Abstract
This study investigated the expression of C-C chemokine receptor type 3 (CCR3), transcription factor SOX5 (SOX5) and microtubule-associated protein 1 light chain 3 (LC3) in patients with elderly onset rheumatoid arthritis (EORA) and the clinical significance. Ninety patients with elderly onset rheumatoid arthritis were selected in our hospital from January to December in 2016 to serve as patient group. At the same time, 50 healthy people were selected as control group. Levels of CCR3, SOX5 and LC3 in serum of two groups were detected by enzyme-linked immunosorbent assay (ELISA). Expression levels of CCR3, SOX5 and LC3 mRNA in peripheral blood mononuclear cells (PBMCs) were detected by reverse transcription-PCR (RT-PCR). Expression level of CCR3 mRNA in patient group was 0.752±0.054, which was significantly higher than that in control group (0.287±0.032, t=8.932, P<0.05). Levels of CCR3, SOX5 and LC3 in serum of patients in patient group were significantly higher than those in control group (P<0.05). Positive correlations were found between serum levels of CCR3 and SOX5 (r=0.613, P<0.05), serum levels of CCR3 and LC3 (r=0.637, P<0.05), and serum levels of SOX5 and LC3 (r=0.645, P<0.05). CCR3, SOX5 and LC3 are highly expressed in PBMC and serum, which may be closely related to the occurrence and development of EORA. These indexes may be used as indicators of clinical diagnosis and prognosis of patients with EORA.
Keywords: elderly onset rheumatoid arthritis, C-C chemokine receptor type 3, SOX5, light chain 3
Introduction
Rheumatoid arthritis is a relatively common autoimmune disease, and this disease is usually complicated with pericarditis, lymphadenitis and other complications (1). Attentions should be paid to rheumatoid arthritis. Without treatment in time, rheumatoid arthritis can easily develop into irreversible articular cartilage damage, causing serious adverse effects to patients (2). Early diagnosis of rheumatoid arthritis is beneficial to the clinical treatment and prognosis of this disease. Therefore, it would be of great clinical value to identify serum markers for the diagnosis and treatment of rheumatoid arthritis. C-C chemokine receptor type 3 (CCR3), transcription factor SOX5 (SOX5) and microtubule-associated protein 1 light chain 3 (LC3) are common serum markers. So far, only few reports on the functions of these serum markers in the development of elderly onset rheumatoid arthritis (EORA) have been reported. This study aimed to investigate the expression of CCR3, SOX5 and LC3 in patients with elderly onset rheumatoid arthritis and the clinical significance.
Materials and methods
General information
Ninety patients with elderly onset rheumatoid arthritis were selected in Huaihe Hospital from January to December in 2016 to serve as patient group. Inclusion criteria: i) meet the diagnostic criteria for rheumatoid arthritis (3), and age ≥60 years; ii) received no intervention treatment within 3 months before admission; iii) patients signed written informed consent. Exclusion criteria: i) patients combined with heart, lung and other important organ diseases; ii) patients with incomplete clinical data. In patient group, there were 50 males and 40 females, ages ranged from 61 to 74 years with an average age of 65.2±2.3 years, and the course ranged from 1 to 6 years with an mean value of 4.5±1.2 years. There was no significant difference in gender, age and general information between the two groups (P>0.05). This study was approved by the Ethics Committee of Henan University Huaihe Hospital.
Methods
Major reagents
Human CCR3 enzyme-linked immunosorbent assay (ELISA) kit (RunyuBio, Shanghai, China); human SOX5 ELISA kit (Shanghai Jiang Lai Biotechnology Co., Ltd., Shanghai, China); human LC3 ELISA kit (Shanghai Yanjin Biotechnology Co., Ltd., Shanghai, China); TRIzol (Bioss, Beijing, China); reverse transcription kit (Shenzhen Bao An Kang Biological Co., Ltd., Shenzhen, China), DNA polymerase (JiNingShiYe, Ltd., Shanghai, China).
Serum collection and processing
Fasting venous blood (5 ml) was collected in the morning, and heparin was added for anticoagulation. Blood was stored at −70°C before use.
ELISA to detect CCR3, SOX5 and LC3 proteins in serum
Serum levels of CCR3, SOX5 and LC3 were measured by ELISA according to the instructions of the kits.
