Summary
Lowering total tau levels is an attractive therapeutic strategy for Alzheimer's disease and other tauopathies. High-throughput screening in neurons derived from human induced pluripotent stem cells (iPSCs) is a powerful tool to identify tau-targeted therapeutics. However, such screens have been hampered by heterogeneous neuronal production, high cost and low yield, and multi-step differentiation procedures. We engineered an isogenic iPSC line that harbors an inducible neurogenin 2 transgene, a transcription factor that rapidly converts iPSCs to neurons, integrated at the AAVS1 locus. Using a simplified two-step protocol, we differentiated these iPSCs into cortical glutamatergic neurons with minimal well-to-well variability. We developed a robust high-content screening assay to identify tau-lowering compounds in LOPAC and identified adrenergic receptors agonists as a class of compounds that reduce endogenous human tau. These techniques enable the use of human neurons for high-throughput screening of drugs to treat neurodegenerative disease.
Keywords: human induced pluripotent stem cells, human neurons, Tau-lowering, neurogenin 2, high-content screening, adrenergic receptor, Alzheimer’s disease, tau, frontotemporal dementia, neurodegeneration
Highlights
-
•
Inducible, isogenic, and integrated Ngn2 iPSCs were differentiated to pure neurons
-
•
A simple and scalable two-step protocol ensures minimal differentiation variability
-
•
An HCS assay was established to screen for tau-lowering compounds in LOPAC
-
•
AR agonists were identified as a class of compounds that reduce human tau
Gan and colleagues developed a simple and scalable technology to generate a large quantity of homogeneous glutamatergic cortical neurons by engineering a neurogenin 2-expressing cassette to the AAVS1 locus of iPSCs. They developed a high-content screening assay and identified adrenergic receptor agonists as a class of compounds that lower endogenous human tau, a key pathogenic factor in Alzheimer's disease.
Introduction
The microtubule-associated neuronal protein tau stabilizes microtubules and mediates axon outgrowth and axonal transport. Abnormal tau is strongly implicated in Alzheimer's disease (AD) and other neurodegenerative tauopathies (Wang and Mandelkow, 2016). Although intraneuronal aggregates of insoluble tau fibrils, known as neurofibrillary tangles, are a hallmark of tauopathies and correlate with cognitive decline in AD (Nelson et al., 2012), soluble tau may also play a key pathogenic role (Brunden et al., 2008, Spires-Jones et al., 2011). In Drosophila and mouse models, overexpression of wild-type human tau induces neurodegeneration (Wittmann et al., 2001), axonopathy (Spittaels et al., 1999), and extensive cell death (Andorfer et al., 2005) independently of tangle formation. In two regulatable tauopathy mouse models, suppressing soluble tau expression resulted in memory recovery (Santacruz et al., 2005, Sydow et al., 2011) and stabilized neuron numbers (Santacruz et al., 2005) without reducing the level of neurofibrillary tangles, suggesting that soluble forms of tau promote neurodegeneration. Lowering endogenous tau levels reduces amyloid β (Aβ)-induced behavioral deficits in AD mouse models (Roberson et al., 2007, Vossel et al., 2010), and lowering total tau levels by inhibiting tau acetylation or phosphorylation rescues tau-related memory deficits in PS19 transgenic mice (Lasagna-Reeves et al., 2016, Min et al., 2015). Since tau knockout mice appear to be cognitively normal, lowering total tau levels in neurons appears to be safe and will likely have a high therapeutic index (Morris et al., 2013). Thus, soluble tau is a promising therapeutic target. However, identifying selective, non-toxic tau-lowering compounds has proven to be difficult (Gruninger, 2015).
Cell-based “phenotypic” high-throughput screening (HTS) is a powerful unbiased tool to identify gene targets or small-molecule compounds exerting desired effects. However, HTS requires large numbers of cells and has been largely restricted to immortalized human neuronal lines, such as neuroblastoma SH-SY5Y(Jain et al., 2012) and glioma H4 (Albrecht et al., 2004) cells, or non-neuronal lines, such as HeLa cells (Fatokun et al., 2013). Since these cells differ physiologically from post-mitotic neurons, hits identified in these cells might not work in neurons. This may be particularly true for tau, a neuronal protein that is abundant in axons but is mainly expressed in the cytosol in non-neuronal cells (Uberti et al., 1997). Rodent primary neurons are more physiologically relevant, but challenges in scalability preclude their use for HTS, and certain compounds may differ in activity between human and rodent cells.
Human induced pluripotent stem cells (iPSCs) are a promising alternative because they can be used to generate large numbers of subtype-specific human neurons that are relevant to neurodegenerative disease. However, iPSC-derived neurons currently have limited utility in HTS assays (D'Aiuto et al., 2014), as traditional differentiation methods are difficult to scale up and usually yield a heterogeneous population of neurons and glia-like cells over a protracted timeline (Muratore et al., 2014, Nicholas et al., 2013). More homogeneous neuronal populations can be produced by overexpressing pro-neuronal transcription factors (Chanda et al., 2014, Pang et al., 2011). Neurogenin 2 (NGN2)-induced neurons from various human embryonic stem cell and iPSC lines show robust morphological, transcriptional, and functional homogeneity (Busskamp et al., 2014, Zhang et al., 2013). However, this method has shortcomings for HTS. First, it entails a labor-intensive multi-step differentiation procedure that is difficult to apply to microplates. Second, it is subject to cell-to-cell and well-to-well variability due to different viral infection and puromycin selection rates, uneven cell distribution, which might affect cell survival and image quantification, and experiment-to-experiment variability due to differences in viral titers and qualities of primary mouse glia from different batches. Third, it is costly to scale up.
In this study, we engineered a clonal iPSC line that stably harbors a doxycycline-inducible mouse Ngn2 transgene at an adeno-associated virus integration site 1 (AAVS1) safe-harbor locus. This integrated, inducible, and isogenic Ngn2 iPSC line (i3N) can be differentiated into functional glutamatergic cortical neurons by a simplified two-step differentiation protocol. We developed a robust high-content screening (HCS) assay to identify tau-lowering compounds and discovered compounds that target adrenergic receptor (AR) pathways to lower endogenous human tau.
