factors. The aim of the current study was to evaluate the effects of bFGF incorporated into a Dex-PCL-HEMA/PNIPAAm hydrogel on angiogenesis and cardiac health in a rat model of acute MI, induced by coronary artery ligation. Phosphate-buffered solution (PBS group), Dex-PCL-HEMA/PNIPAAm hydrogel (Gel group), bFGF in phosphate-buffered solution (bFGF group) or bFGF in hydrogel (Gel + bFGF group) was injected into a peri-infarcted area of cardiac tissue immediately following MI. On day 30 post-surgery, cardiac function was assessed by echocardiography, apoptosis index by terminal deoxynucleotidyl transferase dUTP nick-end labeling assessment and vascular development by immunohistochemical staining. The findings demonstrated that injection of bFGF along with hydrogel induced angiogenesis, reduced collagen content, MI area and cell apoptosis and improved cardiac function compared with the injection of either bFGF or hydrogel alone. bFGF incorporated with Dex-PCL-HEMA/PNIPAAm hydrogel injection induces angiogenesis, attenuates cardiac remodeling and improves cardiac function following MI.
Keywords: hydrogel, basic fibroblast growth factor, myocardial infarction, angiogenesis
Introduction
Myocardial infarction (MI) remains one of the most serious cardiovascular diseases worldwide and causes high mortality and morbidity (1). Acute blocking of the coronary artery leads to acute ischemia, hypoxia and irreversible death in myocardial cells, eventually inducing adverse cardiac remodeling and progressive heart failure (2–4). Thus, in situ repair and regeneration of vessels to rebuild coronary collateral circulation and improve blood supply to the infarction zone and its periphery have emerged as potential therapeutic approaches for treating MI (5,6).
Growth factors are widely used to treat MI by inducing angiogenesis (7). Fibroblast growth factors (FGFs) are potent mitogens that induce angiogenesis in vascular and capillary endothelial cells. Basic FGF (bFGF), a 16-kD monomeric factor, is the most potent angiogenic factor in the FGF family, and it targets the migration and proliferation of endothelial cells, smooth muscle cells and fibroblasts (8,9). bFGF has been extensively studied in animal (10–12) and clinical (13,14) experiments.
However, as bFGF has a short half-life and because the improper administration of bFGF at high doses is accompanied by side effects, including retinopathy (15,16), hypotension (17), angioma, and edema and thrombocytopenia (8,9), the therapeutic administration of bFGF is largely limited to laboratory and clinical trials.
Thermosensitive Dex-PCL-HEMA/PNIPAAm hydrogel is a type of cross-linked polymer with biocompatibility and nontoxicity (18). In a previous study, hydrogel was identified to be a suitable delivery vehicle and controlled-release system for drugs and cells. He et al (19) combined this hydrogel with high mobility group box 1 and injected it into the peri-infarct zone of a rat heart, which induced the activation of endogenous c-Kit cardiac cells and vascular regeneration. Wan et al (20) combined this hydrogel with short hairpin RNA of angiotensin converting enzyme, which induced enhanced cardioprotective effects in a rat with MI.
Thus, the aim of the present study was to investigate whether the angiogenesis effect induced by bFGF is strengthened or enhanced by incorporating it into a hydrogel (a controlled delivery system) to sustain and localize release in the infarcted heart wall of a rat model of MI, and also to investigate whether bFGF and hydrogel implantation may improve infarcted left ventricle (LV) function, and inhibit LV remodeling.
Materials and methods
Animals
A total of 52 8–10 week old male Sprague Dawley rats (200–250 g) were used. Rats were purchased from Hunan Provincial Center for Disease Control and Prevention (Beijing, China) and maintained in a controlled environment with temperature at 22–25°C, humidity at 45–55%, and a 12:12-h light-dark cycle. Groups of five rats were housed in a cage with food and water provided ad libitum. All experimental procedures involving animal use conformed with the Guide for the Care and Use of Laboratory Animals, as published by the US National Institutes of Health, and the protocol was approved by the Institutional Animal Care Committee from Wuhan University (Wuhan, China).
Synthesis of hydrogel
All the reagents used to synthesize the hydrogel, including N-isopropylacrylamide (NIPAAm), 2-Hydroxylethyl methacrylate (HEMA) and dextran were obtained from Shanghai Chemical Reagent Co., Ltd. (Shanghai, China). As described previously (18), the hydrogel was synthesized with biodegradable dextran chains grafted with a hydrophobic poly (e-caprolactone)-HEMA (PCL-HEMA) chain and the PCL (polycaprolactam)-grafted polysaccharide chains into a thermoresponsive poly-NIPAAm (PNIPAAm) network. The former is hydrophobic and the latter is amphiphilic and thermoresponsive. Thus, the hydrogel is biodegradable, biocompatible and thermoresponsive. The gel solution has a lower critical solution temperature (LCST) of 33°C and is therefore able to shift from solution to gel at 37°C and solidify at a temperature below the LCST. The polymer was mixed with phosphate-buffered solution (PBS) to develop the 1.5% weight (WT) gel solution and was subsequently autoclaved at 120°C for 20 min for sterilization.
In vitro release of bFGF from hydrogel
Initially, 100 units of bFGF was dissolved in 1.0 ml Dex-PCL-HEMA/PNIPAAm hydrogel solution (1.5% WT), and air bubbles were prevented during the mixing process. The mixture was then combined with 500 ml PBS in a beaker at 37°C. At 1, 5, 10, 15, 20, 25 and 30 days after the combination during the in vitro release experiment, a 1 ml aliquot of the buffer medium was removed for bFGF concentration measurement. The concentration of bFGF released from the hydrogel was analyzed using a human bFGF chemiluminescence immunoassay diagnostic kit (xy-CL-H0394c; Siemens AG, Munich, Germany); and a chemiluminescence immunoassay analyzer machine (IMMULITE® 2000; Siemens AG) according to the manufacturer's protocol. Dex-PCL-HEMA/PNIPAAm hydrogel without bFGF was used as a control. Cumulative bFGF release (%) from the hydrogel was calculated as follows: Cumulative release (%) = (Mt / M0) × 100, where Mt is the amount of bFGF released from the hydrogel at time (t) and M0 is the concentration of 100 U of bFGF dissolved in 500 ml PBS. Assessments of the bFGF release from the hydrogel were repeated three times.
