Abstract
Objective
To evaluate the effect and toxicity of alprostadil combined with thioctic acid injection in the treatment of patients with diabetic nephropathy (DN).
Methods
Sixty two patients with DN were included in this study and randomly divided into control group (n=32) and experiment group (n=30). Patients in the control group were given alprostadil 20ug+NS 100ml ivgtt, qd and patients in the experiment group were given alprostadil 20ug+NS 100ml ivgtt combined with thioctic acid injection of 0.45g+100ml ivgtt, qd for 14 days. After treatment, the renal function and serum level of CRP, IL-6 and TNF-α were compared between the two groups.
Results
After two weeks of treatment, the serum level of CysC and UAER significant decreased for both control and experiment group with statistical difference of p<0.05. After treatment, the serum level of CysC were 1.40 ±0.46 mg/L and 1.02±0.33 for control and experiment group respectively (p<0.05). The post-treatment UAER in experiment group was significantly lower than those of control group with statistical difference (81.02±0.33 vs112.45±20.32, p<0.05) ug/min. The serum level of CRP, IL-6 and TNF-α were significantly decreased after treatment for both control and experiment group (p<0.05). And the post-treatment serum CRP, IL-6 and TNF-α in experiment group were significantly lower than those of control group with statistical difference (p<0.05). No significant side effects were found for the two groupsin the course of treatment.
Conclusion
Alprostadil combined with α-lipoic acid may improve renal function in patients with diabetic nephropathy by decreasing the levels of serum inflammatory factors.
Keywords: Alprostadil, α-lipoic acid, Diabetic nephropathy, Inflammatory factors
1. Introduction
Diabetic nephropathy (DN) is a common chronic complication of diabetes, and this condition is the first etiological factor leading to end-stage renal disease in developed countries [1,2]. Inflammatory reactions and oxidative stress play a key role in renal injury related to DN [3-5]. It is also closely associated with disease progression, but the early onset of this diabetic complication is hidden, and disease progression cannot be controlled easily in the advanced stages. Therefore, DN should be detected and treated in the early stages. Alprostadil [6], α-lipoic acid [7,8], and other drugs can decrease the level of inflammatory cytokines in patients with DN and improve their renal functions. However, the combination of these drugs has been rarely reported. This study aimed to observe the effect of combined alprostadil-lipoic acid treatment on the renal functions and inflammatory cytokines of elderly patients who suffer from early DN.
2. Material and methods
2.1. Patients
Sixty two elderly patients who were diagnosed with early DN and admitted to the Nephrology Department of Lishui People’s Hospital from February 2015 to January 2017 were selected as study objects. Patients who satisfied the following criteria were included: age above 60 years; characteristics consistent with the diagnostic criteria presented by WHO in 1999; with signed informed consent and willingness to participate in the clinical study; and who were diagnosed with early DN in accordance with Mogensen’s DN staging criteria (UAER of 20–200μg/min or total urinary albumin for 24h of 30–300 mg). The following exclusion criteria were considered: uncontrolled or untreated acute or chronic infection, acute heart failure, gout, or ketoacidosis; systemic diseases or autoimmune diseases; and malignant tumors or bowel diseases. This study was approved by the ethical committee of Lishui People’s Hospital.
2.2. Treatment
The included 62 patients were randomly divided into two groups; a control group (n=32) and an experiment (n=30) group. Differences in gender, age, diabetic duration, blood sugar, serum creatinine, blood pressure, and other baseline data between the groups were not significant (P>0.05). All of the patients controlled their blood sugar through proper diet and insulin intake and their glycosylated hemoglobin was maintained at 5%–8%.The patients also used ACEI or ARB drugs to control the blood pressure. Patients in the control group were given alprostadil (Peking Detai Pharmaceutical Co., Ltd.) 20ug+NS 100ml ivgtt, qd and patients in the experiment group were given alprostadil 20ug+NS 100ml ivgtt combined with thioctic acid injection (YantaiZhichu Pharmaceutical Co., Ltd.) of 0.45g+100ml ivgtt, qd for 14 days.