Results
Engineered iPSCs for Scalable Production of Homogeneous Excitatory Neurons
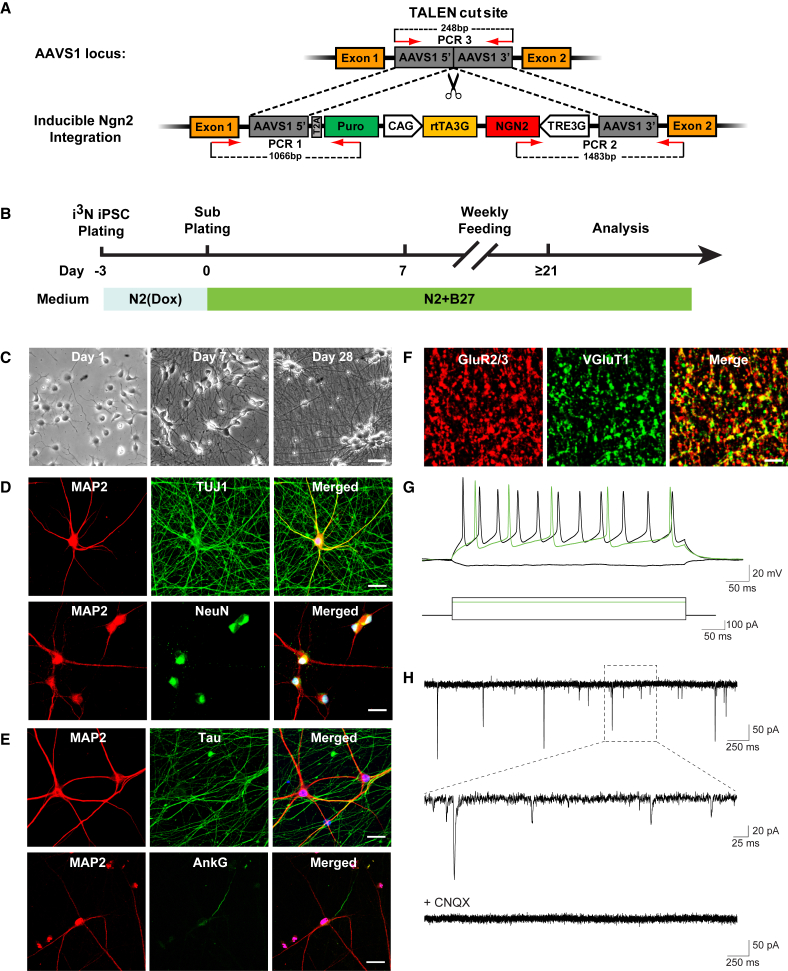
Lentivirus-mediated NGN2 expression induces rapid differentiation of iPSCs into excitatory neurons (Zhang et al., 2013). To avoid viral transduction-induced toxicity and variability in NGN2 expression, we engineered isogenic iPSC lines with an integrated Ngn2 expression cassette. A doxycycline-inducible Ngn2 transgene was integrated into the AAVS1 safe harbor of a well-characterized control human iPSC line (WTC11) (Miyaoka et al., 2014) by TALEN-mediated integration of a donor cassette containing a puromycin-resistance gene (Figure 1A). Six puromycin-resistant clones were picked, and integration of the Ngn2 transgene into the AAVS1 locus was confirmed with two sets of primers (PCR1 and PCR2) (Figures 1A and S1A). Transgene integration into both alleles was confirmed by the absence of the wild-type allele, determined by a third set of primers (PCR3) (clones 1 and 4). These iPSC clones have isogenic, integrated, and inducible NGN2 expression (i3N); neurons derived from them are called i3Neurons. Further characterization of clone 1 in the absence of doxycycline showed homogeneous expression of the pluripotency markers OCT4, SOX2, and TRA-1-81, indicating no leakage of NGN2 expression (Figure S1B). The cells also had a normal karyotype (Figure S1C).
Figure 1.
Engineering of i3N iPSCs and Generation of Homogeneous Functional Glutamatergic Neurons by a Simplified Two-Step Procedure
(A) Schematic of the targeting of the AAVS1 locus with pUCM.Puro-CAG.rtTA3G-TRE3G.Ngn2 donor vector by TALEN-mediated integration. The third-generation doxycycline-inducible reverse transcriptional activator (rtTA3G) is driven by the CAG promoter and followed by rbGlob polyA tail. Mouse Ngn2 is driven by the tet response element (TRE3G) and followed by SV40 polyA tail. It is oriented tail-to-tail with rtTA3G. Orange boxes are exons of the PPP1R12C gene; gray boxes are regions of homology. PCR1 and PCR2 primers are used for 5′ and 3′ junction PCR screening and generate 1.1-kb and 1.5-kb PCR products, respectively. PCR3 primers (product size, 248 base pairs) are used to detect the nonintegrated allele at the AAVS1 locus.
(B) Flow diagram of the two-step procedure for generating i3Neurons.
(C) Representative phase-contrast images during the differentiation of i3Neurons. The timeline is the same as shown in (B). Scale bar, 50 μm.
(D) Representative images showing immunocytochemical staining for the pan-neuronal marker MAP2, βIII tubulin (TUJ1 antibody), and NeuN in i3Neurons after 4 weeks of differentiation. Nuclei were labeled by Hoechst. Scale bar, 25 μm.
(E) Representative images of immunocytochemical staining of mature 8-week-old i3Neurons show tau enrichment (detected with HT7) in an axon identified by the axon initiation segment marker ankyrin G (AnkG). Nuclei were labeled by Hoechst. Scale bar, 25 μm.
(F) Representative confocal images of i3Neurons showing immunolabeling of postsynaptic GluR2/3 containing AMPA-type glutamate receptors (red) and the presynaptic vesicular glutamate transporter VGlut1 (green). The colocalization of GluR2/3 and VGlut1 puncta marks glutamatergic synapses formed between i3Neurons. Scale bar, 5 μm.
