Abstract
Objective
Super‐refractory status epilepticus (SRSE) is a life‐threatening form of status epilepticus that continues or recurs despite 24 hours or more of anesthetic treatment. We conducted a multicenter, phase 1/2 study in SRSE patients to evaluate the safety and tolerability of brexanolone (USAN; formerly SAGE‐547 Injection), a proprietary, aqueous formulation of the neuroactive steroid, allopregnanolone. Secondary objectives included pharmacokinetic assessment and open‐label evaluation of brexanolone response during and after anesthetic third‐line agent (TLA) weaning.
Methods
Patients receiving TLAs for SRSE control were eligible for open‐label, 1‐hour brexanolone loading infusions, followed by maintenance infusion. After 48 hours of brexanolone infusion, TLAs were weaned during brexanolone maintenance. After 4 days, the brexanolone dose was tapered. Safety and functional status were assessed over 3 weeks of follow‐up.
Results
Twenty‐five patients received open‐label study drug. No serious adverse events (SAEs) were attributable to study drug, as determined by the Safety Review Committee. Sixteen patients (64%) experienced ≥1 SAE. Six patient deaths occurred, all deemed related to underlying medical conditions. Twenty‐two patients underwent ≥1 TLA wean attempt. Seventeen (77%) met the response endpoint of weaning successfully off TLAs before tapering brexanolone. Sixteen (73%) were successfully weaned off TLAs within 5 days of initiating brexanolone infusion without anesthetic agent reinstatement in the following 24 hours.
Interpretation
In an open‐label cohort of limited size, brexanolone demonstrated tolerability among SRSE patients of heterogeneous etiologies and was associated with a high rate of successful TLA weaning. The results suggest the possible development of brexanolone as an adjunctive therapy for SRSE requiring pharmacological coma for seizure control. Ann Neurol 2017;82:342–352
Status epilepticus (SE) is a life‐threatening condition that involves persistent or recurring seizures.1 Patients with recurrent seizures after first‐line emergency treatment are commonly administered second‐line antiepileptic drugs (AEDs).2 Patients who fail to respond to second‐line agents are said to have refractory SE (RSE) and are commonly administered continuous intravenous (IV) anesthetic third‐line agents (TLAs).2 Super‐refractory status epilepticus (SRSE) is a life‐threatening form of status epilepticus that continues or recurs despite 24 hours or more of TLA treatment.2 Experimental evidence supports that SE becomes more refractory with time,3 and achieving resolution of seizures and return of consciousness takes longer with SRSE than with RSE or SE.4
A major concern for current treatments is the lack of clinical evidence for treatment outcomes.5, 6 Between 26.6% and 36.6% of patients with SE fail to respond to the administration of first‐line agents,7, 8 of which approximately 23% also fail second‐line agents9 and 10% to 15% fail TLAs,10 thus progressing to SRSE. Approximately one third of patients with RSE or SRSE die,2, 9, 11 one third recover, typically with neurological or other deficits, and one third return to baseline.2, 8 A retrospective study revealed that 76.2% of discharged patients with an in‐hospital diagnosis of RSE had a poor functional outcome as defined by modified Rankin score.11
Allopregnanolone is a potent, endogenously produced neuroactive steroid that acts as a positive allosteric modulator (PAM) on a broad range of γ‐aminobutyric acid type A receptor (GABAAR) isoforms12, 13, 14, 15, 16 expressed throughout the brain, including those that mediate either phasic or tonic inhibition.17, 18, 19 Extrasynaptic receptors containing the δ‐subunit that mediate tonic inhibition are particularly sensitive to modulation by neurosteroids.18 The robust effects of allopregnanolone on multiple GABAA receptor populations in vitro produces a potent regulation of cortical function, especially under conditions of hyperexcitability.20
Allopregnanolone has demonstrated significant protection against seizures in several animal models.15, 16, 21 Continuous allopregnanolone IV administration in two murine seizure models predict that anticonvulsant effects should occur at plasma concentrations within the range of 37 to 142ng/ml.22, 23, 24 Short infusions of allopregnanolone that result in plasma concentrations that range from 9.4 to 25.1μg/ml (30 and 80μM) for up to 90 minutes have been previously reported to cause anesthesia in rats, without notable heart or respiratory rate effects.22, 23
In addition to the preclinical evidence, three recent case studies report the successful treatment of SRSE with brexanolone (USAN; formerly SAGE‐547 Injection; Sage Therapeutics, Inc., Cambridge, MA), a proprietary formulation of allopregnanolone, in human patients.25, 26, 27 Given the evidence suggesting potential benefits of allopregnanolone in treating SRSE, the objectives of the present study were to evaluate the safety and tolerability, pharmacokinetics (PK), and response to brexanolone in a cohort of patients with SRSE.
Materials and Methods
Study Design
This open‐label, uncontrolled phase 1/2 study of brexanolone (547‐SSE‐201) in the treatment of SRSE comprised a 24‐hour screening period, a 4‐day treatment period (96 hours), a 1‐day dose taper period (24 hours), a 2‐day acute follow‐up period, and a 3‐week extended follow‐up period (days 8, 15, 22, and 29; Fig). The study population consisted of adults and children aged ≥2 years diagnosed with SRSE who previously underwent therapy with IV TLA(s) for ≥24 hours to achieve seizure or burst suppression. For this study, SRSE was defined by the following criteria and in accord with those used at major epilepsy treatment centers5:
Failure to respond to the administration of at least one first‐line agent (eg, benzodiazepine or other emergent initial treatment), according to primary standard of care, and
Failure to respond to at least one second‐line agent (eg, phenytoin, fosphenytoin, valproate, phenobarbital, levetiracetam, or other urgent control treatment), according to primary standard of care, and
Failure to be weaned off of TLA(s), or presence of one or more breakthrough seizures >6 hours after initiation of at least one TLA (e.g., pentobarbital, midazolam, or propofol) that had to be titrated up to burst suppression or seizure suppression (ie, to include those patients who, despite maximal treatment, were unable to be seizure or burst suppressed).