Model of MI and in vivo bFGF delivery
All 52 male Sprague Dawley rats were anesthetized with 3% sodium pentobarbital (p3761; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) (30 mg/kg) peritoneally, intubated and ventilated at a respiratory rate of 70 times/min. A thoracotomy was then performed at the third left intercostal space. Following stripping of the epicardia and identification of the anatomy, the left coronary artery was ligated with 6-to-0 polypropylene stitching, 2 mm below the left atrial appendage. Electrocardiogram (ECG) monitoring was used and when the ECG read an ST-segment elevation in Leads II and III and pallor appeared in the infarcted area, this indicated successful MI. Rats with MI were randomly assigned to groups receiving multiple intramyocardial injections of 100 µl PBS without bFGF (group PBS), 100 µl hydrogel without bFGF (group Gel), 100 µl PBS containing 2 µg bFGF (PBS + bFGF; group bFGF) or 100 µl hydrogel containing 2 µg bFGF (hydrogel + bFGF; group Gel + bFGF). Each group had 7–10 rats available for testing. The compound was injected via a 32-gauge needle into four sites (25 µl per injection) of the peri-infarct zone immediately following coronary ligation.
Echocardiography
On day 30 following MI, all of the living rats were anesthetized with 3% sodium pentobarbital (25 mg/kg) injected peritoneally and placed in a lateral position, with their chest shaved. In a blind manner, transthoracic echocardiography (Acuson; Siemens Healthcare, Mountain View, CA, USA) equipped with a 3- to 7-MHz probe was used to assess their left ventricular (LV) dimension and function through long-axis and short-axis views. Rats with a visual infarct area <20% at the papillary level in the short-axis view were excluded to minimize the variation in infarct areas. LV end-diastolic diameter (LVEDD), LV end-systolic diameter (LVESD) and LV ejection fraction (LVEF) were measured from at least three consecutive cardiac cycles.
Histopathological examinations
Following the echocardiography measurements on day 30 post-MI, the rats were sacrificed by sodium pentobarbital overdose (200 mg/kg). The heart was quickly removed in diastole with 10% KCl. Ventricles were fixed in 10% (vol/vol) buffered formalin solution at 4°C for 24–48 h. After dehydration with a graded series [75, 85, 90, 95 and 100% (vol/vol)] of ethanol solution, five different transverse levels around the injection site were subsequently embedded. The specimens were sliced into 5-µm thick sections from apex to base and stained with Masson trichrome and underwent immunohistochemistry analysis.
Firstly, the sections were rinsed in peroxidase blocking agent (K4011; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) at room temperature for 5 min to block endogenous peroxidase activity, then washed with PBS 3 times (5 min/wash). Secondly, the sections were incubated with the following primary antibodies at 4°C overnight to analyze neovascularization: Anti-α-smooth muscle actin (αSMA) antibody (MABT381; 1:100 dilution; EMD Millipore, Billerica, MA, USA) and anti-cluster of differentiation (CD)31 antibody (sc-1506; 1:100 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Sections were then washed with PBS. Thirdly, horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (K4011; Dako; Agilent Technologies, Inc.) and HRP-conjugated rabbit anti-goat antibody (14-13-06, KPL, Inc., Gaithersburg, MD, USA) were used as secondary antibodies and the incubation extended for 30–60 min at room temperature. Finally, sections were rinsed in diaminobenzidine substrate-chromogen solution (K4011, Dako; Agilent Technologies, Inc.) for 10 min and subsequently in hematoxylin solution (03971; Sigma-Aldrich; Merck KGaA) for a further 10 sec for counterstaining. Sections were washed with PBS and prepared for testing.
The MI area, collagen content and new blood vessels were examined via light microscopy in sections taken from the border zone (1–2 mm from the edge of the infarction zone) of the four groups. In each LV transverse section, four random microscopic fields were selected in a blind fashion and subsequently examined. The infarct size (%) was calculated from the ratio of the surface area of the infarct wall and the entire surface area of the left ventricle by automated computer image analysis. The collagen content (%) was calculated from the ratio of the collagen area to the area of the entire high-powered field (magnification, ×200) using image analysis software (Image-Pro Plus 6.0; Media Cybernetics, Inc, Rockville, MD, USA). The number of arterioles/high-powered field (magnification, ×200) and capillaries/high-powered field (magnification, ×200) were counted in three tissue sections from each rat. The mean results of data compiled from the four fields of each section were subsequently calculated. Blood vessel density was indicated as the number of vessels at one high-powered field (magnification, ×200).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
On day 30, a TUNEL assay (11684817910; Roche Diagnostics GmbH, Mannheim, Germany) was used to detect apoptotic cardiac cells in the LV. As directed by the manufacturer, this method uses the transferase-mediated dUTP nick-end labeling technique to stain DNA fragments in the nucleus of apoptotic cells, followed by hematoxylin counterstaining. A total of three different fields (magnification, ×200) from each slide were examined by light microscopy. TUNEL-positive nuclei and the total number of nuclei were recorded by a blinded rater. The percentage of TUNEL positive nuclei was deemed as the apoptotic index.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis of the data were performed by one-way analysis of variance followed by the Student-Newman-Keuls' test. P<0.05 was considered to indicate a statistically significant difference.