(G) Representative traces of action potentials evoked by 500-ms current step injections at just above the firing threshold (green trace) and at a higher firing frequency (black trace).
(H) Spontaneous excitatory postsynaptic currents recorded from an i3N neuron (top) were blocked by CNQX, an AMPA receptor antagonist (bottom).
See also Figures S1 and S2.
i3N iPSCs could be differentiated into functional excitatory neurons by the published multi-step differentiation protocol. To overcome the poor scalability and reproducibility of this procedure when adapted to the HTS platform, we established a simplified two-step protocol (Figure 1B). After doxycycline induction and subplating, pre-differentiated i3Neurons became post-mitotic, and exhibited neuron-like morphology in less than 7 days and mature neuronal morphology within 3–4 weeks in the absence of glia (Figure 1C). Differentiated neuron markers βIII tubulin (TUJ1), microtubule-associated protein 2 (MAP2), and neuronal nuclei antigen (NeuN) were detected in i3Neurons after 4 weeks of maturation (Figure 1D). The absence of Olig2-positive oligodendrocytes or glial fibrillary acidic protein (GFAP)-positive astrocytes confirmed the purity of the neuronal population (Figure S2A).
In 8-week-old i3Neurons, tau was abundant in axons identified by the presence of a single axonal initial segment (AIS), as detected with ankyrin G staining (Kordeli et al., 1995) (Figure 1E). More than 90% of i3Neurons contained AIS, supporting the notion that they exhibit mature polarity by 8 weeks (Figure S2B). Tau expression had little overlap with MAP2, a dendritic marker (Figure 1E). Both three-repeat (3R) and four-repeat (4R) tau were detected (Figure S2C). Tau in i3Neurons were also highly phosphorylated, compared with healthy human brain (Figure S2D). Importantly, all i3Neurons expressed vesicular glutamate transporter 1 (VGlut1), a marker of glutamatergic neurons, and were GABA negative, indicating a homogeneous population of excitatory glutamatergic neurons (Figure S2E). The punctate distribution of synapsin-1 staining along the processes revealed abundant synapse formation (Figure S2F). When co-cultured with glia, i3Neurons had mature synapses that contained juxtaposed pre- and postsynaptic markers of glutamatergic synapses (Figure 1F). Whole-cell patch-clamp recordings showed action potential firing in response to current injections (Figure 1G). Spontaneous postsynaptic currents detected in i3Neurons were blocked by CNQX, an AMPA receptor antagonist, confirming functional glutamatergic synaptic transmission (Figure 1H).
i3Neurons Cultured in Microplates Show Homogeneous Gene Expression with Low Variability
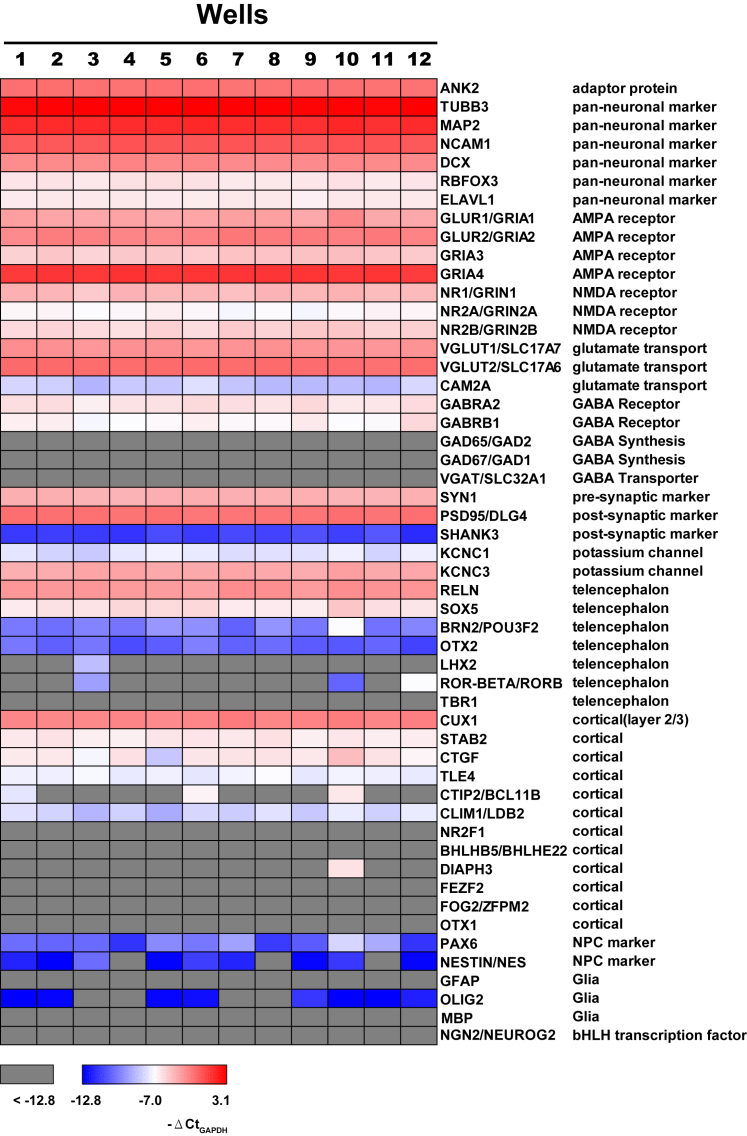
Two key determinants of reliable HTS are scalability and minimal well-to-well variability. We were able to produce large quantities of i3Neurons via i3N iPSC proliferation and a pre-differentiation step. Subsequent subplating enables i3Neurons culture in 96- and 384-well microplates with even cell distribution (Figure 1B). To determine whether i3Neurons homogeneously differentiate in microplates, we quantified the expression of multiple neuronal and glial genes of 4-week-old cells from 12 randomly selected wells of a 96-well plate by RT-qPCR (Figure 2). Consistently high levels of pan-neuronal genes, AMPA receptor genes, glutamate transporter genes, synaptic genes, and CUX1, a marker of cortical layer 2/3 neurons, were observed in all 12 wells, suggesting a uniform differentiation of glutamatergic cortical neurons. In addition, all the i3Neurons showed low expression of two markers of neural progenitor cells, PAX6 and NESTIN, indicating that they were fully differentiated. Ngn2 was undetectable, as expected in the absence of doxycycline. No markers of glial cells or of GABAergic neurons (e.g., GAD65/67) were detected, but the GABA receptors GABRA2 and GABRB1 were expressed at low levels. Thus, i3Neurons can be cultured in microplates with low well-to-well variability and are suitable for HTS.