Reverse transcription PCR (RT-PCR) to detect the expression of CCR3, SOX5 and LC3 mRNAs in peripheral blood mononuclear cells (PBMCs)
Appropriate amount of lymphocyte separation solution was added to serum samples. After centrifugation at 4°C, PBMC was isolated and washed with RPMI-1640. Total RNA was extracted using TRIzol reagent and reverse transcription was carried out in strict accordance with instructions of the kit. Reverse transcription conditions were: 42°C for 60 min, 95°C for 5 min and 5°C for 5 min. The product was stored at −20°C before PCR reaction. Primers used were: 5′-TGGCGGTTGGCGGTGTTTTTCATTTTC-3′ (sense) and 5′-CCGGCTCTGCTGTGGAT-3′ (antisense) for CCR3, the length of product was 315 bp; 5′-GTAGTGTTTGCCCTCACCAACA-3′ (sense) and 5′-ACAGCGTCTGGATGATTCTGA-3′ (antisense) for SOX, the lenth of product was 502 bp; 5′-GAGTGGAAGATGTCCGGCTC-3′ (sense) and 5′-CCAGGAGGAAGAAGGCTTGG-3′ (antisense) for LC3, the length of product was 416 bp. Reaction conditions: 94°C for 5 min, followed by 38 cycles of 94°C for 30 sec; 59°C for 30 sec and 72°C for 60 sec, and 72°C for 5 min.
Ten microliters of PCR produce was subjected to 2% agarose gel electrophoresis and images were taken under ultraviolet light. Optical densities of the bands were measured and the relative expression levels of CCR3, SOX5 and LC3 mRNAs were normalized to the grey scale of β-actin.
Statistical analysis
Using SPSS 20.0 (IBM, Armonk, NY, USA) statistical software was used for statistical analysis, measurement data were expressed as mean ± SD and processed using t-test. P<0.05 was considered to be statistically significant.
Results
Comparison of expression levels of CCR3 mRNA in PBMCs between two groups
RT-PCR results showed that expression level of CCR3 mRNA in patient group was 0.752±0.054, which was significantly higher than that in control group (0.287±0.032, t=8.932, P<0.05) (Fig. 1).
Figure 1.
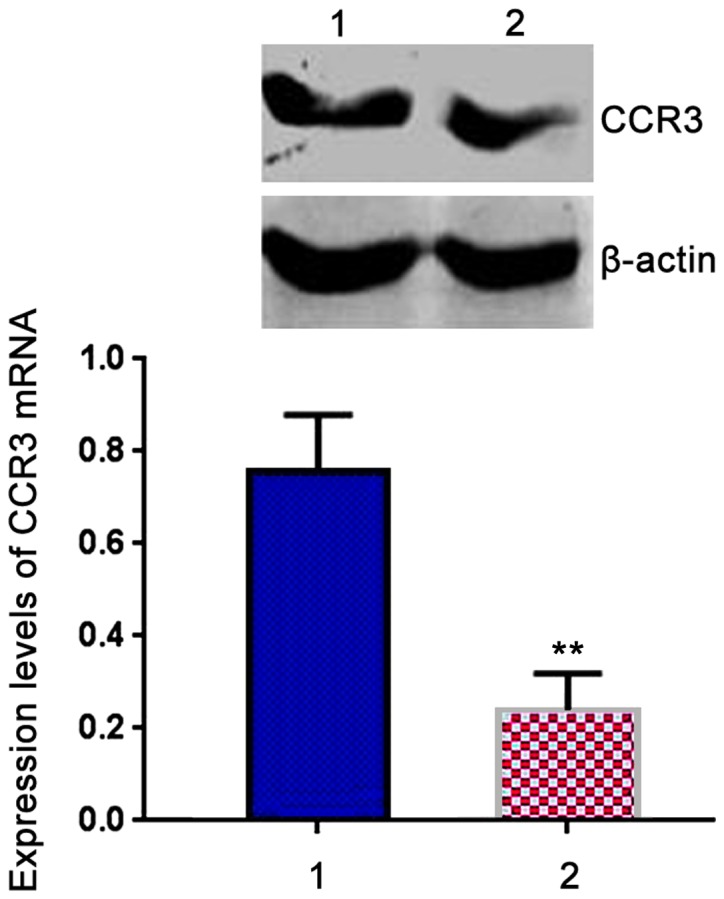
Comparison of expression levels of C-C chemokine receptor type 3 (CCR3) mRNA in peripheral blood mononuclear cells (PBMCs) between two groups. Lane 1, Patient group; lane 2, control group. RT-PCR results showed that expression level of CCR3 mRNA in patient group was significantly higher than that in control group. **P<0.05 compared with patient group.