Results
Animals
A total of 52 rats were initially included in the present study. Prior to surgery, 2 rats succumbed to hyperaesthesia, 5 (1 in PBS, 1 in Gel, 1 in Gel + bFGF and 2 in bFGF group) succumbed to acute left heart failure within 24 h of MI surgery and another 2 (1 in PBS and 1 in Gel group) due to wound infection. A total of 3 rats (1 in PBS, 1 in Gel and 1 in Gel + bFGF group) were excluded because the infarct size was <20% on visual inspection. From each group, 30 days post-MI after echocardiography testing, 7 rats were sacrificed to measure the infarct size, collagen content and angiogeneisis, in addition to a TUNEL assay. Echocardiography was performed in all the rats, 30 days post MI.
In vitro release kinetics of bFGF from the hydrogel
In vitro release rates of bFGF from the hydrogel are presented in Fig. 1. Within 5 days, ~60% of the bFGF was released from the hydrogel and, by day 10, ~72% of the bFGF was released. By day 30, >95% was released.
Figure 1.
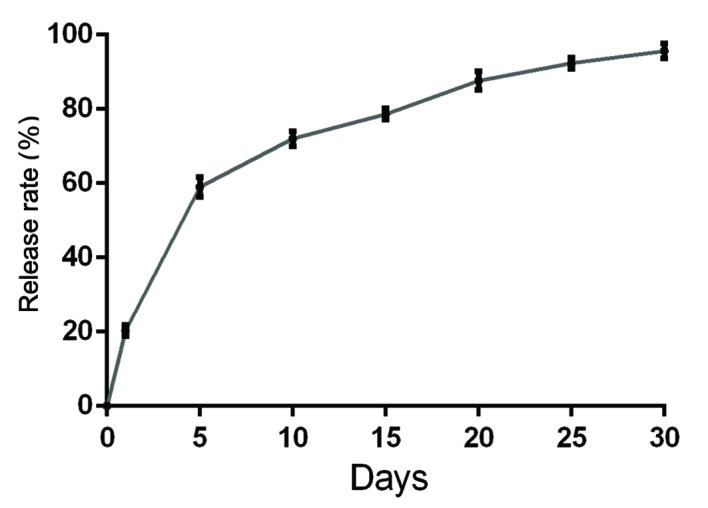
Time course of the in vitro basic fibroblast growth factor release from the Dex-PCL-HEMA/PNIPAAm hydrogel over 30 days.
LV diameter and LVEF
On day 30 post-MI, the Gel, bFGF and Gel + bFGF groups exhibited a significant reduction in LV diameter (P<0.05; Fig. 2A and B) and a significant increase in the LVEF (P<0.05; Fig. 2C), compared with the PBS group. The Gel + bFGF group demonstrated a significant decrease in LVEDD and LVESD and a significant increase in LVEF compared with the Hydrogel and bFGF group (P<0.05; Fig. 2A and B). Differences in the LV diameters or LVEF between the hydrogel and bFGF groups were not significant.
Figure 2.
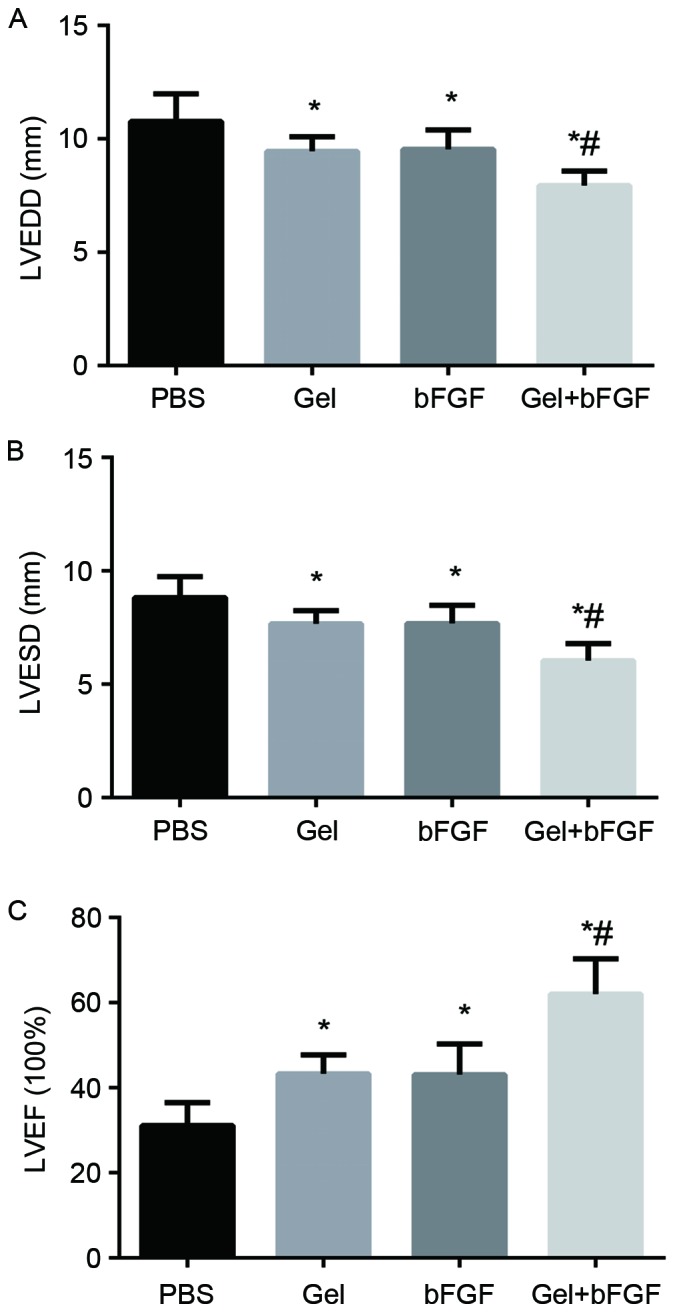
Echocardiographic evaluation of LV function. Evaluation of (A) LVEDD, (B) LVESD and (C) LVEF 30 days post-MI. Data are expressed as the mean + standard deviation, *P<0.05 vs. PBS and Gel; #P<0.05 vs. bFGF (n=7 in each group). LV, left ventricular; LVEDD, LV end-diastolic diameter; LVESD, LV end-systolic diameter; LVEF, LV ejection fraction; MI, myocardial infarction; bFGF, basic fibroblast growth factor; PBS, phosphate buffered saline; Gel, rats treated with 100 µl hydrogel without bFGF; Gel + bFGF, rats treated with 100 µl PBS containing 2 µg bFGF.