Figure 2.
i3Neurons Show Homogeneous Expression of Glutamatergic Cortical Neuronal Genes
Heatmap of RT-qPCR analysis of expression levels of genes listed on the right. Expression levels are normalized to housekeeping gene GAPDH (expressed as −ΔCt) and color coded as shown. mRNA was harvested from 12 random wells of 4-week-old i3Neurons cultured in a 96-well plate. See also Table S1.
Optimization of an HCS Assay to Screen for Tau-Lowering Compounds
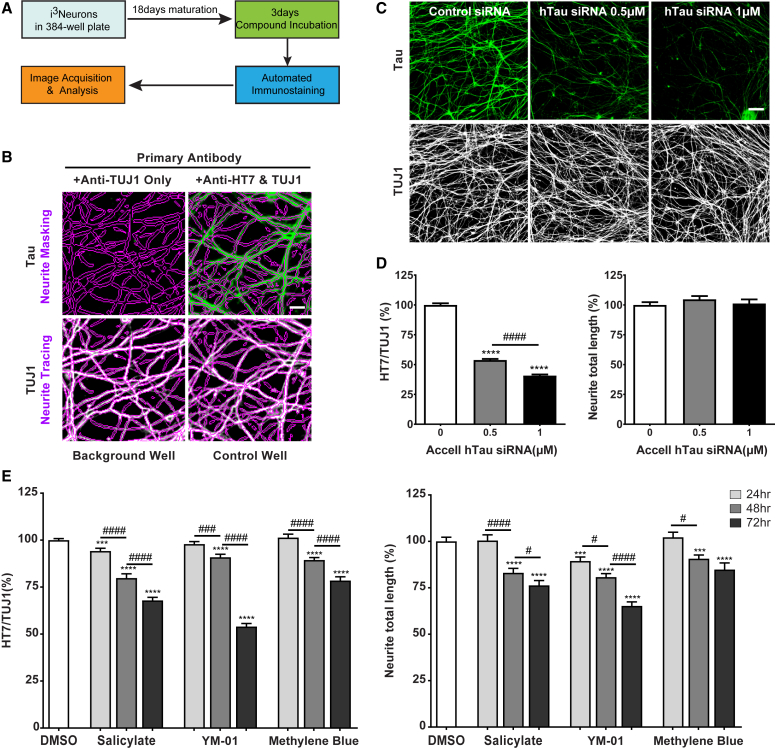
Lowering tau levels has emerged as a key therapeutic opportunity in AD. To our knowledge, no HCS targeting endogenous tau has been performed in post-mitotic human neurons. Because i3Neurons are scalable and highly homogeneous, they are an ideal system to screen for tau-targeted therapeutics. Taking advantage of the highly specific anti-human tau antibody HT7, we set out to develop a 384-well format HCS assay to detect endogenous tau levels in i3Neurons (Figure 3A). Total tau levels were determined by immunoreactivity of HT7 normalized to βIII tubulin intensity in a corresponding well (HT7/TUJ1) (Figure 3B). The background signal, measured in wells omitting HT7, was more than 10-fold lower than those with HT7 (Figures 3B and S3A). The optimal seeding density was 2,000 cells/well (Figure S3A). Neuronal health was judged from neurite total length (Figure S3B) and valid nucleus count (Figure S3C), two highly correlated health parameters (Figure S3D) that are widely used (Harrill et al., 2013, K Hancock et al., 2015). Treatment with a tau-specific siRNA reduced the total tau levels in a dose-dependent manner (Figures 3C and 3D), whereas increased total tau levels were detected when i3Neurons were infected by AAV human tau (Figures S3E and S3F), confirming assay specificity and sensitivity. Salicylate, YM-01, and methylene blue reduce total tau levels (Abisambra et al., 2013, Hosokawa et al., 2012, Min et al., 2015). All three compounds reduced the HT7/TUJ1 signal in a time-dependent manner accompanied by a mild yet significant reduction of neurite total length (Figure 3E). The Z′ factor, a measure of the assay response window (Zhang et al., 1999) and determined by values of control and hTau siRNA, was 0.41, supporting the robustness of the HCS assay.
Figure 3.
Development and Validation of an HCS Assay to Detect Tau Levels in i3Neurons
(A) Schematic of the HCS assay optimized to measure cellular tau levels in neurons treated with small-molecule compounds.
(B) Representative fluorescence high-content images showing tau (green) and βIII tubulin (white) channels from a background well (left, anti-TUJ1 only) and a control well (right, anti-HT7 and TUJ1). Neurite regions (purple) were traced according to the TUJ1 channel and were applied to the tau channel with the neuronal profiling module of Cellomics software. Scale bar, 10 μm.
(C) Representative fluorescence high-content images showing tau (green) and βIII tubulin (white) channels from i3Neurons after 7 days of treatment with control siRNA or human tau siRNA (0.5 or 1 μM). Scale bar, 50 μm.
(D) Automated quantification of human tau levels (left) and neurite total length (right) from i3Neurons treated with human tau siRNA. Data are from three independent experiments, total N = 42 per treatment; values are means ± SEM relative to control siRNA. ∗∗∗∗p < 0.0001 compared with control siRNA, STATA mixed model. ####p < 0.0001, STATA mixed model.
(E) Automated quantification of human tau levels (left) and neurite total length (right) from i3Neurons treated with 5 mM salicylate, 1 μM YM-01, or 1.5 μM methylene blue for 24–72 hr. Data are from three independent experiments, total N = 42 per treatment; values are means ± SEM relative to DMSO. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, compared with DMSO, STATA mixed model; #p < 0.05, ###p < 0.001, ####p < 0.0001, comparison between three time points within each compound treatment, STATA mixed model.
