Abstract
To evaluate the clinical value of serum α-L-fucosidase (AFU), 5’-nucleotidase (5’-NT) and alpha fetoprotein (AFP) as biomarkers for primary hepatocellular carcinoma (PHC) diagnosis. Methods: Thirty six primary hepatocellular carcinoma (PHC) patients and 36 healthy controls were recruited in this study from February 2014 to January 2016 in the Second People’s Hospital of Tianjin. The serum level of AFU, 5’-NT and AFP were examined and compared between the two groups. The diagnostic sensitivity, specificity area under the receiver operating characteristic (ROC) curve were calculated by STATA11.0 software. Results: The serum level of AFU, 5’-NT, AFP were 30.87±10.43(U/L), 5.58±3.89(U/L), 233.60±226.60 (μg/L) respectively for primary hepatocellular carcinoma group and 19.96±6.73 (U/L), 1.87±0.84 (U/L), 16.64±14.17 (μg/L) for healthy control groups. The serum level of AFU, 5’-NT and AFP in primary hepatocellular carcinoma group were significant higher than those of healthy control group (P<0.001). The diagnostic sensitivity and specificity were 0.78 (95%CI:l0.61-0.90), 0.64 (95%CI:0.46-0.79) for serum AFU, 0.75(95%CI:0.58-0.88), 0.72(95%CI:0.55- 0.86) for serum 5’-NT and 0.72 (95%CI:0.55-0.86), 0.92 (95%CI:0.78-0.98) for serum AFP respectively. The AUC under the ROC curve were 0.80 (0.69-0.90), 0.80 (0.69-0.91) and 0.87 (0.780-0.96) for serum AFU, 5’-NT and AFP respectively. Positive correlation between AFU and 5’-NT (rpearson=0.63, P<0.05), AFU and AFP (rpearson=0.49, P<0.05), 5’-NT and AFP(rpearson=0.44, P<0.05) were found in the primary hepatocellular carcinoma patients. Conclusion: Serum AFU, 5’-NT and AFP were higher in PHC patients than those of healthy controls. The difference between PHC patients and healthy controls made serum AFU, 5’-NT and AFP potential biomarker for PHC diagnosis.
Keywords: Primary hepatocellular carcinoma, α-L-fucosidase, 5’-nucleotidase, Alpha fetoprotein, Diagnosis
1. Introduction
Primary hepatocellular carcinoma (PHC) is one of the most diagnosed malignant carcinoma and cause of cancer-related mortality [1]. Hepatitis B virus infection is an important risk factor for the development of primary hepatocellular carcinoma [2]. The incidence of hepatitis B virus infection in China is high, resulting in high morbidity from hepatic carcinoma [2, 3]. Primary hepatocellular carcinoma is characterized by high malignancy and metastasis and poor prognosis. Early detection of hepatic carcinoma plays an important role in the diagnosis and treatment of the disease [4].
Tumor markers are potential screening tools used for early diagnosis of malignancies. Thus, determination of a suitable marker is clinically significant to the early diagnosis of PHC. Alpha fetoprotein (AFP) is the most commonly used serum indicator for the auxiliary diagnosis of PHC [5-7]. Serum AFP level is related to tumor diameter, tumor differentiation, and biological characteristics. However, the AFP level of some patients remains normal in the early or even later period of PHC, whereas some patients with benign hepatic diseases show false-positive AFP results. Alpha-L-fucosidase (AFU) belongs to the lysosome acidic hydrolase group. AFU is highly expressed in patients with liver cirrhosis and hepatic carcinoma [8]. However, limited research is available about the function of AFU in the diagnosis and prognosis of hepatic carcinoma. 5’-Nucleotidase (5’-NT) extensively exists on the cell membranes of various types of tissues and is mainly distributed in the cholangiole and sinusoid in the liver [9]. 5’-NT is released into the blood when hepatic cells are injured or intra- or extra hepatic obstruction occurs. Therefore, changes in the serum level of 5’-NT are not significantly influenced by other extra hepatic diseases. Thus, 5’-NT may serve as an indicator in the diagnosis and risk determination of the postoperative recurrence of hepatic carcinoma.