Infarct size
The Gel, bFGF and Gel + bFGF groups exhibited a significant reduction in infarct size compared with the PBS group on day 30 following MI (P<0.05; Fig. 3), whereas the Gel + bFGF group demonstrated the largest reduction compared with groups treated with either agent alone. The bFGF group demonstrated a slight reduction in infarct size compared with that of the Gel group, but it was not significant.
Figure 3.
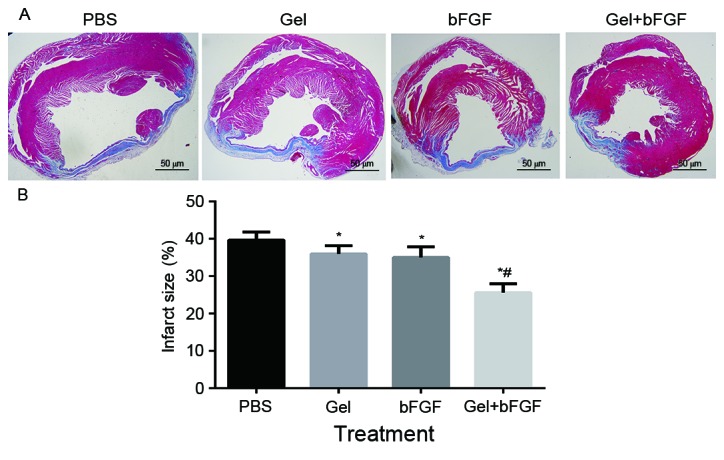
Infarct size on day 30 post-MI. (A) Representative images of left ventricles from each group following Masson's Trichrome staining (magnification, ×10). (B) Infarct size as percentages at 30 days. Infarct size is calculated from the ratio of surface area of infarct wall and the entire surface area of the left ventricle. Data are expressed as the mean + standard deviation, *P<0.05 vs. PBS and Gel; #P<0.05 vs. bFGF (n=7 in each group). MI, myocardial infarction; PBS, phosphate buffered saline; Gel, rats treated with 100 µl hydrogel without bFGF; bFGF, basic fibroblast growth factor; Gel + bFGF, rats treated with 100 µl PBS containing 2 µg bFGF.
Collagen content
On day 30 post-MI, the collagen content was significantly decreased following treatment with bFGF and hydrogel compared with the results of using either agent alone (P<0.05; Fig. 4). Although the PBS, Gel and bFGF groups indicated no significant differences in collagen content, the bFGF group exhibited a slightly decreased collagen content compared with that of the Gel and PBS group.
Figure 4.
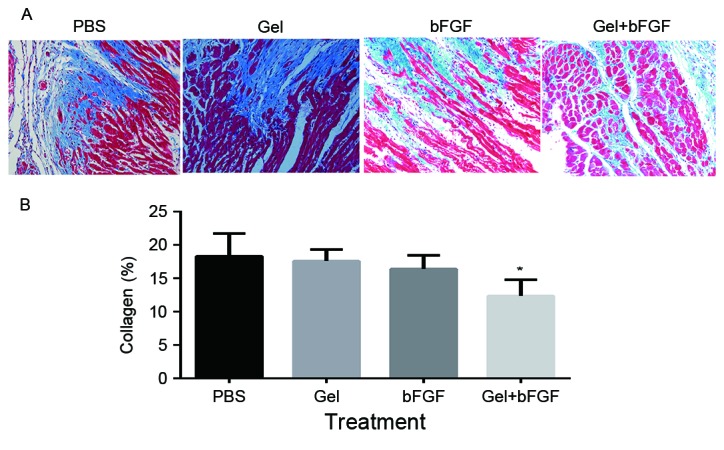
Collagen content 30 days post-MI. (A) Representative images of left ventricles from each group following Masson's Trichrome staining (magnification, ×200). (B) Collagen content as percentages on day 30. Collagen content is calculated from the ratio of the collagen area to the area of the entire high-power field. Data are expressed as the mean + standard deviation, *P<0.05 vs. PBS and Gel (n=7 in each group). MI, myocardial infarction; PBS, phosphate buffered saline; Gel, rats treated with 100 µl hydrogel without bFGF; bFGF, basic fibroblast growth factor; Gel + bFGF, rats treated with 100 µl PBS containing 2 µg bFGF.
Apoptosis
The bFGF group and the Gel + bFGF group exhibited a significant decrease (P<0.05; Fig. 5) in the apoptosis index when compared with the PBS and Gel groups 30 days after MI, whereas the Gel + bFGF group had a significantly reduced apoptosis index (P<0.05; Fig. 5) compared with the bFGF group. There was no significant difference in the apoptotic index between the PBS group and the Gel group (Fig. 5).
Figure 5.