See also Figure S3.
Identification of Tau-Lowering Compounds via HCS
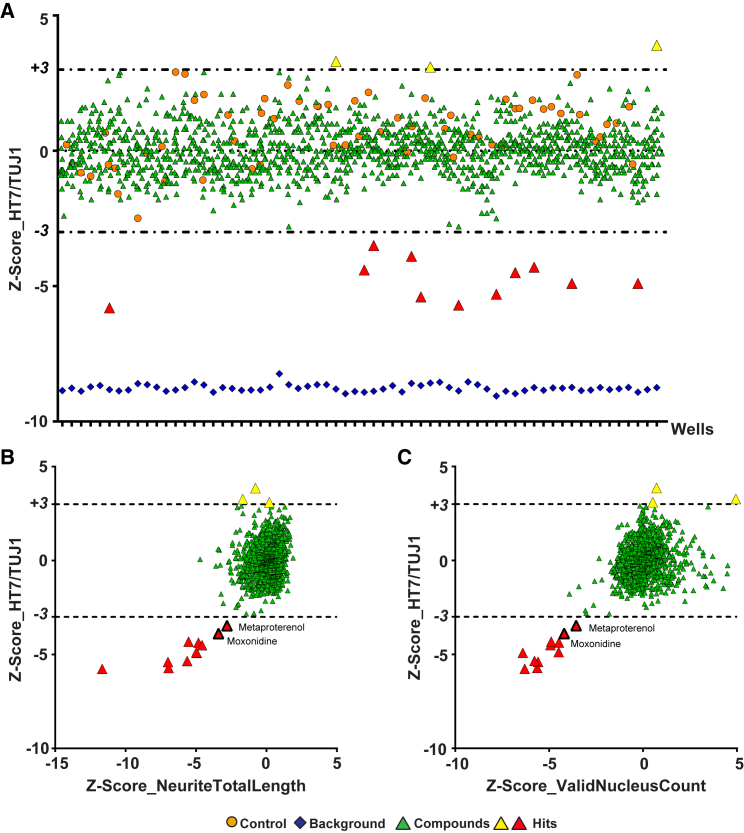
Next, we used the HCS assay to screen LOPAC (Library of Pharmacologically Active Compounds) for tau-lowering compounds. This library contains 1,280 bioactive small molecules, including inhibitors, receptor ligands, marketed drugs, and pharmaceutically relevant structures, that affect most signaling pathways and cover major drug target classes. Since overwhelming majority compounds did not change tau levels (Figure 4A), we used sample compounds as their own control and calculated Z scores based on LOPAC compounds on each plate (Brideau et al., 2003). Compounds that changed tau (as judged from the HT7/TUJ1 ratio) were defined as hits if their Z scores were greater than 3 or less than −3, a stringent cut-off correlates to a p value of 0.00135 (Zhang et al., 2006). All hits were then ranked by neuronal health parameters, including neurite total length (Figure 4B) and valid nucleus count (Figure 4C).
Figure 4.
Primary Screening and Hit Selection from LOPAC
(A) Overview of automated quantification of human tau levels from i3Neurons incubated with compounds from LOPAC at 10 μM for 3 days (green triangles). The library was distributed to three and one-half 384-well plates; each plate contains 16 control wells (DMSO, orange circles) and 16 background wells (blue diamonds). Results are shown as the Z score, calculated based on LOPAC compounds on each plate. Compounds above (yellow triangles) and below (red triangles) the cut-offs of +3 or −3 (dotted lines) are considered hits.
(B and C) Hits are ranked by neurite total length (B) and valid nucleus count (C). Results are shown as Z score, calculated based on LOPAC compounds on each plate. Moxonidine and metaproterenol are the top two tau-lowering hits from both rankings.
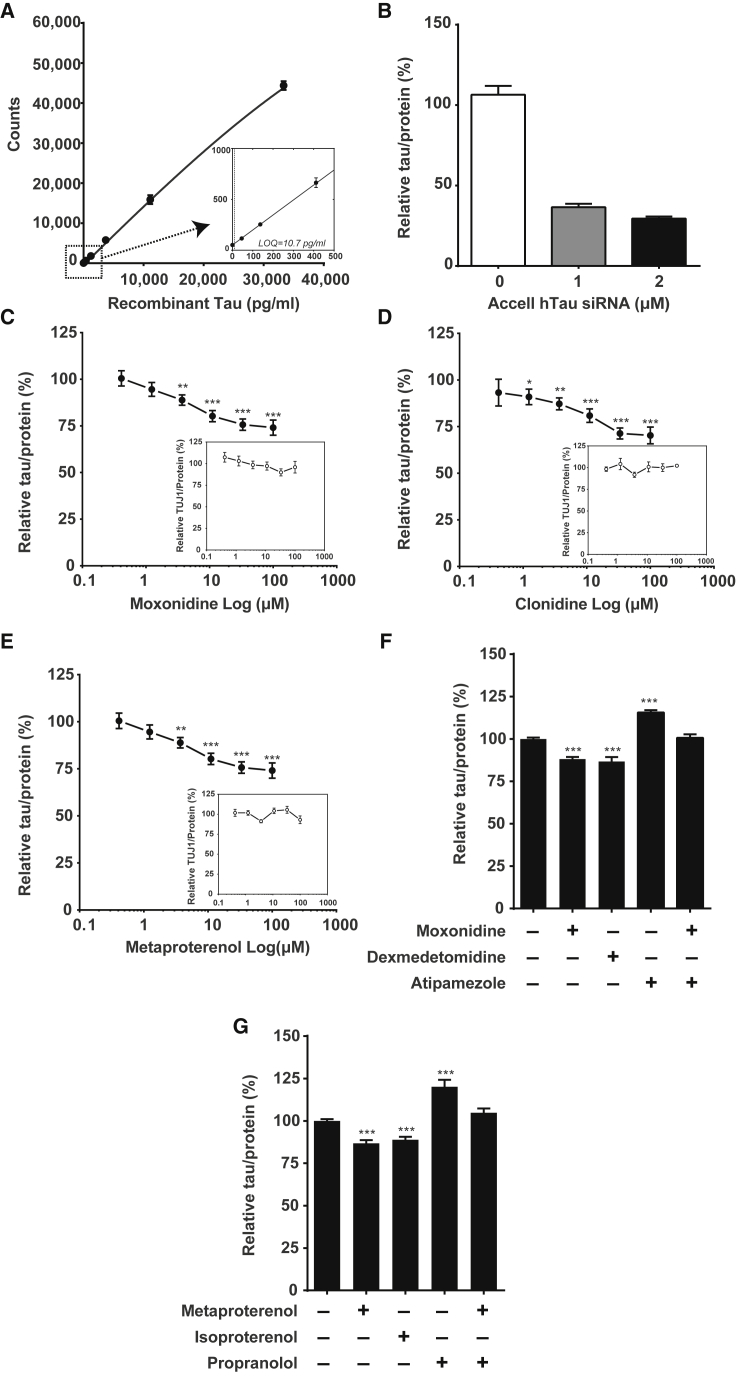
The top two tau-lowering hits that show least cytotoxicity, measured by neurite total length and valid nucleus count, are two functionally related AR agonists, moxonidine and metaproterenol (Figures 4B and 4C). To validate these hits, we adapted a sensitive human tau ELISA that uses HT7 as the capture antibody and Tau5 as the detection antibody (Meredith et al., 2013). The dynamic range of the assay was >3,000, and the detection limit was ∼10.7 pg/mL (Figure 5A). This assay readily detected siRNA-induced reduction of endogenous human tau by >50% (Figure 5B). We then confirmed that moxonidine, clonidine (a moxonidine-related adrenergic agonist), and metaproterenol reduced tau levels in a dose-dependent manner. None of the three compounds induced a significant loss of βIII tubulin, as measured with a βIII tubulin ELISA, indicating a lack of toxicity (Figures 5C–5E).
Figure 5.
Activation of α- and β-AR Reduces Total Tau Levels in i3Neurons
(A) Representative calibration curve of HT7-Tau5 ultra-sensitive human tau ELISA. Inset shows the assay's limit of quantification (LOQ).
(B) 7-day incubation of human tau siRNA significantly lowered total tau levels in i3Neurons. Human tau levels were quantified by HT7-Tau5 ELISA and normalized to protein level. Values are means ± SEM relative to control siRNA. Data are from one experiment, N = 6 wells per treatment.
(C–E) Concentration-response curve of moxinidine (C), clonidine (D), and metaproterenol (E) determined by HT7-Tau5 ELISA. Insets show the βIII tubulin level for each concentration as determined by βIII tubulin ELISA. Both tau and βIII tubulin levels are normalized to protein levels. Values are means ± SEM relative to DMSO control. Data are from four independent experiments performed in triplicate (C, N = 12 per concentration) and three independent experiments performed in triplicate (D and E, N = 9 per concentration). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; STATA mixed model.