2. Material and methods
2.1. Patients inclusion
A total of 36 patients who were admitted into the Tianjin Second People’s Hospital and diagnosed with primary hepatocellular carcinoma from February 2014 to January 2016 were selected as study participants (Case group). And another 36 healthy controls that received regular physical examination were included in this study as control group. The PHC patient inclusion criteria are as follows: diagnosis of primary hepatocellular carcinoma through iconography or cytology and no prior treatment before diagnosis of hepatocellular carcinoma. Meanwhile, patients with other malignant tumors and hepatic metastasis were excluded. Those who received hepatocellular carcinoma treatment, including surgery, intervention, or chemotherapy, were also excluded. The treatment group included 36 patients comprising 21 males and 15 females with an average age of 48.8±18.2 years. The control group comprised 36 cases, including 19 males and 17 females with an average age of 50.20±22.12.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent: Informed consent has been obtained from all individuals included in this study.
2.2. Serum AFU, 5’-NT, and AFP detection
All participants were subjected to fasting blood sampling; serum was separated and stored in the refrigerator at −80 °C until use. All tests were carried out in strict accordance with the standard operating procedure or reagent instruction. Examination of AFU (Sichuan Maccura Co., Ltd.) and 5’-NT (Ningbo Medical System Biotechnology Co., Ltd.) was carried out using a fully automatic biochemical analyzer (Beckman AU5800) through the velocity method. AFP was examined (Roche Co., Ltd.) through a fully automatic chemiluminescent analyzer (Roche Co., Ltd) via chemiluminescent immunoassay.
2.3. Statistical analysis
The measurement data were expressed by x ± s and the comparison between groups was made based on the student t-test of the sample mean. The enumeration data were expressed with a relative number, and the comparison between groups was made based on the c2 or fisher’s exact test. The correlation between serum AFU, 5’-NT and AFP was calculated by the Pearson correlation test. Diagnostic sensitivity and specificity was calculated by the equation: sensitivity=true positive/(true positive+ false negative), specificity=true negative/( true negative+ false positive). The area under the receiver operating characteristic (ROC) curve was used to evaluate the diagnostic efficacy of serum AFU, 5’-NT and AFP as biomarkers for hepatocellular carcinoma. All the data was calculated through SPSS17.0 software.
3. Results
3.1. Serum AFU, 5’-NT and AFP
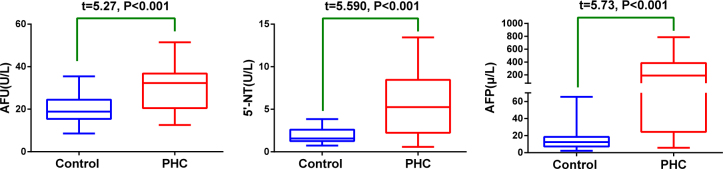
The serum level of AFU, 5’-NT, AFP were 30.87±10.43(U/L), 5.58±3.89(U/L), 233.60±226.60(μg/L) respectively for primary hepatocellular carcinoma group and 19.96±6.73(U/L), 1.87±0.84(U/L), 16.64±14.17(μg/L) for healthy control groups. The serum level of AFU, 5’-NT and AFP in primary hepatocellular carcinoma group were significant higher than those of healthy control group (P<0.001), (Figure 1).
Figure 1.
The box plot of serum AFU, 5’-NT and AFP of PHC and healthy control groups
3.2. Diagnostic value of serum AFU, 5’-NT and AFP
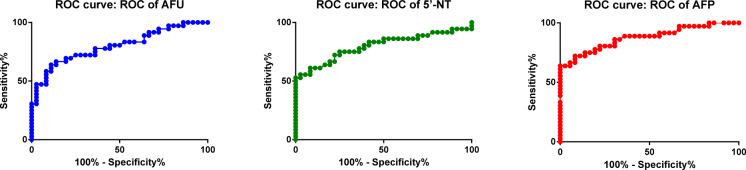
The diagnostic sensitivity and specificity were 0.78 (95%CI:l0.61-0.90), 0.64(95%CI:0.46-0.79) for serum AFU, 0.75(95%CI:0.58-0.88), 0.72(95%CI:0.55-0.86) for serum 5’-NT and 0.72(95%CI:0.55-0.86), 0.92(95%CI:0.78-0.98) for serum AFP respectively, (Table 1). The AUC under the ROC curve were 0.80(0.69-0.90), 0.80(0.69-0.91) and 0.87(0.780-0.96) for serum AFU, 5’-NT and AFP respectively, (Figure 2).
Table 1.