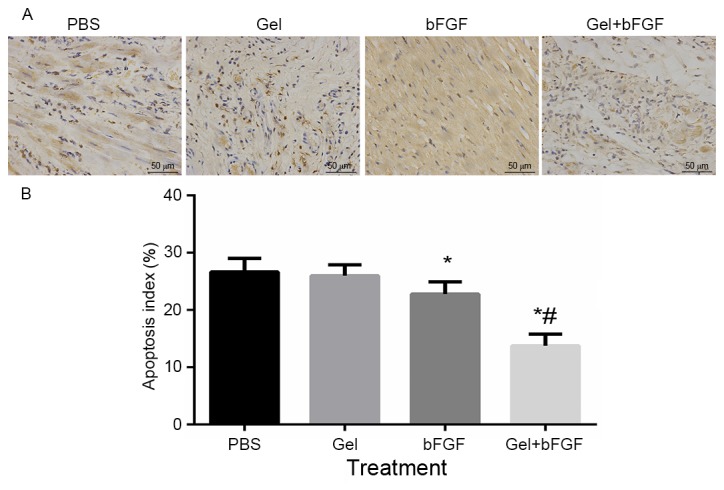
Cardiomyocyte apoptosis 30 days post-MI. (A) Immunohistochemistry of terminal deoxynucleotidyl transferase dUTP nick-end labeling in the border zone (magnification, ×200). (B) Apoptotic index was quantified at 30 days. Data are expressed as the mean ± standard deviation, *P<0.05 vs. PBS and Gel; #P<0.05 vs. bFGF (n=7 in each group). MI, myocardial infarction; PBS, phosphate buffered saline; Gel, rats treated with 100 µl hydrogel without bFGF; bFGF, basic fibroblast growth factor; Gel + bFGF, rats treated with 100 µl PBS containing 2 µg bFGF.
Neovascularization: α-SMA and CD31
On day 30 post-MI, the arterial density and capillary density in the peri-infarct area was significantly increased in the bFGF group and the Gel + bFGF group compared with the other groups (P<0.05; Figs. 6 and 7), whereas the Gel + bFGF group exhibited a significant increase in arterioles, compared with the bFGF group (P<0.05; Figs. 6 and 7). No significant difference in arteriole density was observed between the PBS group and the Gel group.
Figure 6.
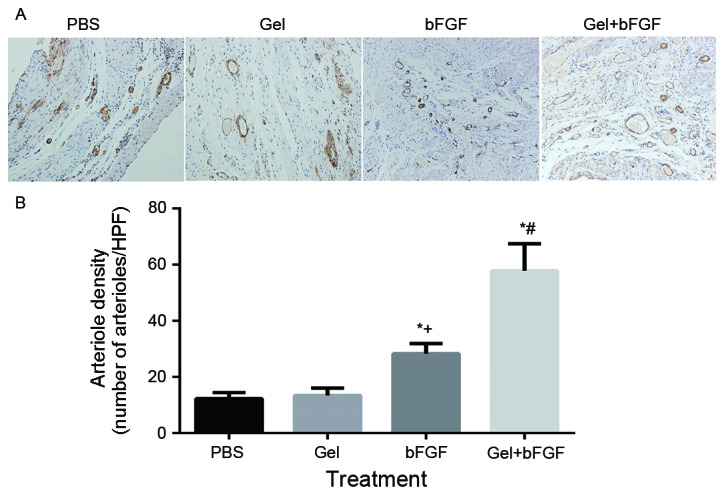
Arterial density 30 days post-MI. (A) Representative immunohistochemistry images of α-SMA staining in the border zone (magnification, ×200). (B) Arterial density per HPF (magnification, ×200) at 30 days. Data are expressed as the mean ± standard deviation, *P<0.05 vs. PBS; +P<0.05 vs. Gel; #P<0.05 vs. bFGF (n=7 in each group). MI, myocardial infarction; α-SMA, α-smooth muscle actin; PBS, phosphate buffered saline Gel, rats treated with 100 µl hydrogel without bFGF; bFGF, basic fibroblast growth factor; Gel + bFGF, rats treated with 100 µl PBS containing 2 µg bFGF; HPF, high power field.
Figure 7.
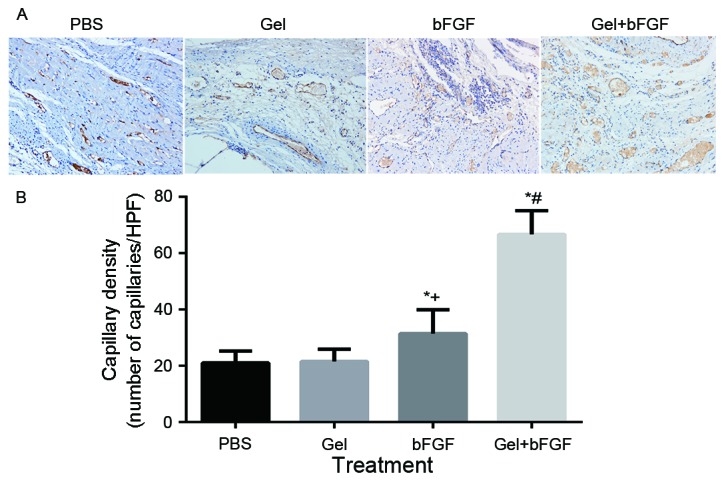
Capillary density 30 days post-MI. (A) Representative images of Immunohistochemistry of CD31 staining in the border zone (magnification, ×200). (B) Capillary density per HPF (magnification, ×200) at 30 days. Data are expressed as the mean ± standard deviation, *P<0.05 vs. PBS; +P<0.05 vs. Gel; #P<0.05 vs. bFGF (n=7 in each group). MI, myocardial infarction; CD31, cluster of differentiation 31; PBS, phosphate buffered saline; Gel, rats treated with 100 µl hydrogel without bFGF; bFGF, basic fibroblast growth factor; Gel + bFGF, rats treated with 100 µl PBS containing 2 µg bFGF; HPF, high power field.
Discussion
The present study is, to the best of our knowledge, the first to demonstrate that an intramyocardial injection of bFGF along with a Dex-PCL-HEMA/PNIPAAm hydrogel immediately following MI in rats significantly decreases the size of the infarct zone for up to 30 days, inhibits the apoptosis of cardiomyocytes, increases angiogenesis, attenuates ventricular remodeling, and improves cardiac function compared with injection of either alone or PBS treatment.