Figure 1.
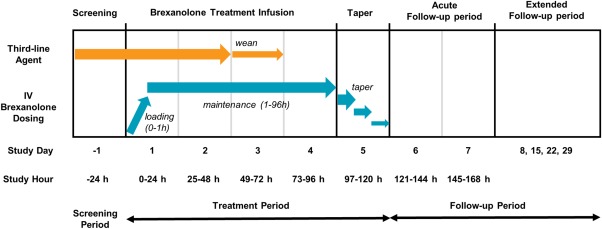
Clinical study design showing brexanolone administration and patient evaluation time points for open‐label phase 1/2 study.
Specifically, failure to respond to TLAs (criterion #3 above) was allowed in diagnoses at all sites when breakthrough seizures occurred during active infusion of TLAs >6 hours after initiation, for example, requiring addition of two TLAs for seizure control. If seizures initially abated during active infusion of an TLA, SRSE could alternatively be diagnosed if seizures recurred upon weaning a TLA after 24 hours of administering the TLA. This definition differs somewhat from a recent definition of Shorvon et al,2 in that SRSE was allowed to be diagnosed in patients in whom seizure activity persisted despite TLA administration, making them clinically inappropriate for a wean. As such, this SRSE definition incorporates other versions of the SRSE definition promulgated by Shorvon and Trinka defining SRSE as “the stage reached when the seizures are either not controlled by initial anesthesia or recur after initial control.”28 Patients in whom SRSE achieved at least seizure suppression upon TLA administration nevertheless required 24 hours of TLA administration preceding a wean.2
After approval by a local hospital‐based institutional review board, written informed consent by a legally authorized representative was obtained before commencement of any study‐related procedures not considered standard of care. Patient eligibility based on inclusion and exclusion criteria was confirmed by the clinical investigator and medical monitor (Table 1). A safety review committee (SRC), which included independent, noninvestigator, nonsponsor, critical care specialists in addition to sponsor representatives, reviewed safety, treatment response, and PK data for each patient after treatment with brexanolone and determined whether the study could continue as planned. Serious adverse events (SAEs) were reported to the SRC on an ongoing basis throughout the study.
Table 1.
Patient Eligibility
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
• ≥2 years of age • EEG‐confirmed SRSE diagnosis under concomitant IV TLA treatment for ≥24 hours • Failure to control seizures after ≥6 hours on TLAs • Failure to be weaned from TLAs after ≥24 hours |
• Pregnant or lactating • Known allergies to progesterone or allopregnanolone • SRSE attributed to anoxic/hypoxic encephalopathy • Children (patients <18 years) with an encephalopathy attributed to an underlying progressive neurological disorder • Clinically significant ECG abnormalities • Significant medical or surgical condition that could compromise vital organ systems, or other conditions that could place the patient at increased risk • Patients who receive IV treatment for seizure or burst suppression, which would require more than 24 hours to wean • Patients who have been exposed to other investigational medications or devices within 30 days • Patients previously enrolled in the trial |
EEG = electroencephalogram; ECG = electrocardiogram; SRSE = super‐refractory status epilepticus; IV = intravenous; TLAs = anesthetic third‐line agent.
Treatment and Procedures
Assessments and laboratory samples were collected from prospective patients during the screening period in order to determine eligibility. All patients were in a pharmacologically induced coma and were being treated with TLAs.
A 5‐day infusion of brexanolone was added to primary standard‐of‐care treatment for SRSE. Brexanolone is a solution of 5mg/ml allopregnanolone in 250mg/ml of sulfobutylether‐beta‐cyclodextrin (Ligand Pharmaceuticals, Inc., La Jolla, CA) diluted in sterile water for injection and administered intravenously. Brexanolone was administered in the form of a loading dose (286.6μg/kg for 1 hour), followed by a 4‐day maintenance infusion (86 or 156μg/kg/h).
The standard maintenance infusion rate of brexanolone to be studied in this trial was chosen to achieve a mean allopregnanolone exposure of approximately 47ng/ml. This is equivalent to the highest endogenous concentrations measured during pregnancy (approximately 50ng/ml during the third trimester),29 which is well tolerated by women without apparent adverse events (AEs). The brexanolone dose selected to achieve this target concentration was 86μg/kg/h, which is considered the standard maintenance dose.
The high maintenance dose was targeted to produce plasma concentrations of approximately 94ng/ml and was modeled to achieve 156μg/kg/h. This high‐dose regimen was initiated after safety and tolerability of the standard dose regimen were reviewed. The plasma concentrations observed with the standard and high‐dose regimens of brexanolone are in line with the range suggested by PK modeling (data on file; Sage Therapeutics, Inc.).
The initial loading dose and first 48 hours of brexanolone maintenance served to assess safety and assure that seizure activity remained controlled. If the patient's seizure activity was controlled with no SAEs requiring study drug discontinuation, the TLA was weaned per hospital standard of care after 48 hours of the maintenance infusion of brexanolone (ie, study day 3; Fig), which was then continued for the remainder of the 96‐hour treatment period to assess the ability of brexanolone to maintain seizure suppression. Continuous electroencephalogram (EEG) monitoring was used to determine whether the seizure activity had stopped. If significant seizure activity resumed while weaning off the TLA, the patient was treated as medically appropriate, with resumption of a concomitant TLA if needed. Repeated weaning attempts were encouraged, if medically appropriate. After 96 hours (4 days) of therapy with brexanolone, the dose was tapered and discontinued over 24 hours. If, at any time during the study, the clinical investigator determined that brexanolone treatment should be terminated, the 24‐hour taper would have occurred immediately.