(F and G) 3-day incubation with moxonidine (30 μM) and the α-AR agonist dexmedetomidine (100 μM) (F) or metaproterenol (30 μM) and the β-AR agonist isoproterenol (30 μM) (G) significantly reduced total tau levels in i3Neurons. The α-AR antagonist atipamezole (100 μM) (F) and the β-AR antagonist propranolol (50 μM) (G) increased total tau levels and abolished the tau-lowering effect of moxonidine (F) or metaproterenol (G), respectively. Human tau levels were quantified by HT7-Tau5 ELISA and normalized to protein level. Values are means ± SEM relative to DMSO control. Data are from three independent experiments performed in triplicate (F, N = 9 per treatment) and four independent experiments performed in triplicate (G, N = 12 per treatment) ∗∗∗p < 0.001, STATA mixed model.
See also Figure S4.
Additional pharmacologic modulators of α- and β-AR activity were used to further confirm the involvement of α- and β-adrenergic signaling in modulating endogenous human tau levels. i3Neurons were treated with nonselective α- or β-AR agonists (dexmedetomidine or isoproterenol) and their respective antagonists. Activation of α- or β-ARs significantly reduced endogenous human tau levels, and inhibition by nonselective antagonists caused tau accumulation (Figures 5F and 5G). Moreover, the tau-lowering effects of moxonidine and metaproterenol were abolished by pre-incubation with the corresponding antagonists (atipomezole and propranol, respectively) (Figures 5F and 5G). The ability of α- or β-AR agonists to reduce endogenous tau levels was further confirmed in an independent line of i3Neurons (Figures S4A and S4B). Our results showed that activation of α- and β-adrenergic signaling pathways could represent tau-lowering therapeutic strategies in human neurons.
Discussion
In this study, we integrated a doxycycline-inducible mouse Ngn2 cassette into the AAVS1 locus and established iPSC lines that can be converted efficiently to a uniform population of glutamatergic neurons by a simplified two-step protocol. We then developed a robust, scalable, and simple HCS assay and used it to identify compounds that lower tau in post-mitotic human neurons. This proof-of-principle screen identified AR agonists as a class of compounds that decrease endogenous human tau levels.
i3Neurons have several features that make them suitable for HTS, particularly imaging-based HCS. First, the population of differentiated neurons is homogeneous, which may reflect the uniform genetic background of the clonal i3N iPSCs. As all of the iPSC-derived cells are neurons, sensitivity and specificity of HTS screens is increased. Other safe-harbor loci besides the AAVS1 locus (e.g., citrate lyase beta-like) can be used in human iPSCs (Cerbini et al., 2015). By targeting multiple safe-harbor sites with P2A or IRES elements, it may be possible to integrate multiple inducible transcription factors to differentiate iPSCs into other neuronal subtypes, such as dopaminergic neurons (Amamoto and Arlotta, 2014) or motor neurons (Hester et al., 2011), for use in HTS. Second, our simplified glia-free differentiation protocol overcomes hurdles, such as scalability, variability, complexity, and cost, that previously hindered the use of iPSC-derived neurons in drug discovery pipelines. For example, instead of generating and freezing large quantities of neuronal precursor cells, our approach involves a single cost-effective 3-day pre-differentiation procedure. The pre-differentiated neurons are post-mitotic and can be subplated onto poly-D-lysine/laminin-coated microplates with high consistency. Differentiation of i3Neurons does not require viral infection, puromycin selection, or frequent medium change, thereby reducing the possibility of introducing variability and contamination during HTS. Thus, standardized HTS procedures can be established regardless of location, time, or users, facilitating future use of i3Neurons for ultra-HTS of large libraries of chemical compounds.