Comparison of expression levels of SOX5 mRNA in PBMCs between two groups
Expression of SOX5 mRNA in patient group was 0.674±0.032, which was significantly higher than that in control group (0.223±0.012, t=7.541, P<0.05) (Fig. 2).
Figure 2.
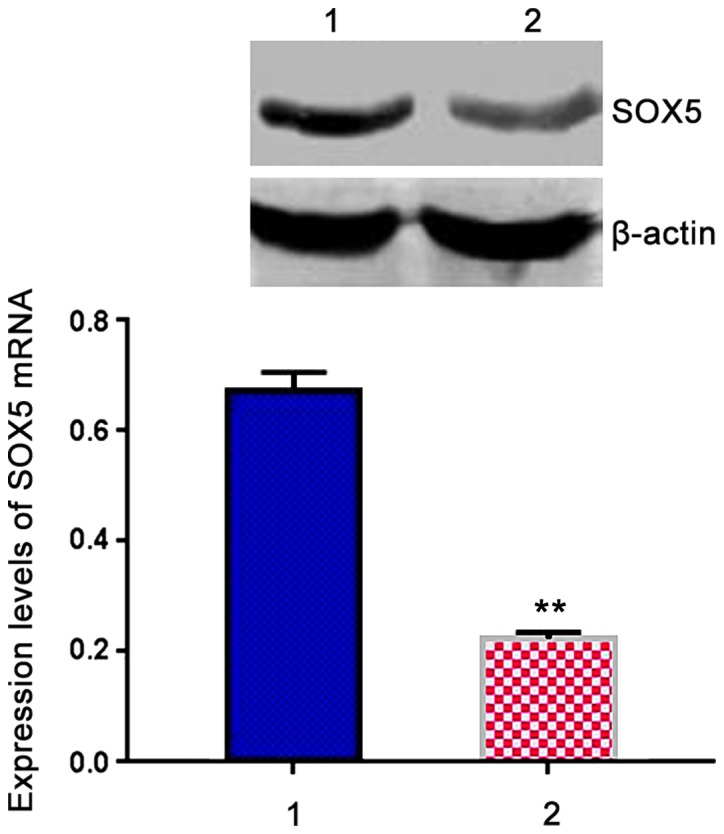
Comparison of expression levels of SOX5 mRNA in peripheral blood mononuclear cells (PBMCs) between two groups. Lane 1, Patient group; lane 2, control group. RT-PCR results showed that expression level of SOX5 mRNA in patient group was significantly higher than that in control group. **P<0.05 compared with patient group.
Comparison of expression levels of LC3 mRNA in PBMCs between two groups
Expression of LC3 mRNA in patient group was 0.674±0.032, which was significantly higher than that in control group (0.223±0.012, t=7.541, P<0.05) (Fig. 3).
Figure 3.
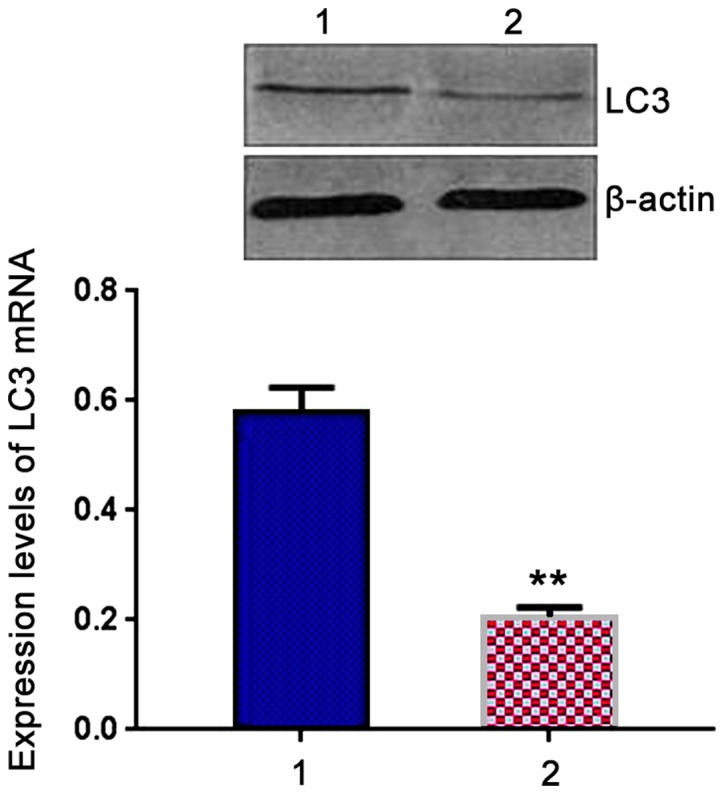
Comparison of expression levels of light chain 3 (LC3) mRNA in peripheral blood mononuclear cells (PBMCs) between two groups. Lane 1, Patient group; lane 2, control group. RT-PCR results showed that expression level of LC3 mRNA in patient group was significantly higher than that in control group. **P<0.05 compared with patient group.