Attempts to wean the patient from TLA(s) were made on days 3 and 4 during the maintenance infusion (Fig). Primary standard‐of‐care treatment for SRSE and ongoing treatment(s) for all underlying medical conditions were given concomitantly with brexanolone treatment in all patients. All patients receiving treatment with brexanolone were followed up with study assessments to day 29.
The prespecified primary outcomes included safety and tolerability measures, including monitoring of treatment‐emergent AEs, vital signs, physical exam, 12‐lead electrocardiogram (ECG), and clinical laboratory values (serum chemistry, hematology, and urinalysis). Safety outcomes, including AEs, were evaluated throughout the course of the study (screening through day 29). The safety population, defined as all patients who had an infusion of brexanolone initiated, was used to summarize all disposition, baseline characteristic, and safety data.
The prespecified secondary response outcomes were based on the response assessment population, defined as all patients who completed the brexanolone infusion and who had at least one TLA wean attempt before the start of the taper of brexanolone. Key response measures included treatment response and duration of response. Responders were defined as all patients for whom the wean attempt was successful and reinitiation of TLA(s) for seizure control during administration of the brexanolone maintenance dose was not required. The PK population was defined as all patients who received the study drug and had at least one sample taken for PK analysis. PK modeling was performed by an independent clinical research organization (Covance, Dedham, MA).
Outcomes were evaluated over the 5‐day treatment and the 3‐week follow‐up period, with baseline scores (as applicable) established during the screening period. Status Epilepticus Severity Score (STESS; range of 0–6), a validated tool for systematic evaluation of the outcome of SE patients, was tabulated by survival endpoints.30 Functional status of patients was monitored using Modified Rankin Scale‐9Q (mRS‐9Q; range of 0–5),31 Clinical Global Impression‐Severity/Improvement (CGI‐S/CGI‐I; range of 1–7),32 Richmond Agitation‐Sedation Scale (RASS; adults only; range of –5 to 4),33 Glasgow Coma Scale (GCS; range, 3–15),34 and the National Institutes of Health Stroke Scale (NIHSS; range of 0–42)35 scores.
Recurrence of SE for patients successfully weaned off TLA(s), but subsequently requiring reinitiation of any TLA treatment following conclusion of the brexanolone treatment regimen was monitored, along with plasma allopregnanolone concentration and overall survival during the 29‐day study period. Response data were summarized at each time point collected. For all data, the visit or start date was used to calculate study day, defined according to the number of hours postinitiation of brexanolone infusion. The first 24‐hour brexanolone infusion administration was considered day 1.
Statistical Analysis
The safety population, defined as all subjects who received an infusion of brexanolone, was used to summarize patient disposition, baseline demographics and characteristics, and safety data. The response assessment population, defined as all subjects who completed brexanolone infusion and had at least one attempt to wean the TLA(s) before the start of the taper of brexanolone infusion, was used to summarize all response variables.
No imputation process was used to estimate missing data. Baseline values were defined as the last observed value before initiation of brexanolone infusion. No formal statistical testing was performed. Outcome variables and changes from baseline were summarized at each time point and at the end of treatment observation by dose regimen using descriptive statistics. For continuous outcome measures, descriptive statistics included the number of subjects (n), mean, standard deviation (SD), and range, whereas n and percentage (%) were included for categorical/binary variables
Results
Patient Characteristics and Demographics
Twenty‐five (25) eligible patients with confirmed diagnoses of SRSE by study criteria were enrolled at 12 of 18 open sites (Table 2). Although the SRSE study criteria differed slightly from the SRSE definition proposed by Shorvon et al by allowing for either failure upon a wean following 24 hours of TLA administration or alternatively persistent seizure activity despite ongoing TLA efforts at seizure suppression (see Materials and Methods), enrolled subjects had a mean duration of SRSE at baseline of 6.1 days (range, 1, 19).2 The underlying etiologies of SRSE included cerebral meningitis or encephalitis (n = 6; 24%); intracranial hemorrhage (n = 4; 16%); worsening of a known seizure disorder and idiopathic causes (n = 3; 12% each); unknown causes (n = 3; 12% each); primary/metastatic brain tumors and toxic ingestion (n = 2 each; 8%); and anti‐N‐methyl‐d‐aspartate receptor (anti‐NMDA‐R) encephalitis, stroke, sickle cell anemia, posterior reversible encephalopathy syndrome (PRES), and lupus (n = 1 each; 4%). All patients had been treated with TLAs, which included a history of midazolam, pentobarbital, propofol, and ketamine, or combinations thereof (Table 2).
Table 2.
Summary of Baseline Demographics and Clinical Characteristics
| Patient Profile | Mean | SD | Range | n/N (%) |
|---|---|---|---|---|
| Sex | ||||
| Male | — | — | — | 16/25 (64.0) |
| Female | — | — | — | 9/25 (36.0) |
| Intubated | — | — | — | 25/25 (100) |
| Age, y | 47.6 | 19.52 | 10, 76 | 25/25 (100) |
| Overall duration, days | 9.2 | 5.70 | 3, 20 | 25/25 (100) |
| SE | 2.2 | 2.30 | 1, 12 | 25/25 (100) |
| RSE | 3.0 | 2.63 | 1, 13 | 25/25 (100) |
| SRSE | 6.1 | 5.16 | 1, 19 | 25/25 (100) |
| NIHSS total score | 33.4 | 4.87 | 20, 38 | 25/25 (100) |
| STESS total score | 3.4 | 1.00 | 1, 5 | 25/25 (100) |
| GCS total score | 3.8 | 1.65 | 3, 10 | 24/25 (96) |
| RASS score | –4.8 | 0.49 | –5, –3 | 23/25 (92) |
| mRS‐9Q score | 5.0 | 0.00 | 5, 5 | 24/25 (96) |
| CGI‐S score | 6.9 | 0.28 | 6, 7 | 25/25 (100) |
| Past wean attempts | 2.0 | 1.97 | 0, 8 | 25/25 (100) |
| TLA History a (Safety Population; N = 25) | Standard Dose | High Dose | Total | Total % |
| Ketamine | 4 | 0 | 4 | 16 |
| Midazolam | 13 | 4 | 17 | 68 |
| Pentobarbital | 6 | 3 | 9 | 36 |
| Propofol | 16 | 4 | 20 | 80 |
SD = standard deviation; CI = confidence interval; SE = status epilepticus; RSE = refractory status epilepticus; SRSE = super‐refractory status epilepticus; NIHSS = National Institutes of Health Stroke Scale; STESS = Status Epilepticus Severity Score; GCS = Glasgow Coma Scale; RASS = Richmond Agitation‐Sedation Scale; mRS‐9Q = Modified Rankin Scale‐9Q; CGI‐S = Clinical Global Impression‐Severity.