Tau-targeted approaches have been proposed as an alternative therapeutic strategy for AD and other tauopathies (Dehdashti et al., 2013, DeVos et al., 2013, Panza et al., 2016). Previous tau-targeted small-molecule strategies—including kinase inhibitors against hyperphosphorylated tau, tau aggregation inhibitors, microtubule stabilizers, and compounds that enhance clearance of tau aggregates—have had limited success (Brunden et al., 2009, Gruninger, 2015, Medda et al., 2016, Min et al., 2015, Panza et al., 2016). Lowering total tau levels may have beneficial effects. For example, the MAPT H1c haplotype increases tau expression and is associated with increased risk of progressive supranuclear palsy, corticobasal degeneration, and AD (Baker et al., 1999, Di Maria et al., 2000, Myers et al., 2005, Myers et al., 2007). Tau lowering in transgenic mouse models rescued functional deficits and tau-mediated neurodegeneration (Andrews-Zwilling et al., 2010, Ittner et al., 2010, Lasagna-Reeves et al., 2016, Min et al., 2015, Roberson et al., 2007, Roberson et al., 2011, Vossel et al., 2010) and appears to be safe (Morris et al., 2013). However, discovering compounds that reduce total tau levels has been challenging. Tau is natively unstructured and has proved a difficult target for rational drug design. Several tau-lowering candidates that reduce tau gene transcription were identified by small-scale screening with in-cell western analysis (Dickey et al., 2006). AlphaLISA and homogeneous time-resolved fluorescence assays were used in SH-SY5Y cells to screen for tau-lowering compounds (Dehdashti et al., 2013). However, these studies were done and validated only in tumor cell lines that lack well-defined axons highly enriched in tau. Previous tau-lowering compounds also caused significant cytotoxicity (Dehdashti et al., 2013). In addition, the regulation of tau homeostasis may differ in rodent and human neurons. Indeed, methylene blue, which reduces the levels of human tau overexpressed in mouse neurons in vivo and in vitro (Congdon et al., 2012, Hosokawa et al., 2012), had minor effect in human neurons in our assay.
Using i3Neurons in a robust HCS assay, we identified AR agonists as a class of tau-lowering compounds. Both α- and β-AR signaling appeared to regulate tau levels in similar fashion, as AR activation led to tau reduction and AR inhibition led to tau accumulation. Besides moxonidine hydrochloride (α-adrenergic agonist) and metaproterenol hemisulfate (β-adrenergic agonist), three other α- or β-adrenergic agonists (clonidine, dexmedetomidine, and isoproterenol) also reduced tau levels in human neurons. The tau-lowering effects of moxonidine and metaproterenol were abolished by their corresponding antagonists atipomezole and propranol, which elevated tau levels by themselves. In agreement with our findings, the selective β2-AR antagonist ICI 118,551 increased tau phosphorylation and accumulation in an AD mouse model (Branca et al., 2014). Paradoxically, genetic suppression of β2-ARs reduced tau pathology (Wisely et al., 2014). One likely explanation could be that complete removal of β2 AR prevented harmful effects of dysregulated β2-ARs, such as binding to Aβ.
Deficiency in α- or β-AR signaling has been implicated in AD. Levels of high-affinity α2-ARs are markedly reduced in AD patients (Pascual et al., 1992). Polymorphisms of β-ARs are linked to increased risk of late-onset AD (Yu et al., 2008). The natural ligand of ARs is norepinephrine, whose major source in the CNS is noradrenergic neurons in the locus coeruleus (LC); these neurons project widely to the forebrain, which includes two regions especially affected by tauopathies, the hippocampus and neocortex (Mather and Harley, 2016). Patients with AD have significant degeneration of neurons in the LC and much lower levels of norepinephrine. Interestingly, abnormal tau lesions emerged predominantly in LC regions even in individuals in their 20s, 30s, and 40s (Braak and Del Trecidi, 2015, Braak et al., 2011), suggesting that low levels of norepinephrine in the LC may lead to tau accumulation in young susceptible people and can eventually propagate to other brain regions.
How adrenergic signaling affects tau homeostasis is not known. Interestingly, some AR-modulating compounds may have a beneficial effect that is independent of their AR agonist activities. For example, norepinephrine and isoproterenol, whose chemical backbones contain 1,2-dihydroxybenzene, reduce insoluble tau levels by directly binding cysteine residues in tau to prevent tau oligomerization, independent of their AR agonist activities (Soeda et al., 2015). Modulating adrenergic signaling could affect other aspects of AD pathology as well. Chronic activation of β-ARs increases Aβ production (Ni et al., 2006) and protects against the detrimental effects of Aβ on hippocampal function (Li et al., 2013). AR activation also reduces lipopolysaccharide-induced expression of tumor necrosis factor alpha in microglia (Schlachetzki et al., 2010, Szabo et al., 1997) and interferon-γ-induced expression of class II antigens in astrocytes (Frohman et al., 1988). More studies are needed to dissect the molecular mechanisms underlying the role of adrenergic signaling in AD and to further validate AR agonists as a potential therapeutic strategy for AD and related tauopathies.
Experimental Procedures
Chemicals and Reagents
All medium, reagents, and supplements for iPSC culture and differentiation were from Invitrogen unless otherwise specified. Doxycycline, DMSO, cytosine β-D-arabinofuranoside (Ara-C), salicylate, LOPAC library, AR agonists and antagonists, and electrophysiology related chemicals were from Sigma.