The diagnostic value of serum AFU, 5’-NT and AFP for hepatocellular carcinoma
| Factors | AFU | 5’-NT | AFP |
|---|---|---|---|
| Sen (95%CI) | 0.78 (0.61-0.90) | 0.75(0.58-0.88) | 0.72(0.55-0.86) |
| Spe(95%CI) | 0.64(0.46-0.79) | 0.72(0.55-0.86) | 0.92(0.78-0.98) |
| LR | 2.15 | 2.70 | 8.67 |
| AUC(95%CI) | 0.80(0.69-0.90) | 0.80(0.69-0.91) | 0.87(0.780-0.96) |
| Cut-off | value | 20.19 2. | 41 35.08 |
Sen: Sensitivity; Spe: Specificity; LR: likelihood ratio; AUC: area under the roc curve;
Figure 2.
The ROC curve of serum AFU, 5’-NT and AFP for hepatocellular carcinoma diagnosis
3.3. Correlation between serum AFU, 5’-NT and AFP
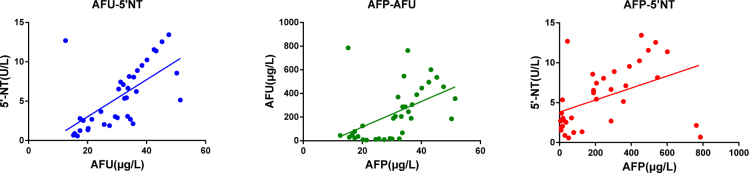
Positive correlation between AFU and 5’-NT (rpearson =0.63, P<0.05), AFU and AFP (rpearson =0.49, P<0.05), 5’-NT and pearson AFP(rpearson=0.44, P<0.05) were found in the primary hepatocellular carcinoma patients, (Figure 3).
Figure 3.
The scatter plot of correlation between serum AFU, 5’-NT and AFP in hepatocellular carcinoma patients
4. Discussion
China has the highest incidence of morbidity and mortality from PHC, accounting for approximately 320,000 deaths annually [10]. In 2008, morbidity from PHC in China ranked third among digestive system cancer related deaths, second to gastric carcinoma and esophagus cancer [2]. PHC is a malignant tumor with a high degree of malignancy, fast progression, poor prognosis, and high risk metastasis. Preventing hepatic carcinoma is severely needed, and new methods for diagnosis and treatment, including the use of efficient laboratory markers, are urgently necessary in clinical practice [11, 12]. Generally, serum AFP, γ-glutamyl transpeptidase, TGFβ1, 5’-NT and AFU were widely used as biomarker for PHC auxiliary diagnosis. However, AFP is the only tumor marker clinically widely used for the diagnosis, treatment, and monitoring of carcinomas [5]. This marker has drawbacks, including high possibility of false-negative results, high cost of reagent, and long processing time for single detection [13].
5’-NT is abundant in the liver, and intrahepatic 5’-NT is mainly distributed in cholangiole and hepatic sinusoid. In PHC, liver infiltration causes intrahepatic cholestasis, leading to significantly increased 5’-NT activity. Therefore, 5’-NT has an important value for hepatocellular carcinoma diagnosis. AFU is a lysosome acidic hydrolase that catalyzes the catabolism of oligose, glycopeptides, glycoprotein, and glucoside of fucosidase. AFU extensively exists in human cells, and its level in hepatic and renal tissues is particularly high. Under normal conditions, AFU is continuously released into peripheral blood as a product of cell metabolism. Except for pregnant women, the serum AFU level of healthy persons is relatively low. Meanwhile, the serum AFU level of patients with hepatic carcinoma is increased because the AFU inhibitor generated by hepatocellular carcinoma reduces the hydrolysis capability for substrate, causing substrate accumulation and compensated increase in AFU level. Therefore, AFU serum concentration can be a diagnosis index for primary hepatic carcinoma [8, 14].
The receiver operating characteristic (ROC) curve is the currently recognized comprehensive assessment method for diagnosis. The ROC curve reflects integrated indexes of sensitivity and specificity and correlation between sensitivity and specificity through composition rule. AUC below 0.5 indicates that the test has no diagnostic value; AUC within 0.5–0.7 means relatively low accuracy; AUC within 0.7–0.9 means certain accuracy; and AUC >0.9 means high accuracy. The more the curve deviates to the top left corner, the larger the AUC is and the larger the diagnostic value is. AUC is intuitional and favorable for comparison among different indexes [15, 16].