2.3. Serum CysC, UAER, and SCr and inflammatory cytokines detection
Renal function indexes (CysC, UAER, and SCr) and inflammatory cytokines (IL-6, TNF-α, CRP) were compared between the two groups. Serum CysC was examined with an automatic biochemical analyzer. UAER was determined through radioimmunoassay, and serum IL-6 and TNF-α were detected through an enzyme linked immune sorbent assay. The tests were conducted in accordance with the manufacturers’ instructions.
2.4. Statistical analysis
STATA11.0 statistical software (http://www.stata.com) was used to do the data analysis. The measurement data were expressed with X ± S and the comparison between groups was made based on the t-test of the sample mean. The enumeration data were expressed with a relative number, and the comparison between groups was made based on the χ2 test. Two tails P<0.05 meant a statistical difference.
3. Results
3.1. General characteristics of the two groups
Eighteen male and 14 female DN patients with the mean age of 65.82±11.63 year were included in the control group. And there were 16 male, 14 female DN patients with the mean age of 67.24±10.81years were included in the experiment group. The mean age, gender, course of disease and serum creatinine level of the two groups were not statistical different (P>0.05), (Table 1).
Table 1.
General characteristics of the two groups
| Age | Gender | Course of disease | Serum creatinine | |
|---|---|---|---|---|
| Groups | (year) | (M/F) | (year) | (umol/L) |
| Control(n=32) | 65.82±11.63 | 18/14 | 10.66±4.65 | 70.22±12.56 |
| Experiment(n=30) | 67.24±10.81 | 16/14 | 11.74±6.23 | 68.36±15.44 |
3.2. Renal function of two groups after treatment
After two weeks treatment, the serum level of CysC and UAER significantly decreased for both control and experiment group (P<0.05), (Table 2). The serum level of CysC were 1.40 ±0.46 mg/L and 1.02±0.33 for control and experiment group respectively with statistical difference (P<0.05) after treatment. The post-treatment UAER in experiment group was significant lower than those of control group with statistical difference (81.02±0.33 vs112.45±20.32, P<0.05) ug/min.
Table 2.
The serum level of CysC, UAER, SCr before and after treatment for both control and experiment group
| Group | CysC (mg/L) | SCr(umol/L) | UAER(ug/min) | |||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Control(n=32) | 1.78±0.51 | 1.40 ±0.46# | 70.22±12.56 | 69.55±11.66 | 152.11±26.88 | 112.45±20.32# |
| Experiment(n=30) | 1.80 ±0.56 | 1.02±0.33 #* | 68.36±15.44 | 68.23± 14.55 | 147.69±29.65 | 81.02±0.33#* |
Compared to before treatment, P < 0. 05;
Compared to control group after treatment, P < 0. 05
3.3. Serum level of CRP, IL-6 and TNF-α
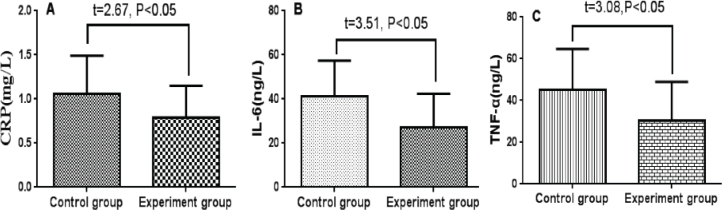
The serum level of CRP, IL-6 and TNF-α were significant decreased after treatment in both control and experiment group (P<0.05), (Table 3). And the post-treatment serum CRP, IL-6 and TNF-α in the experiment group were significantly lower than that of the control group with statistical difference (P<0.05), (Figure 1).
Table 3.
Serum level of CRP, IL-6 and TNF-α before and after treatment for both control and experiment group
| Group | CRP(mg/L) | IL-6(ng/L) | TNF-α(ng/L) | |||
|---|---|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Control(n=32) | 1.69 ±0.51 | 1.06±0.43# | 55.33±17.36 | 41.20 ±16.21# | 60.60±22.26 | 45.23±19.45# |
| Experiment(n=30) | 1.67±0.49 | 0.79±0.36#* | 56.47 ±18.22 | 27.14 ±15.27#* | 63.21±24.74 | 30.33±18.56#* |
Compared to before treatment, P < 0. 05;
Compared to control group after treatment, P < 0. 05
Figure 1.