Comparison of serum levels of CCR3, SOX5 and LC3 between two groups
Levels of CCR3, SOX5 and LC3 in serum of patient group were significantly higher than those in control group (P<0.05) (Table I).
Table I.
Comparison of serum levels of CCR3, SOX5 and LC3 between two groups (ng/l).
| Groups | n | CCR3 | SOX5 | LC3 |
|---|---|---|---|---|
| Patient group | 90 | 732.6±37.3 | 694.2±47.8 | 637.7±34.5 |
| Control group | 50 | 187.2±25.5 | 165.4±21.2 | 146.1±17.8 |
| t-value | 9.446 | 10.152 | 8.953 | |
| p-value | <0.05 | <0.05 | <0.05 |
CCR3, C-C chemokine receptor type 3; LC3, light chain 3.
Correlation analysis
There was a positive correlation between serum levels of CCR3 and SOX5 in patients (r=0.613, P<0.05) (Fig. 4). Serum level of CCR3 was positively correlated with serum level of LC3 (r=0.637, P<0.05) (Fig. 5). Serum level of SOX5 was positively correlated with serum level of LC3 (r=0.645, P<0.05) (Fig. 6).
Figure 4.
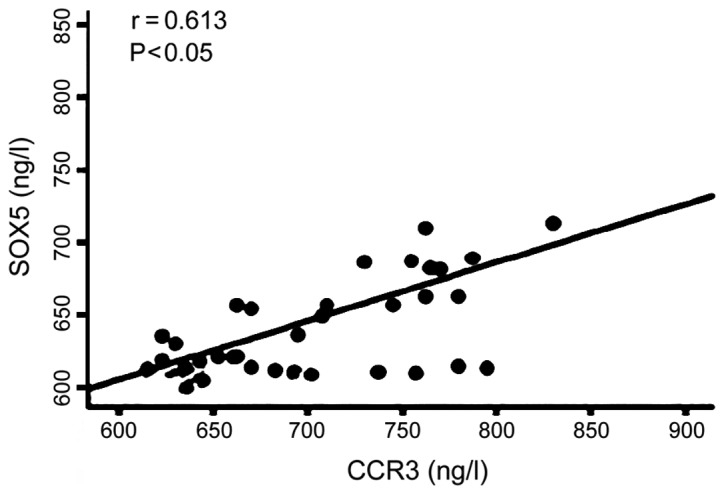
Serum level of C-C chemokine receptor type 3 (CCR3) was positively correlated with serum level of SOX5.
Figure 5.
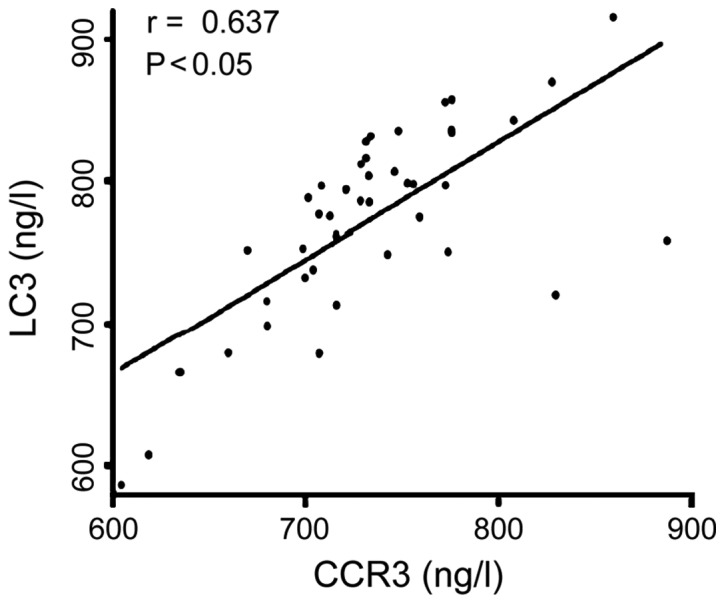
Serum level of C-C chemokine receptor type 3 (CCR3) was positively correlated with serum level of light chain 3 (LC3).