Angiogenic therapy is emerging as a potential strategy for the treatment of ischemic heart disease. As growth factors have a short half-life, numerous approaches have been used to deliver them in vivo, including intracoronary delivery, intravenous delivery and left atrial administration. (8,9,21,22) However, these methods cannot ensure an adequate concentration of the growth factors in the targeted myocardium as general blood circulation accelerates their metabolism and degradation (23). Lazarous et al (24) demonstrated that when ~100 µg/kg bFGF was injected per animal, only 3–5% of the total dose was recovered from the heart following intracoronary administration, 1.3% following left atrial administration, 0.5% following intravenous or Swanz Ganz delivery, and 19% 150 min after pericardial administration. Furthermore, such delivery methods with a high dose of bFGF have side effects, including proatherogenic effects, plaque expansion and instability, rheumatoid arthritis, hemangiomas and atherosclerosis (25). An intramyocardial route provides a high myocardial uptake of angiogenesis factors. Kawasuji et al (26) demonstrated that intramyocardial administration of bFGF increased regional myocardial blood flow and improved cardiac function in acute MI, indicating that intramyocardial administration of bFGF may be a possible therapeutic approach for patients with acute MI. Similarly, in the current study when compared with the PBS group, the bFGF group presented with a smaller infarct size, less collagen deposition, a smaller apoptosis index, more angiogenesis and more preserved cardiac function. Furthermore, the dose of bFGF in the current study was only 8 µg/kg per rat, which was much less than the doses used in previous studies.
Biomaterials including collagen (27), fibrin glue (28), alginate (29), matrigel and self-assembling peptides (30) and hydrogel (31) are widely used in tissue engineering and they are more likely to be used as a delivery and sustained release system for growth factors to induce enhanced myocardial protective effects. Garbern et al (32) used a pH-responsive hydrogel carrying bFGF to induce angiogenesis and achieve therapeutic effects on regional blood flow and cardiac function in infarcted myocardium. However, the dual ability of this hydrogel to respond to both pH and temperature makes it uncontrollable in the presence of an infarcted myocardium with a pathological microenvironment. The sustained release of bFGF with an alginate microsphere induced a valid improvement in treating MI but the alginate is a polysaccharide with poor biodegradability (33). A combination of polyvinyl alcohol-dextran blended hydrogel with bFGF stimulated angiogenesis and increased wall thickness in an infarcted myocardium but did not increase cardiac function 2 months after MI (34). In a study by Nie et al (7), bFGF integrated with a fibrin glue in a canine infarct model improved myocardial perfusion and cardiac function, but the fibrin interacted with cells from the blood and vessel walls, which would inevitably impair the bindings of bFGF to relevant receptors (7).
Compared with these materials, the hydrogel used in the current study has a number of advantages. The Dex-PCL-HEMA/PNIPAAm hydrogel is biodegradable, biocompatible and nontoxic. Furthermore, this hydrogel is thermosensitive and exhibits a LCST (~33°C) and solidifies at 37°C (18,35). Thus, in vitro the liquid hydrogel uniformly mixed with bFGF and, when it was injected into the heart tissue, the hydrogel solidified instantly in situ at body temperature. The rapid gelation kinetics of the hydrogel guaranteed the effective entrapment of biologically active additives at the injection site. The solid hydrogel serves as a controlled release system and caused local and sustained release of bFGF at a relatively high concentration. As the release kinetics of bFGF from the hydrogel in vitro demonstrated, even 30 days following mixing, bFGF was still being released from the hydrogel.
Furthermore, the hydrogels are cross-linked and porous polymer networks that may absorb a large amount of water and exchange oxygen, nutrients and other metabolites, thus making hydrogels similar to the cardiac extracellular matrix (ECM). The hydrogelism ECM may replace certain damaged ECM functions and provide a suitable microenvironment for the infarcted myocardiums, which results in a smaller infarct size and less collagen deposition. The solid hydrogel also has a controllable mechanical strength, which may provide structural and mechanical support for the infarcted wall and prevent paradoxical motion at the infarct site.
As a previous study demonstrated, a MI of the Dex-PCL-HEMA/PNIPAAm hydrogel may significantly inhibit infarct ventricle remodeling and improve cardiac function by thickening and toughening the infarcted wall and reducing paradoxical motion (35). Therefore, this hydrogel serves as an effective controlled release system to deliver proteins, genes and cells at the infarct site in a sustained and local manner and to induce enhanced cardiac protective effects compared with using either hydrogel or bioactive additives alone (19,20,36). For example, a MI of a hydrogel combined with vascular endothelial growth factor-165 in the rat heart 30 days following MI stimulation has been demonstrated to enhance angiogenesis and improve cardiac function (37).
Similarly, the present study indicated that the delivery of bFGF with a Dex-PCL-HEMA/PNIPAAm hydrogel may also improve angiogenesis and cardioprotection in infarcted myocardium. In the bFGF + Gel group, the biocompatibility and thermosensitivity of the hydrogel served as a controlled release system and caused bFGF to accumulate at relatively high concentrations in the peri-infarct areas. It induced more angiogenesis than that of the bFGF group. Furthermore, the porous polymer physical structure of the hydrogel allows the absorbion of a large amount of water and nutrients, and it may serve as a supporting wall at the peri-infarct site. The bFGF + Gel group also had a smaller infarct size, less collagen deposition and a smaller apoptosis index. However, in the Gel and bFGF groups, the supporting function of the hydrogel and the angiogenesis activity of the bFGF are less effective than in the combined bFGF + Gel group. Thus, the hydrogel may prolong the release of bFGF and increase the local concentration of bFGF by protecting against extracellular degradation. The inherent function of the hydrogel itself may be the mechanism by which, an intramyocardial injection of hydrogel with bFGF into the peri-infarcted hearts of rats has a more synergistic effect than using bFGF or hydrogel either alone.