Bar plot for serum level of CRP, IL-6 and TNF-α in experiment and control group(A: serum CRP; B: serum IL-6; C: serum -TNF-α
3.4. Drug related toxicity
No obvious drug related toxicity was found in the two groups. And all the patients successfully finished the treatment plan.
4. Discussion
The exact pathogenesis of DN remains unclear. Hyperglycemia and abnormal increase levels of TNF-α [9, 10], IL-6 [11,12], and other inflammatory cytokines can damage pancreatic β cells, induce insulin resistance, and facilitate oxidative stress. As a result, a vicious cycle is formed, endothelial cells and tissues are damaged, and the permeability of glomerular basement membrane is increased. These conditions consequently cause proteinuria and trigger the onset of DN. Under hyperglycemic conditions, various factors induce the overproduction of reactive oxygen species (ROS), and ROS levels exceed the scavenging activity of antioxidants [13-16]. The accumulation of high ROS concentrations caninduce oxidative stress oninnate renal cells, activate relevant signal pathways within cells, promote the transcription and high expression of fibroblast growth factors, and stimulate the excessive deposition of extracellular matrix and renal fibrosis [9,10]. Therefore, inflammatory reaction and oxidative stress play a crucial role in the occurrence and development of DN. Inflammatory reaction inhibition and oxidative stress relief are also optimum targets for the clinical treatment of DN.
Luo et.al performed an animal study to evaluate the therapeutic effect of alprostadil in diabetic nephropathy. They found that alprostadil treatment can protect renal function by reducing proteinuria. These effects are mediated, at least in part, through down-regulation of Ang-2 and IL-18 expression [6]. However, the clinical effects of alprostadil for DN patients was seldom reported. Liu et.al evaluate the clinical efficacy of lipoicascid combined with alprostadil in the treatment of diabetic nephropathy. The results indicated that the fasting blood glucose, 24 h urine protein, blood urea nitrogen and creatinine decreased significantly in the treatment group compared with the control group [17]. In our present study, the combined treatment could significantly reduce inflammatory indexes, such asIL-6, CRP, and TNF-α, in patients who suffered from early DN. The combined treatment could also decrease UAER and CysC, a marker of early renal injury. These results indicated that alprostadil and α-lipoic acid could improve patients’ renal functions by reducing their inflammatory cytokine levels which was in accordance with Liu et.al [17]. Prostaglandin E1 serves as the main component of alprostadil. This compound can inhibit the activity of rennin-angiotensin system, dilate blood vessels, resist platelet aggregation, adjust microcirculation, increase renal blood flow, and block inflammatory cytokines to reduce urine protein content and protect the kidneys. As a well-known clinical antioxidant, α-lipoic acid can prevent oxidative stress, enhance vascular endothelial functions, reduce UAER, alleviate glomerular sclerosis and renal tubule interstitial fibrosis, maintain residual renal functions, and delay progressive renal damage in patients.