Figure 6.
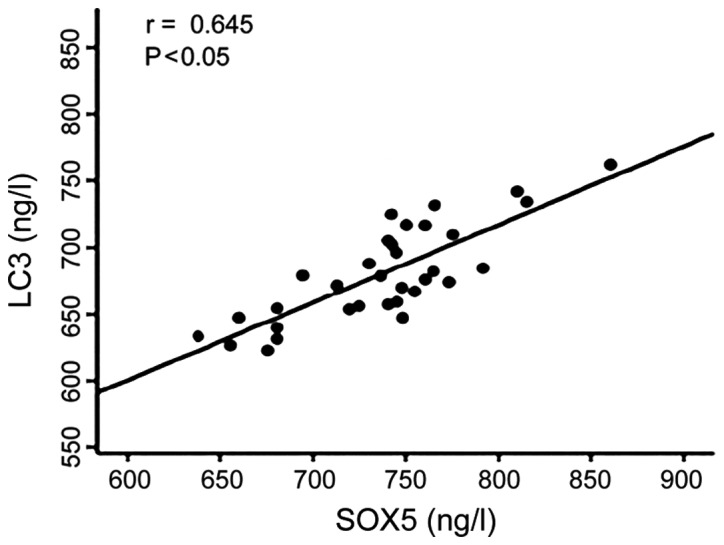
Serum level of SOX5 was positively correlated with serum level of light chain 3 (LC3).
Discussion
Immune deficiency is common is patients with rheumatoid arthritis, and immunosuppressive agents were usually used to treat this disease (4,5). In elderly patients, rheumatoid arthritis is usually combined with low fever, cough and other symptoms or even tuberculosis infection. The complex clinical symptoms of this disease bring difficulties to diagnose (6,7). The pathogenesis of EORA in is still unclear. Rheumatoid arthritis is a genetic factor, infection and immune response mediated complex process (8–10).
Inflammatory response usually occurs in joints and the whole body of patients with EORA. A variety of cytokines will be produced during the development of disease, so those cytokines are actively involved in the progression of disease conditions and development of pulmonary fibrosis (11–14). Related studies have shown that (15,16), chemokines can promote the development of inflammatory factors, as well as the activation of white blood cells. Some studies have also shown that chemokine LKN-1 can induce the chemotactic reaction of endothelial cells through its receptor CCR1 and CCR3, which in turn promote the differentiation of endothelial cells and the formation of external blood vessels (17). It is worth mentioning that, as a G protein-coupled receptor, CCR3 can be expressed in both eosinophils and Th2 lymphocytes, and CCR3 can also mediate cell response to chemokines and promote a variety of biological activity (17). Related studies have shown that (18), CCR3 is closely related to asthma, allergies and other immune diseases. As a newly discovered important transcription factor, SOX5 plays an important role in cartilage formation and regulation, SOX5 also has important functions in regulating the proliferation and differentiation of chondrocyte (19). As an important indicator of autophagosomes, LC3 is mainly located in the cytoplasm and surface of autophagic membrane. LC3 can participate in the formation of autophagosome (20).
Results of this study showed that expression levels of CCR3, SOX5 and LC3 mRNAs in patient group were significantly higher than those in control group (P<0.05). Levels of CCR3, SOX5 and LC3 in serum patient group were significantly higher than those in control group (P<0.05). Positive correlations were found between serum levels of CCR3 and SOX5 (r=0.613, P<0.05), serum levels of CCR3 and LC3 (r=0.637, P<0.05), and serum levels of SOX5 and LC3 (r=0.645, P<0.05). Those results suggest that CCR3, SOX5 and LC3 are very likely to be involved in the pathogenesis of EORA, and have great clinical values in the diagnosis of this disease.
In conclusion, CCR3, SOX5 and LC3 are highly expressed in PBMC and serum, which may be closely related to the occurrence and development of EORA. These indexes may be used as indicators of clinical diagnosis and prognosis of patients with EORA.