A limitation of the present study is that the release kinetics of sustained bFGF delivery from the hydrogel in vivo was not analyzed. Future studies should investigate this and assess different concentrations of bFGF to determine the optimal dosage.
In conclusion, the present study is, to the best of our knowledge, the first to demonstrate that a combination of bFGF and Dex-PCL-HEMA/PNIPAAm hydrogel has synergistic benefits that are superior to the administration of either treatment alone. This combination is able to induce angiogenesis, attenuate cardiac remodeling and improve cardiac function in rats with MI.
Acknowledgements
The present study was supported by grants from the National Nature Science Foundation of China (grant no. 81170307), National Key Basic Research Program of China (grant no. 2005CB623903) and The Fundamental Research Funds for the Central Universities (grant no. 2042014kf0130). The authors would also like to thank The Nature Publishing Group Language Editing company for editing the original manuscript.
References
- 1.Zhang BH, Guo CX, Wang HX, Lu LQ, Wang YJ, Zhang LK, Du FH, Zeng XJ. Cardioprotective effects of adipokine apelin on myocardial infarction. Heart Vessels. 2014;29:679–689. doi: 10.1007/s00380-013-0425-z. [DOI] [PubMed] [Google Scholar]
- 2.Mann DL. Mechanisms and models in heart failure: A combinatorial approach. Circulation. 1999;100:999–1008. doi: 10.1161/01.CIR.100.9.999. [DOI] [PubMed] [Google Scholar]
- 3.Gierach J, Gierach M, Świątkiewicz I, Woźnicki M, Grześk G, Sukiennik A, Koziñski M, Kubica J. Admission glucose and left ventricular systolic function in non-diabetic patients with acute myocardial infarction. Heart Vessels. 2016;31:298–307. doi: 10.1007/s00380-014-0610-8. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Torabi A, Khan NK. Epidemiology and management of heart failure and left ventricular systolic dysfunction in the aftermath of a myocardial infarction. Heart. 2005;91(Suppl 2)(ii7-13):ii43–48. doi: 10.1136/hrt.2005.062026. ii31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jośko J, Gwóźdź B, Jedrzejowska-Szypułka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6:1047–1052. [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 7.Nie SP, Wang X, Qiao SB, Zeng QT, Jiang JQ, Liu XQ, Zhu XM, Cao GX, Ma CS. Improved myocardial perfusion and cardiac function by controlled-release basic fibroblast growth factor using fibrin glue in a canine infarct model. J Zhejiang Univ Sci B. 2010;11:895–904. doi: 10.1631/jzus.B1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, Stiber JA, Lobo AD, Hunsberger S, Guetta E, et al. Comparative effects of basic fibroblast growth factor and vascular endothelial growth factor on coronary collateral development and the arterial response to injury. Circulation. 1996;94:1074–1082. doi: 10.1161/01.CIR.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 9.Lazarous DF, Scheinowitz M, Shou M, Hodge E, Rajanayagam S, Hunsberger S, Robison WG, Jr, Stiber JA, Correa R, Epstein SE, et al. Effects of chronic systemic administration of basic fibroblast growth factor on collateral development in the canine heart. Circulation. 1995;91:145–153. doi: 10.1161/01.CIR.91.1.145. [DOI] [PubMed] [Google Scholar]
- 10.Si HB, Zeng Y, Lu YR, Cheng JQ, Shen B. Control-released basic fibroblast growth factor-loaded poly-lactic-co-glycolic acid microspheres promote sciatic nerve regeneration in rats. Exp Ther Med. 2017;13:429–436. doi: 10.3892/etm.2016.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin Z, Hu Y, Wang Z, Pan S, Zhang H, Ye L, Zhang H, Fang M, Jiang H, Ye J, et al. Intranasal basic fibroblast growth factor attenuates endoplasmic reticulum stress and brain injury in neonatal hypoxic-ischaemic injury. Am J Transl Res. 2017;9:275–288. [PMC free article] [PubMed] [Google Scholar]
- 12.Ran F, Liu C, Liu Z, Shang T, Zhou M, Qiao T. Preventive effects of basic fibroblast growth factor on vascular restenosis after balloon angioplasty. Exp Ther Med. 2014;7:1193–1196. doi: 10.3892/etm.2014.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanazawa T, Kurakami K, Kashima K, Konomi U, Komazawa D, Nakamura K, Matsushima K, Akagi Y, Misawa K, Nishino H, Watanabe Y. Injection of basic fibroblast growth factor for unilateral vocal cord paralysis. Acta Otolaryngol. 2017;137:962–967. doi: 10.1080/00016489.2017.1314550. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai M, Marui A, Tabata Y, Takeda T, Yamamoto M, Yonezawa A, Tanaka S, Yanagi S, Ito-Ihara T, Ikeda T, et al. Safety and efficacy of sustained release of basic fibroblast growth factor using gelatin hydrogel in patients with critical limb ischemia. Heart Vessels. 2016;31:713–721. doi: 10.1007/s00380-015-0677-x. [DOI] [PubMed] [Google Scholar]
- 15.Floege J, Kriz W, Schulze M, Susani M, Kerjaschki D, Mooney A, Couser WG, Koch KM. Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J Clin Invest. 1995;96:2809–2819. doi: 10.1172/JCI118351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaki T, Jyo Y, Tanda N, Kawakami Y, Nohno T, Tamai H, Osawa G. Changes in glomerular epithelial cells induced by FGF2 and FGF2 neutralizing antibody in puromycin aminonucleoside nephropathy. Kidney Int. 1997;51:301–309. doi: 10.1038/ki.1997.37. [DOI] [PubMed] [Google Scholar]