Current episode of SE.
Safety and Tolerability
Safety was evaluated in all 25 participating patients. Mean overall duration of persistent or recurring seizures at baseline was 9.2 days (SD, 5.7; range, 3–20; Table 2). Patients entered the study with SRSE because of a range of etiologies and exhibited a number of additional comorbidities (Table 3). Additionally, at baseline, all patients were in a medically induced anesthetic coma and were receiving multiple medications, with an average of 12.9 total concurrent medications (range, 2–14), including an average of 3.0 AEDs (range, 1–5) and 1.4 TLAs (range, 1–2; Table 3). Twenty‐three patients completed the treatment period. Six patients died during the study and 4 patients died after completing the brexanolone treatment, in each case from causes related to underlying medical conditions and independent of treatment. The cause of death for the 2 patients who died before completion of treatment were metastatic brain cancer after a decision by family to limit life‐sustaining therapies and fulminant cardiac sequelae of organophosphate ingestion.
Table 3.
Baseline Medical Diagnoses and Treatmentsa (N = 25)
| SRSE Etiology | n | % of Patients |
|---|---|---|
| Infection | 6 | 24 |
| Intracranial hemorrhage | 4 | 16 |
| Decompensated seizures | 3 | 12 |
| Unknown | 3 | 12 |
| Primary/secondary brain tumor | 2 | 8 |
| Toxic ingestion | 2 | 8 |
| Anti‐NMDA‐R encephalitis | 1 | 4 |
| Lupus | 1 | 4 |
| Posterior reversible encephalopathy syndrome (PRES) | 1 | 4 |
| Sickle cell anemia | 1 | 4 |
| Stroke | 1 | 4 |
| Most Frequent Comorbid Conditions | n | % of Patients |
| Anemia | 11 | 44 |
| Hypotension | 10 | 40 |
| Convulsion | 9 | 36 |
| Hypertension | 9 | 36 |
| Drug hypersensitivity | 7 | 28 |
| Pneumonia | 7 | 28 |
| Pyrexia | 7 | 28 |
| Deep vein thrombosis | 6 | 24 |
| Subdural hematoma | 6 | 24 |
| Baseline b treatments | Mean | Range |
| Total concurrent medications | 12.9 | 2–14 |
| Antiepileptic medications | 3.0 | 1–5 |
| TLAs | 1.4 | 1–2 |
| Baseline b AEDs | n | % of Patients |
| 1–2 | 8 | 32.0 |
| 3–5 | 17 | 68.0 |
| Baseline b TLAs | n | % of Patients |
| 1 | 15 | 60.0 |
| 2 | 10 | 40.0 |
Patients may have multiple diagnoses and baseline treatments.
At start of the brexanolone infusion.
AEDs = antiepileptic medications; anti‐NMDA‐R = anti‐N‐methyl‐d‐aspartate receptor (anti‐NMDA‐R); SRSE = super‐refractory status epilepticus; TLAs = anesthetic third‐line agent.
SAEs reported in at least 2 individual patients were respiratory failure, pulmonary embolism, sepsis, convulsion, cardiac arrest, and acute renal failure (Table 4). In total, 16 patients (64%) experienced at least one SAE, none of which were deemed by the sponsor and SRC (which included independent, noninvestigator, nonsponsor, and critical care specialists) to be drug related in the context of treatment by good clinical practice (GCP) guidelines.
Table 4.
Most Frequent Adverse Events (AEs) and Serious Adverse Events (SAEs) (N = 25)
| AEs (over 10%) | n | % of Patients |
|---|---|---|
| Pyrexia | 5 | 20 |
| Anemia | 4 | 16 |
| Blood urea increased | 4 | 16 |
| Diarrhea | 4 | 16 |
| Hypotension | 4 | 16 |
| Edema peripheral | 4 | 16 |
| Convulsion | 3 | 12 |
| Decubitus ulcer | 3 | 12 |
| Deep vein thrombosis | 3 | 12 |
| Hematuria | 3 | 12 |
| Hypertension | 3 | 12 |
| Metabolic acidosis | 3 | 12 |
| Pneumonia | 3 | 12 |
| Respiratory failure | 3 | 12 |
| Sepsis | 3 | 12 |
| Sinus tachycardia | 3 | 12 |
| Urinary tract infection | 3 | 12 |
| SAEs a (over 8%) | n | % of Patients |
| Respiratory failure | 3 | 12 |
| Convulsion | 2 | 8 |
| Pulmonary embolism | 2 | 8 |
| Renal failure acute | 2 | 8 |
| Sepsis | 2 | 8 |
None assessed as drug related.