In this study, we found that the serum level of AFU, 5’-NT and AFP in hepatocellular carcinoma group were significant higher than those of healthy control group. And the diagnostic sensitivity and specificity were relative high for serum AFU, 5’-NT and AFP as biomarkers in diagnosis of hepatocellular carcinoma. The AUC under the ROC curve were 0.80(0.69-0.90), 0.80(0.69-0.91) and 0.87(0.780-0.96) for serum AFU, 5’-NT and AFP respectively. For all the three markers, the AUC were higher than 0.7 which indicating high accuracy for PHC diagnosis. So, according to the present results, serum AFU, 5’-NT and AFP were higher in PHC patients than those of healthy controls which made them promising biomarkers for PHC diagnosis.
Footnotes
Conflict of interest statement: Authors state no conflict of interest.
Reference
- [1].Zhu RX, Seto WK, Lai CL. et al. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region[J] Gut Liver. 2016;10(3):332–339. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tanaka M, Katayama F, Kato H. et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures[J] J Epidemiol. 2011;21(6):401–416. doi: 10.2188/jea.JE20100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zeng F, Guo P, Huang Y. et al. Epidemiology of hepatitis B virus infection: results from a community-based study of 0.15 million residents in South China[J] Sci Rep. 2016;6:36186. doi: 10.1038/srep36186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ozer ED, Suna N, Boyacioglu AS.. Management of Hepatocellular Carcinoma: Prevention, Surveillance, Diagnosis, and Staging[J] Exp Clin Transplant. 2017;15(Suppl 2):31–35. doi: 10.6002/ect.TOND16.L9. [DOI] [PubMed] [Google Scholar]
- [5].Sauzay C, Petit A, Bourgeois AM. et al. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma[J] Clin Chim Acta. 2016;463:39–44. doi: 10.1016/j.cca.2016.10.006. [DOI] [PubMed] [Google Scholar]
- [6].Barletta E, Tinessa V, Daniele B.. [Screening of hepatocellular carcinoma: role of the alpha-fetoprotein (AFP) and ultrasonography][J] Recenti Prog Med. 2005;96(6):295–9. quiz 328. [PubMed] [Google Scholar]
- [7].Aoyagi Y, Yanagi M, Asakura H.. [Early diagnosis of hepatocellular carcinoma by serum AFP and PIVKA-II analysis][J] Nihon Naika Gakkai Zasshi. 1995;84(12):2003–2007. [PubMed] [Google Scholar]
- [8].Gan Y, Liang Q, Song X.. Diagnostic value of alpha-L-fucosidase for hepatocellular carcinoma: a meta-analysis[J] Tumour Biol. 2014;35(5):3953–3960. doi: 10.1007/s13277-013-1563-8. [DOI] [PubMed] [Google Scholar]
- [9].Zimmermann H.. 5’-Nucleotidase: molecular structure and functional aspects[J] Biochem. J. 1992;285(2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen W, Zheng R, Baade PD. et al. Cancer statistics in China, 2015[J] CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- [11].Masuda T, Miyoshi E.. Cancer biomarkers for hepatocellular carcinomas: from traditional markers to recent topics[J] Clin Chem Lab Med. 2011;49(6):959–966. doi: 10.1515/CCLM.2011.152. [DOI] [PubMed] [Google Scholar]
- [12].Wright LM, Kreikemeier JT, Fimmel CJ.. A concise review of serum markers for hepatocellular cancer[J] Cancer Detect Prev. 2007;31(1):35–44. doi: 10.1016/j.cdp.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [13].Park SJ, Jang JY, Jeong SW. et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma[J] Medicine (Baltimore) 2017;96(11):e5811. doi: 10.1097/MD.0000000000005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Deugnier Y, David V, Brissot P. et al. Serum alpha-L-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma?[J] Hepatology. 1984;4(5):889–892. doi: 10.1002/hep.1840040516. [DOI] [PubMed] [Google Scholar]
- [15].Cerda J, Cifuentes L.. [Using ROC curves in clinical investigation: theoretical and practical issues][J] Rev Chilena Infectol. 2012;29(2):138–141. doi: 10.4067/S0716-10182012000200003. [DOI] [PubMed] [Google Scholar]
- [16].Brickley MR, Prytherch IM, Kay EJ. et al. A new method of assessment of clinical teaching: ROC analysis[J] Med Educ. 1995;29(2):150–153. doi: 10.1111/j.1365-2923.1995.tb02819.x. [DOI] [PubMed] [Google Scholar]