- 17.Sellke FW, Laham RJ, Edelman ER, Pearlman JD, Simons M. Therapeutic angiogenesis with basic fibroblast growth factor: Technique and early results. Ann Thorac Surg. 1998;65:1540–1544. doi: 10.1016/S0003-4975(98)00340-3. [DOI] [PubMed] [Google Scholar]
- 18.Wu DQ, Qiu F, Wang T, Jiang XJ, Zhang XZ, Zhuo RX. Toward the development of partially biodegradable and injectable thermoresponsive hydrogels for potential biomedical applications. ACS Appl Mater Interfaces. 2009;1:319–327. doi: 10.1021/am8000456. [DOI] [PubMed] [Google Scholar]
- 19.He YY, Wen Y, Zheng XX, Jiang XJ. Intramyocardial delivery of HMGB1 by a novel thermosensitive hydrogel attenuates cardiac remodeling and improves cardiac function after myocardial infarction. J Cardiovasc Pharmacol. 2013;61:283–290. doi: 10.1097/FJC.0b013e31827ecd50. [DOI] [PubMed] [Google Scholar]
- 20.Wan WG, Jiang XJ, Li XY, Zhang C, Yi X, Ren S, Zhang XZ. Enhanced cardioprotective effects mediated by plasmid containing the short-hairpin RNA of angiotensin converting enzyme with a biodegradable hydrogel after myocardial infarction. J Biomed Mater Res A. 2014;102:3452–3458. doi: 10.1002/jbm.a.35014. [DOI] [PubMed] [Google Scholar]
- 21.Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, Udelson JE, Gervino EV, Pike M, Whitehouse MJ, et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2: Double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- 22.Rajanayagam MA, Shou M, Thirumurti V, Lazarous DF, Quyyumi AA, Goncalves L, Stiber J, Epstein SE, Unger EF. Intracoronary basic fibroblast growth factor enhances myocardial collateral perfusion in dogs. J Am Coll Cardiol. 2000;35:519–526. doi: 10.1016/S0735-1097(99)00550-1. [DOI] [PubMed] [Google Scholar]
- 23.Laham RJ, Rezaee M, Post M, Sellke FW, Braeckman RA, Hung D, Simons M. Intracoronary and intravenous administration of basic fibroblast growth factor: Myocardial and tissue distribution. Drug Metab Dispos. 1999;27:821–826. [PubMed] [Google Scholar]
- 24.Lazarous DF, Shou M, Stiber JA, Dadhania DM, Thirumurti V, Hodge E, Unger EF. Pharmacodynamics of basic fibroblast growth factor: Route of administration determines myocardial and systemic distribution. Cardiovasc Res. 1997;36:78–85. doi: 10.1016/S0008-6363(97)00142-9. [DOI] [PubMed] [Google Scholar]
- 25.Kornowski R, Fuchs S, Leon MB, Epstein SE. Delivery strategies to achieve therapeutic myocardial angiogenesis. Circulation. 2000;101:454–458. doi: 10.1161/01.CIR.101.4.454. [DOI] [PubMed] [Google Scholar]
- 26.Kawasuji M, Nagamine H, Ikeda M, Sakakibara N, Takemura H, Fujii S, Watanabe Y. Therapeutic angiogenesis with intramyocardial administration of basic fibroblast growth factor. Ann Thorac Surg. 2000;69:1155–1161. doi: 10.1016/S0003-4975(99)01557-X. [DOI] [PubMed] [Google Scholar]
- 27.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: A novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 28.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 30.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang XJ, Wang T, Li XY, Wu DQ, Zheng ZB, Zhang JF, Chen JL, Peng B, Jiang H, Huang C, Zhang XZ. Injection of a novel synthetic hydrogel preserves left ventricle function after myocardial infarction. J Biomed Mater Res A. 2009;90:472–477. doi: 10.1002/jbm.a.32118. [DOI] [PubMed] [Google Scholar]
- 32.Garbern JC, Minami E, Stayton PS, Murry CE. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32:2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez JJ, Edelman ER, Stamler A, Hibberd MG, Prasad P, Caputo RP, Carrozza JP, Douglas PS, Sellke FW, Simons M. Basic fibroblast growth factor in a porcine model of chronic myocardial ischemia: A comparison of angiographic, echocardiographic and coronary flow parameters. J Pharmacol Exp Ther. 1997;282:385–390. [PubMed] [Google Scholar]
- 34.Fathi E, Nassiri SM, Atyabi N, Ahmadi SH, Imani M, Farahzadi R, Rabbani S, Akhlaghpour S, Sahebjam M, Taheri M. Induction of angiogenesis via topical delivery of basic-fibroblast growth factor from polyvinyl alcohol-dextran blend hydrogel in an ovine model of acute myocardial infarction. J Tissue Eng Regen Med. 2013;7:697–707. doi: 10.1002/term.1460. [DOI] [PubMed] [Google Scholar]
- 35.Wang T, Wu DQ, Jiang XJ, Zhang XZ, Li XY, Zhang JF, Zheng ZB, Zhuo R, Jiang H, Huang C. Novel thermosensitive hydrogel injection inhibits post-infarct ventricle remodelling. Eur J Heart Fail. 2009;11:14–19. doi: 10.1093/eurjhf/hfn009. [DOI] [PubMed] [Google Scholar]
- 36.Li XY, Wang T, Jiang XJ, Lin T, Wu DQ, Zhang XZ, Okello E, Xu HX, Yuan MJ. Injectable hydrogel helps bone marrow-derived mononuclear cells restore infarcted myocardium. Cardiology. 2010;115:194–199. doi: 10.1159/000281840. [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Jiang X, Li X, Hu M, Wan W, Wen Y, He Y, Zheng X. Intramyocardial delivery of VEGF165 via a novel biodegradable hydrogel induces angiogenesis and improves cardiac function after rat myocardial infarction. Heart Vessels. 2016;31:963–975. doi: 10.1007/s00380-015-0710-0. [DOI] [PubMed] [Google Scholar]