Treatment with brexanolone did not appear to have a significant effect on vital signs such as heart rate and systolic/diastolic blood pressure. A total of 207 AEs were reported in 23 patients (92%). The most common AEs (n ≥ 4) were fever, hypotension, diarrhea, peripheral edema, anemia, and blood urea nitrogen (BUN) increase (Table 4). The relationships of AEs to study drug were determined by the clinical investigators in the context of ongoing patient care consistent with GCP guidelines. Within these most common AEs, two events (fever and BUN increase, in 1 patient each) were deemed by the investigators to be “possibly related” to the administration of brexanolone, although both of these events resolved without dose changes after occurring during maintenance dosing.
Pharmacokinetics
Patients administered the standard dose regimen had an average allopregnanolone concentration (Cav) of 66.2ng/ml (SD, 21.93; range, 30.2–114.0), with an average maximum concentration (Cmax) of 144.2ng/ml (SD, 65.26; range, 38.2–306.0). The area under the curve (AUC) values for 24, 1 to 96, and 1 to 144 hours were 1,590, 6,290, and 7,680ng.h/ml, respectively. Patients administered the high‐dose brexanolone regimen had an allopregnanolone Cav of 113.6ng/ml (SD, 42.08; range, 57.5–159.0), with a Cmax of 200.8ng/ml (SD, 71.00; range, 80.9–267.0). AUC values for 24, 1 to 96, and 1 to 144 hours were 2,730, 10,800, and 14,300ng.h/ml, respectively.
Treatment Response
The response assessment population included 22 patients, of whom 16 received the standard dose regimen and 6 received the high‐dose regimen of brexanolone. Reasons for exclusion from the response assessment population were failure to complete brexanolone treatment (2 aforementioned patients who died from the inciting cause of SRSE) and lack of a wean attempt of TLAs (1 patient because of a protocol violation related to physician preference for a markedly extended duration of burst‐suppression). Within the response assessment population, 17 patients (77%) previously unable to be successfully weaned off TLAs had a clinical response and were successfully weaned off all TLAs during the brexanolone maintenance phase. Of the 17 responders, 13 patients received the standard brexanolone dose and 4 patients received the high‐dose brexanolone regimen. In addition, 16 patients (73%) were successfully weaned off TLAs and brexanolone within 5 days of starting the brexanolone infusion without the need to reinstate anesthetic agents in the following 24 hours. Among the 16 responders, 4 patients (23.5%) had recurrence of SE within the 3‐week extended follow‐up period.
No differences in patient characteristics, underlying treatments, or concomitant treatments were observed across patients who were responders versus nonresponders. For example, among responders (n = 17), 10 were male and 7 were female, 2 were aged ≤20 years and 15 were aged >20 years, and 4 were of black descent and 13 were of white descent. Among nonresponders, 3 were male and 2 were female, 1 was aged ≤20 years and 4 were aged >20 years, and 1 was of black descent and 4 were of white descent.
Overall functional status improved in the response assessment population, as measured by an assessment battery at follow‐up day 29. Mean NIHSS, GCS, RASS, mRS‐9Q, and CGI‐S scores improved by −17.8, +6.5, +2.9, −0.9, and −2.9, respectively (Table 5). At screening, all patients had an mRS‐9Q score of 5, and the overall distribution of day 29 mRS‐9Q scores was: zero: no patients; one: 2 low dose, 0 high dose; two: 0 low dose, 1 high dose; three: 1 low dose, 0 high dose; four: 2 low dose, 1 high dose; and five: 8 low dose, 2 high dose.
Table 5.
Summary of Measured Outcomes as Changes From Baseline at Day 29
| Assessment | n | Mean (SD) | Median | Range |
|---|---|---|---|---|
| NIHSS total score | 17 | −17.8 (14.42) | −16.0 | −38, 1 |
| GCS total score | 17 | +6.5 (5.30) | +7.0 | −2, 12 |
| RASS | 17 | +2.9 (2.14) | +4.0 | +0, 5 |
| mRS‐9Q | 17 | −0.9 (1.43) | +0.0 | −4, 0 |
| CGI‐S | 18 | −2.9 (2.01) | −3.0 | −6, 0 |
SD = standard deviation; NIHSS = National Institutes of Health Stroke Scale; GCS = Glasgow Coma Scale; RASS = Richmond Agitation‐Sedation Scale; mRS‐9Q = Modified Rankin Scale‐9Q; CGI‐S = Clinical Global Impression‐Severity.
Discussion
This phase 1/2 study is the first clinical trial of brexanolone in the treatment of SRSE and demonstrates the feasibility of studying novel therapeutic drug candidates in a critically ill population with SRSE of various etiologies. Results of this study demonstrate that IV administration of the neuroactive steroid brexanolone was well tolerated in a heterogeneous cohort of adults and children with SRSE and was associated with successful weaning of TLAs before the brexanolone taper in 77% of patients undergoing at least one wean attempt despite past dependence on pharmacologic coma for seizure control.