In summary, the combined alprostadil-lipoic acid treatment was more effective than single treatments in reducing inflammatory cytokine levels, inhibiting oxidative stress and protecting the kidneys of patients in early stage of DN. However, in this study all inflammatory factors significantly decreased, the benefit for the DN is not clear. The decrease of cytokines may be beneficial for DN patients but this has not definitely been proven in the present work and in previously published studies. Therefore, the detailed mechanism of this combined treatment should be further investigated.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- [1].Kitada M, Koya D.. [Epidemiology of diabetic nephropathy] Nihon Rinsho. 2012;70 Suppl 5:384–388. [PubMed] [Google Scholar]
- [2].Kawanami D, Utsunomiya K.. [Epidemiology of diabetic nephropathy] Nihon Rinsho. 2016;74 Suppl 2:153–157. [PubMed] [Google Scholar]
- [3].Lim AK, Tesch GH.. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146–154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wada J, Makino H.. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- [5].Duran-Salgado MB, Rubio-Guerra AF.. Diabetic nephropathy and inflammation. World J Diabetes. 2014;5:393–398. doi: 10.4239/wjd.v5.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Luo C, Li T, Zhang C, Chen Q, Li Z, Liu J. et al. Therapeutic effect of alprostadil in diabetic nephropathy: possible roles of angiopoietin-2 and IL-18. Cell Physiol Biochem. 2014;34:916–928. doi: 10.1159/000366309. [DOI] [PubMed] [Google Scholar]
- [7].Yi X, Nickeleit V, James LR, Maeda N.. α-Lipoic acid protects diabetic apolipoprotein E-deficient mice from nephropathy. J Diabetes Complications. 2011;25:193–201. doi: 10.1016/j.jdiacomp.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cicek M, Yildirir A, Okyay K, Yazici AC, Aydinalp A, Kanyilmaz S. et al. Use of alpha-lipoic acid in prevention of contrast-induced nephropathy in diabetic patients. Ren Fail. 2013;35:748–753. doi: 10.3109/0886022X.2013.790298. [DOI] [PubMed] [Google Scholar]
- [9].Meng N, Zhang Y, Li H. et al. Association of tumor necrosis factor alpha promoter polymorphism (TNF-alpha 238 G/A and TNF-alpha 308 G/A) with diabetic mellitus, diabetic retinopathy and diabetic nephropathy: a meta-analysis[J] Curr Eye Res. 2014;39(2):194–203. doi: 10.3109/02713683.2013.834942. [DOI] [PubMed] [Google Scholar]
- [10].Gupta S, Mehndiratta M, Kalra S. et al. Association of tumor necrosis factor (TNF) promoter polymorphisms with plasma TNF-alpha levels and susceptibility to diabetic nephropathy in North Indian population[J] J Diabetes Complications. 2015;29(3):338–342. doi: 10.1016/j.jdiacomp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [11].Svensson MK, Eriksson JW.. Change in the amount of body fat and IL-6 levels is related to altered insulin sensitivity in type 1 diabetes patients with or without diabetic nephropathy[J] Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2011;43(3):209–215. doi: 10.1055/s-0031-1271622. [DOI] [PubMed] [Google Scholar]
- [12].Rakitianskaia IA, Riabov SI, Dubrova AG. et al. [The role of IL-6 in the development of morphological changes in renal tissue in elderly patients with type 2 diabetes complicated by diabetic nephropathy][J] Advances in gerontology = Uspekhi gerontologii / Rossiiskaia akademiia nauk, Gerontologicheskoe obshchestvo. 2012;25(4):632–637. [PubMed] [Google Scholar]
- [13].Kohan DE.. Reactive oxygen species and endothelins in diabetic nephropathy. J Lab Clin Med. 2000;135:300–302. doi: 10.1067/mlc.2000.105972. [DOI] [PubMed] [Google Scholar]
- [14].J Am Soc Nephrol. Vol. 14. Proceedings of the Hyonam Kidney Laboratory, Soon Chun Hyang University International Diabetes Symposium; Seoul, Korea: Jan-19. 2003. 2003. Reactive Oxygen Species and Diabetic Nephropathy; pp. S209–296. [PubMed] [Google Scholar]
- [15].Ha H, Hwang IA, Park JH, Lee HB.. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82 Suppl 1:S42–45. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- [16].Bondeva T, Wolf G.. Reactive oxygen species in diabetic nephropathy: friend or foe. Nephrol Dial Transplant. 2014;29:1998–2003. doi: 10.1093/ndt/gfu037. [DOI] [PubMed] [Google Scholar]
- [17].Liu Y, Dong S, Yang X, Jing S, Wang X. Clinical Efficacy of Lipoic Acid Combined with alprostadil in the treatment of Diabetic Nephropathy. China Continuing Medical Education. 2016;8:153–154. [Google Scholar]