References
- 1.Zou YQ, Li YS, Ding XN, Ying ZH. The clinical significance of HRCT in evaluation of patients with rheumatoid arthritis-associated interstitial lung disease: A report from China. Rheumatol Int. 2012;32:669–673. doi: 10.1007/s00296-010-1665-1. [DOI] [PubMed] [Google Scholar]
- 2.Lee HK, Kim DS, Yoo B, Seo JB, Rho JY, Colby TV, Kitaichi M. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127:2019–2027. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 3.Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, Golzarri MF, Traslosheros A, Chu SG, Frits ML, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. 2015;191:1403–1412. doi: 10.1164/rccm.201411-1950OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yin Y, Liang D, Zhao L, Li Y, Liu W, Ren Y, Li Y, Zeng X, Zhang F, Tang F, et al. Anti-cyclic citrullinated peptide antibody is associated with interstitial lung disease in patients with rheumatoid arthritis. PLoS One. 2014;9:e92449. doi: 10.1371/journal.pone.0092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, Lee JS, King TE, Jr, Col-lard HR. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 6.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease: The relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keith RC, Powers JL, Redente EF, Sergew A, Martin RJ, Gizinski A, Holers VM, Sakaguchi S, Riches DW. A novel model of rheumatoid arthritis-associated interstitial lung disease in SKG mice. Exp Lung Res. 2012;38:55–66. doi: 10.3109/01902148.2011.636139. [DOI] [PubMed] [Google Scholar]
- 8.Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Løkke A, Bendstrup E, Ellingsen T. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: Comorbidity and mortality. Ann Rheum Dis. 2017 Jun 13; doi: 10.1136/annrheumdis-2017-211138. (Epub ahead of print). doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 9.Gizinski AM, Mascolo M, Loucks JL, Kervitsky A, Meehan RT, Brown KK, Holers VM, Deane KD. Rheumatoid arthritis (RA)-specific autoantibodies in patients with interstitial lung disease and absence of clinically apparent articular RA. Clin Rheumatol. 2009;28:611–613. doi: 10.1007/s10067-009-1128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, Dawson J, Sathi N, Ahmad Y, Koduri G, et al. British Rheumatoid Interstitial Lung (BRILL) Network: Rheumatoid arthritis-related interstitial lung disease: Associations, prognostic factors and physiological and radiological characteristics - a large multicentre UK study. Rheumatology (Oxford) 2014;53:1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 11.Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, Oddis CV, Ascherman DP. Iden-tification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated inter-stitial lung disease. Arthritis Rheum. 2013;65:869–879. doi: 10.1002/art.37881. [DOI] [PubMed] [Google Scholar]
- 12.Hallowell RW, Horton MR. Interstitial lung disease in patients with rheumatoid arthritis: Spon-taneous and drug induced. Drugs. 2014;74:443–450. doi: 10.1007/s40265-014-0190-z. [DOI] [PubMed] [Google Scholar]
- 13.Solomon JJ, Chung JH, Cosgrove GP, Demoruelle MK, Fernandez-Perez ER, Fischer A, Frankel SK, Hobbs SB, Huie TJ, Ketzer J, et al. Predictors of mortality in rheumatoid arthritis-associated inter-stitial lung disease. Eur Respir J. 2016;47:588–596. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 14.Zamora-Legoff JA, Krause ML, Crowson CS, Ryu JH, Matteson EL. Risk of serious infection in patients with rheumatoid arthritis-associated interstitial lung disease. Clin Rheumatol. 2016;35:2585–2589. doi: 10.1007/s10067-016-3357-z. [DOI] [PubMed] [Google Scholar]
- 15.Mori S. Management of rheumatoid arthritis patients with interstitial lung disease: Safety of biological antirheumatic drugs and assessment of pulmonary fibrosis. Clin Med Insights Circ Respir Pulm Med. 2015;9(Suppl 1):41–49. doi: 10.4137/CCRPM.S23288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, New-man W, et al. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 17.Danilova E, Skrindo I, Gran E, Hales BJ, Smith WA, Jahnsen J, Johansen FE, Jahnsen FL, Baekkevold ES. A role for CCL28-CCR3 in T-cell homing to the human upper airway mucosa. Mucosal Immunol. 2015;8:107–114. doi: 10.1038/mi.2014.46. [DOI] [PubMed] [Google Scholar]
- 18.Nagai N, Ju M, Izumi-Nagai K, Robbie SJ, Bainbridge JW, Gale DC, Pierre E, Krauss AH, Ad-amson P, Shima DT, et al. Novel CCR3 antagonists are effective mono- and combination inhibitors of choroidal neovascular growth and vascular permeability. Am J Pathol. 2015;185:2534–2549. doi: 10.1016/j.ajpath.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefebvre V. The SoxD transcription factors - Sox5, Sox6, and Sox13 - are key cell fate modulators. Int J Biochem Cell Biol. 2010;42:429–432. doi: 10.1016/j.biocel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]