The mechanism of action of brexanolone, which is a selective PAM of GABAAR, offers a novel mechanism and therapeutic target for development as a potential treatment for SRSE. Benzodiazepines are the current first‐line therapy for early and established SE. Similar to allopregnanolone, benzodiazepines, such as midazolam, act as PAMs of the GABAAR. Prolonged seizure activity during SE leads to an activity‐dependent internalization and reduction in synaptic, benzodiazepine‐sensitive GABAA receptors,36, 37 which may contribute to benzodiazepines' inability to halt SE in SRSE patients.38, 39
Extrasynaptic GABAARs, including those containing α4 and δ subunits, however, do not internalize with prolonged seizure activity and are believed to mediate tonic inhibition.36, 40 It has been shown in vitro (electrophysiology using human receptors in Xenopus expression system) and in animal models (whole‐cell and extracellular recordings in murine hippocampal slice preparations) that endogenous neuroactive steroid allopregnanolone enhances both synaptic and extrasynaptic GABAAR function,18, 41 suggesting potential for modulation of extrasynaptic GABAAR function in the treatment of SRSE. Unlike benzodiazepines, the site of allopregnanolone binding to the GABAaR for PAM activity is on the α subunit. Unlike benzodiazepines, which bind at the interface between α and β subunits, the binding site for allopregnanolone allows for modulation of both synaptic GABAARs and extrasynaptic GABAARs, the latter not being internalized during SE.15, 16
Human clinical data for administration of exogenous allopregnanolone have been limited to case reports, most being in conscious subjects, in whom concentrations up to 150nM produced feelings of sedation, intoxication, flushing, and mild headache, but with no severe AEs reported.42, 43, 44, 45, 46, 47 The first successful allopregnanolone treatment of new‐onset refractory SE syndrome was in the treatment of SRSE in a 23‐year‐old man.26 After multiple unsuccessful TLA wean attempts, the patient received 24 hours of IV allopregnanolone after which the ninth pentobarbital wean attempt was successful. Allopregnanolone treatment was continued for 4 days, and the patient achieved complete clinical seizure resolution and was eventually discharged from the hospital.
Allopregnanolone infusion was subsequently reported in the successful treatment of other pediatric and adult SRSE cases25, 27 An 11‐year‐old female was treated with allopregnanolone after multiple days of treatment and unsuccessful TLA wean attempts. Treatment resulted in cessation of SE, cognitive improvement, and eventual discharge.25 Another 2‐year‐old female received allopregnanolone after multiple trials of AEDs and numerous unsuccessful TLA wean attempts. After treatment with allopregnanolone, TLAs were tapered over the next 24 to 72 hours, and allopregnanolone was continued for 4 days and tapered on the fifth day. The patient remained free of seizures over the next 12 subsequent days and was eventually discharged.25 Another successful allopregnanolone treatment was reported in a 28‐year‐old adult male with SRSE.27 After multiple days of treatment and unsuccessful TLA wean attempts, the patient was given allopregnanolone over 5 days. Starting at around 60 hours after the initial infusion through 7 days after allopregnanolone was stopped, the EEG progressively resembled normal waveform morphology. At 7 days, the patient's seizures recurred as primarily right temporal seizures.27
Limitations of the current study relate to the open‐label design, lack of control, small sample size, variation in institutional guidelines for type and maximum dose of AEDs and TLAs, and lack of standardization for treatment goals for TLAs and their weaning. Nevertheless, the high response rate in this study among patients with SRSE not previously responding to other treatments stands in comparison to previous observational studies. In a retrospective study of 42 patients with RSE and SRSE, (s)‐ketamine infusion for a median of 3 days resulted in a survival rate of 54.8% and resolution of SRSE in 64% of patients.48 Another retrospective case series demonstrated a 57% control rate and 57% survival rate when ketamine was administered in 58 subjects with 60 episodes, although no subject responded when ketamine was introduced 8 days beyond status epilepticus onset, compared to a large proportion of patients in our cohort responding after a 9.2‐day mean duration of status epilepticus before brexanolone administration.49 A randomized, single‐blind trial recruiting 24 patients demonstrated a 43% response rate and 57% survival rate among 14 patients assigned to propofol and a 22% response rate and a 56% survival rate among 9 patients assigned to barbiturates.50 Definitions and criteria for SRSE can vary between studies, and each study's unique criteria should be considered in any comparison. In the present study, the SRSE definition encompasses definitions of Shorvon and Trinka, in which seizures occur after 24 hours of TLA administration2 as well as those in which seizures continue despite ongoing TLA administration.28 Nevertheless, the mean duration of SRSE at baseline was 6.1 (range, 1, 19 days) among recruited subjects, demonstrating that the population included patients consistent with the definition proposed by Shorvon et al. Several of the limitations of the present study will largely be addressed in the ongoing SAGE‐547 Treatment as Adjunctive Therapy Utilized in Status Epilepticus (STATUS) Trial (NCT02477618), which is a larger, randomized, placebo‐controlled, multinational phase 3 clinical trial in SRSE that utilizes clinical standardization of key elements of standard of care across the participating sites.
Conclusions
The results of this study demonstrate that despite the rare nature of SRSE and the variability of clinical treatment, an interventional study in critically ill SRSE patients is feasible, and brexanolone appears well tolerated in the sample of patients studied. Furthermore, this open‐label trial demonstrated the ability to observe the clinical response of SRSE during and following brexanolone infusion; clinical improvement was observable despite significant baseline neurological and systemic morbidity, past refractoriness to multiple baseline AEDs, and past dependence on one or more baseline TLAs for seizure control. The data demonstrate that brexanolone may offer a new approach as adjunctive therapy to TLAs in the management of children and adults with SRSE. This hypothesis is being tested in a larger, controlled trial that is currently ongoing in 15 countries.
Author Contributions
E.S.R., J.C., M.W., A.M.H., V.H., H.C., J.J.D., and S.J.K. contributed to the conception and design of the study. E.S.R., J.C., M.W., A.M.H., V.H., H.C., J.J.D., S.R., E.H., and S.J.K. contributed to the acquisition and analysis of data. E.S.R., M.S.W., E.H., H.C., J.J.D., and S.J.K. drafted the manuscript.
Potential Conflicts of Interest
E.H., J.D., and S.K. are employees and stockholders, with employee stock options, of Sage Therapeutics. H.C. is an employee, with stock options, of Sage Therapeutics. S.R. is a paid consultant for Sage Therapeutics. A.H. has received consultants' fees from Sage Therapeutics, Marinus Pharmaceuticals, Inc., and UCB; and has received grant support from UCB in relation to epilepsy; is an editor for the Journal of Clinical Neurophysiology; has served on a clinical standardization team for an ongoing phase III trial of brexanolone for SRSE; and has received compensation as part of a research contract with Sage Therapeutics. E.S.R. is principal investigator of the phase 3 study of brexanolone for SRSE. E.S.R. has received consulting fees for from GLG Consulting, ExpertConnect, and Guidepoint Global, Inc., in relation to epilepsy management. E.S.R. receives no direct salary support from Sage Therapeutics. He receives salary from Massachusetts General Hospital, which participates in a Clinical Research Support Clinical Trial Agreement with Sage Therapeutics. Through this agreement, E.S.R. serves as the co‐lead principal investigator of the phase 3 study of brexanolone for SRSE, chair of the study's clinical standardization team, co‐academic lead of the study's publication committee comprised of a majority of academic researchers as members, and co‐chair of the study's academic writing committee for publication of the primary outcome results of the phase 3 study and prespecified secondary analysis publications. Of his salary support and fringe benefits, 4.17% is paid to E.S.R. by Massachusetts General Hospital as part of this research contract between Massachusetts General Hospital and Sage Therapeutics, and E.S.R. performs 4.17% certified effort of a 40‐hour week of certified clinical, administrative, educational, and research effort. H.V. has received consultants' fees from Sage Therapeutics. J.C. is currently on the advisory board for study planning for Sage Therapeutics; he has served on a clinical standardization team for an ongoing phase III trial of brexanolone for SRSE and received compensation as part of a research contract with Sage Therapeutics. M.W. has received consulting fees from GLG Consulting and from Sage Therapeutics and is currently on the Clinical Advisory Board for Sage Therapeutics; he has served on a clinical standardization team for an ongoing phase III trial of brexanolone for SRSE and received compensation as part of a research contract with Sage Therapeutics.
Sage Therapeutics funded the trial and was involved in the design of the trial, data collection, statistical analysis, and the interpretation. The study investigators were involved in the design of the trial and data collection. Study investigators (E.S.R., M.S.W., S.R., E.H., H.C., J.J.D., and S.J.K.) had access to and reviewed the primary data. Specific contributions of study authors to the study conception and design, data acquisition and analysis, and drafting of the manuscript are noted under “Author Contributions.”
Brexanolone has been granted both Fast Track and orphan drug designations by the US Food and Drug Administration (FDA) for the treatment of SRSE. The active pharmaceutical ingredient and treatment Investigational New Drug (IND) were contributed under agreement by the Regents of the University of California and the University of California‐Davis.
Acknowledgment
Sage Therapeutics, Inc., funded the trial. Secretarial services were provided by Boston Strategic Partners, Inc., and statistical analysis was provided by SR, with support from Sage Therapeutics, Inc. The active pharmaceutical ingredient for this study was contributed under agreement with the Regents of the University of California and the University of California Davis.
References
- 1. Hocker S, Tatum WO, LaRoche S, Freeman WD. Refractory and super‐refractory status epilepticus—an update. Curr Neurol Neurosci Rep 2014;14:452. [DOI] [PubMed] [Google Scholar]
- 2. Ferlisi M, Shorvon S. The outcome of therapies in refractory and super‐refractory convulsive status epilepticus and recommendations for therapy. Brain 2012;135(pt 8):2314–2328. [DOI] [PubMed] [Google Scholar]
- 3. Karunakaran S, Grasse DW, Moxon KA. Changes in network dynamics during status epilepticus. Exp Neurol 2012;234:454–465. [DOI] [PubMed] [Google Scholar]
- 4. Kamppi L, Ritvanen J, Mustonen H, Soinila S. Delays and factors related to cessation of generalized convulsive status epilepticus. Epilepsy Res Treat 2015;2015:591279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shorvon S, Ferlisi M. The treatment of super‐refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain 2011;134:2802–2818. [DOI] [PubMed] [Google Scholar]
- 6. Brophy GM, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care 2012;17:3–23. [DOI] [PubMed] [Google Scholar]
- 7. Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Eng J Med 2012;366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002;43:146–153. [DOI] [PubMed] [Google Scholar]
- 9. Novy J, Logroscino G, Rossetti AO. Refractory status epilepticus: a prospective observational study. Epilepsia 2010;51:251–256. [DOI] [PubMed] [Google Scholar]
- 10. Pugin D, Foreman B, De Marchis GM, et al. Is pentobarbital safe and efficacious in the treatment of super‐refractory status epilepticus: a cohort study. Crit Care 2014;18:R103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hocker SE, Britton JW, Mandrekar JN, Wijdicks EF, Rabinstein AA. Predictors of outcome in refractory status epilepticus. JAMA Neurol 2013;70:72–77. [DOI] [PubMed] [Google Scholar]
- 12. Holzbauer M, Birmingham MK, De Nicola AF, Oliver JT. In vivo secretion of 3 alpha‐hydroxy‐5 alpha‐pregnan‐20‐one, a potent anaesthetic steroid, by the adrenal gland of the rat. J Steroid Biochem 1985;22:97–102. [DOI] [PubMed] [Google Scholar]
- 13. Ottander U, Poromaa IS, Bjurulf E, et al. Allopregnanolone and pregnanolone are produced by the human corpus luteum. Mol Cell Endocrinol 2005;239:37–44. [DOI] [PubMed] [Google Scholar]
- 14. Paul SM, Purdy RH. Neuroactive steroids. FASEB J 1992;6:2311–2322. [PubMed] [Google Scholar]
- 15. Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: A summary of the Twelfth Eilat Conference (EILAT XII). Epilepsy Res 2015;111:85–141. [DOI] [PubMed] [Google Scholar]
- 16. Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Thirteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIII). Epilepsia 2017;58:181–221. [DOI] [PubMed] [Google Scholar]
- 17. Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Brain Res Rev 2001;37:68–80. [DOI] [PubMed] [Google Scholar]
- 18. Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology 2002;43:651–661. [DOI] [PubMed] [Google Scholar]
- 19. Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 2005;6:565–575. [DOI] [PubMed] [Google Scholar]
- 20. Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 2012;73:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patil CY, Jadhav SA, Doifode SM, Baig MS. Neuroactive steroids and their role in epilepsy. Int J Basic Clin Pharmacol 2012;1:150–159. [Google Scholar]
- 22. Zhu D, Birzniece V, Backstrom T, Wahlstrom G. Dynamic aspects of acute tolerance to allopregnanolone evaluated using anaesthesia threshold in male rats. Br J Anaesth 2004;93:560–567. [DOI] [PubMed] [Google Scholar]
- 23. Zhu D, Wang MD, Backstrom T, Wahlstrom G. Evaluation and comparison of the pharmacokinetic and pharmacodynamic properties of allopregnanolone and pregnanolone at induction of anaesthesia in the male rat. Br J Anaesth 2001;86:403–412. [DOI] [PubMed] [Google Scholar]
- 24. Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine‐ and kainic acid‐induced limbic seizures and status epilepticus in mice. Neuropharmacology 1996;35:1049–1056. [DOI] [PubMed] [Google Scholar]
- 25. Broomall E, Natale JE, Grimason M, et al. Pediatric super‐refractory status epilepticus treated with allopregnanolone. Ann Neurol 2014;76:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaitkevicius H, Ng M, Moura L, et al. Successful allopregnanolone treatment of new onset refractory status epilepticus (NORSE) syndrome: first in man experience. Epilepsia 2013;54(suppl 6):19. [Google Scholar]
- 27. Bobb W, Kolls B, Ummat M, Husain A, eds. Allopregnanolone to Treat Refractory Status Epilepticus. ACNS Annual Meeting; February 4–9, 2014, Atlanta, GA.
- 28. Shorvon S, Trinka E. Status epilepticus—making progress. Epilepsia 2011;52(suppl 8):1–2. [DOI] [PubMed] [Google Scholar]
- 29. Luisi S, Petraglia F, Benedetto C, et al. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab 2000;85:2429–2433. [DOI] [PubMed] [Google Scholar]
- 30. Rossetti AO, Logroscino G, Milligan TA, et al. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol 2008;255:1561–1566. [DOI] [PubMed] [Google Scholar]
- 31. Patel N, Rao VA, Heilman‐Espinoza ER, et al. Simple and reliable determination of the modified rankin scale score in neurosurgical and neurological patients: the mRS‐9Q. Neurosurgery 2012;71:971–975; discussion, 5. [DOI] [PubMed] [Google Scholar]
- 32. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]
- 33. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation‐Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 2002;166:1338–1344. [DOI] [PubMed] [Google Scholar]
- 34. Teasdale G, Maas A, Lecky F, et al. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol 2014;13:844–854. [DOI] [PubMed] [Google Scholar]
- 35. Waterhouse E. Predictors of early seizures after stroke. Epilepsy Curr 2002;2:75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodkin HP, Sun C, Yeh JL, Mangan PS, Kapur J. GABA(A) receptor internalization during seizures. Epilepsia 2007;48(suppl 5):109–113. [DOI] [PubMed] [Google Scholar]
- 37. Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005;25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenfield LJ, Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 2013;22:589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C. Neuroactive steroids for the treatment of status epilepticus. Epilepsia 2013;54(suppl 6):93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit‐specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci 2008;28:2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit‐containing GABAA receptors. Proc Natl Acad Sci U S A 2003;100:14439–14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Timby E, Balgard M, Nyberg S, et al. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology (Berl) 2006;186:414–424. [DOI] [PubMed] [Google Scholar]
- 43. van Broekhoven F, Backstrom T, van Luijtelaar G, et al. Effects of allopregnanolone on sedation in men, and in women on oral contraceptives. Psychoneuroendocrinology 2007;32:555–564. [DOI] [PubMed] [Google Scholar]
- 44. Kask K, Backstrom T, Nilsson LG, Sundstrom‐Poromaa I. Allopregnanolone impairs episodic memory in healthy women. Psychopharmacology (Berl) 2008;199:161–168. [DOI] [PubMed] [Google Scholar]
- 45. Kask K, Backstrom T, Lundgren P, Sundstrom Poromaa I. Allopregnanolone has no effect on startle response and prepulse inhibition of startle response in patients with premenstrual dysphoric disorder or healthy controls. Pharmacol Biochem Behav 2009;92:608–613. [DOI] [PubMed] [Google Scholar]
- 46. Timby E, Hedstrom H, Backstrom T, et al. Allopregnanolone, a GABAA receptor agonist, decreases gonadotropin levels in women. A preliminary study. Gynecol Endocrinol 2011;27:1087–1093. [DOI] [PubMed] [Google Scholar]
- 47. Navarro PA, Kaddouz D, de Ziegler D, Silva de Sa MF, Ferriani RA. Vaginal administration of allopregnanolone to postmenopausal women undergoing estrogen replacement therapy: preliminary results. Maturitas 2003;46:147–152. [DOI] [PubMed] [Google Scholar]
- 48. Hofler J, Rohracher A, Kalss G, et al. (S)‐ketamine in refractory and super‐refractory status epilepticus: a retrospective study. CNS Drugs 2016;30:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gaspard N, Foreman B, Judd LM, et al. Intravenous ketamine for the treatment of refractory status epilepticus: a retrospective multicenter study. Epilepsia 2013;54:1498–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rossetti AO, Milligan TA, Vulliemoz S, Michaelides C, Bertschi M, Lee JW. A randomized trial for the treatment of refractory status epilepticus. Neurocrit Care 2011;14:4–10. [DOI] [PubMed] [Google Scholar]