Generating and Selecting i3N iPSC Clones
Ngn2 transgene was subcloned to a pUCM donor vector containing an AAVS1 homology arm. The Tet-ON 3G-controlled Ngn2 transgene was integrated to the AAVS1 locus of human iPSC lines through a TALEN nuclease pair. Genomic DNA from puromycin-selected and expanded clones were purified and genotyped by three PCR reactions. We generated i3N iPSC lines from two independent wild-type genetic background human iPSC lines: WTC11 (Miyaoka et al., 2014) and F12486.13 (female, white, age at biopsy 48 years, reprogrammed by Sendai virus, generated by Dr. Celeste Karch, Washington University in St. Louis). The iPSC protocol was approved by the Committee on Human Research at the University of California, San Francisco (15-15798). A detailed protocol is described in Supplemental Experimental Procedures.
i3Neuron Differentiation
i3Neurons were differentiated with a simplified two-step protocol (pre-differentiation and maturation). For pre-differentiation, i3N iPSCs were incubated with doxycycline (2 μg/mL) for 3 days at a density of 2.0–2.5 × 106 cells/well in six-well plates coated with Matrigel in knockout Dulbecco’s modified Eagle’s medium (KO-DMEM)/F12 medium containing N2 supplement, non-essential amino acids (NEAA), mouse laminin (0.2 μg/mL), brain-derived neurotrophic factor (BDNF, 10 ng/mL), neurotrophin-3 (NT3,10 ng/mL; Peprotech), and Y-27632. The medium was changed daily, and Y-27632 was removed from day 2. For maturation, pre-differentiated i3N precursor cells were dissociated, counted, and subplated at the desired density on plates coated with poly-D-lysine (PDL)/laminin in maturation medium containing 50% DMEM/F12, 50% Neurobasal-A medium, 0.5× B27 supplement, 0.5× N2 supplement, GlutaMax, NEAA, mouse laminin (1 μg/mL), BDNF (10 ng/mL), and NT3 (10 ng/mL). Half of the medium was replaced on day 7 and again on day 14, and the medium volume was doubled on day 21. Thereafter, one-third of the medium was replaced weekly until the cells were used. For electrophysiological recording, i3N precursor cells were subplated on Matrigel-coated coverslips, and mouse glia were added on day 1 in maturation medium containing 5% heat-inactivated fetal bovine serum and 2 μM Ara-C.
Immunocytochemistry
i3N iPSCs or i3Neurons in coverslips were fixed with conditioned medium containing 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and incubated for 1 hr in blocking solution containing PBS, 0.01% Triton X-100, and 5% normal goat serum. The cells were then incubated in blocking solution containing primary antibody overnight at 4°C, followed by incubation with secondary antibody for 1 hr. Images were acquired with an LSM880 confocal system (Zeiss) with Airyscan and a 20× or 63× oil-immersion objective lens. Antibodies used for immunocytochemistry were those against SOX2 (sc-17320S; Santa Cruz Biotechnology), OCT4 (sc-5279; Santa Cruz Biotechnology), TRA-1-81 (sc-21706; Santa Cruz Biotechnology), MAP2 (AB5622 or MAB3418; Millipore), VGlut1 (MAB5502; Millipore), βIII tubulin (TUJ1; Aves Labs), neuronal nuclear antigen (MAB377; Millipore), GABA (A2052; Sigma), HT7 (MN1000; Thermo Fisher), ankyrin G (N106/36; NeuroMa), synapsin-1 (D12G5; Cell Signaling), Olig2 (AB9610; Millipore), GFAP (MAB3402; Millipore), and GluR2/3 (AB1506; Millipore).
Reverse Transcription and Real-Time qPCR
i3Neurons were cultured in 96-well PDL plates (655946, Greiner) at a density of 10,000 cells/well for 4 weeks. cDNA from 12 random wells was obtained with Cells-to-CT Kits (Ambion) as recommended by the manufacturer. qPCR reactions were done in duplicate with SYBR Green Real-Time PCR master mixes (Applied Biosystems) and the Applied Biosystems 7900HT fast real-time PCR system. All the primers (Table S1) have been validated with human brain RNA (Zhang et al., 2013). RNAs without reverse transcription were used as negative control, and the dissociation curve from each gene was reviewed to ensure the desired amplification. Expression levels were normalized to GAPDH.
HCS Assay to Determine Total Tau Levels
After pre-differentiation, i3N precursor cells were placed in 384-well plates at a density of 2,000 cells/well. Fresh maturation medium was added weekly. On day 18, human tau siRNA, known tau-lowering compounds (salicylate, YM-01, and methylene blue) and 1,280 compounds from LOPAC were added and incubated at the desired final concentration for the desired amount of time. Human tau, recognized by HT7 antibody, total neurites, recognized by βIII tubulin antibody (TUJ1; Aves Labs) and nuclei, recognized by Hoechst were detected by a semi-automated immunostaining procedure. A fully automated ArrayScan high-content system (Thermo) was used to acquire images and quantify total tau levels. See Supplemental Experimental Procedures for detailed methods.
Human Tau and βIII Tubulin ELISA
Sensitive human tau and βIII tubulin ELISAs were adapted and modified according to previous reports (Barten et al., 2011, Meredith et al., 2013). Briefly, mouse monoclonal antibody HT7 or rabbit monoclonal βIII tubulin antibody (ab68193; Abcam) was used for capture. The respective analytes were detected with alkaline phosphatase-conjugated mouse monoclonal antibodies Tau5 or TUJ1 (806401, 801201; BioLegend). Recombinant full-length human tau (rPeptide) and recombinant βIII tubulin (Cytoskeleton) were used to generated standard curves for each assay. The CDP-Star substrate (T2214, Invitrogen) was used as a chemiluminescent alkaline phosphatase substrate. See Supplemental Experimental Procedures for detailed methods.
Statistics
The sample size for each experiment was determined on the basis of previous experience. Differences between means were assessed by unpaired Student’s t test (GraphPad Prism, v. 6.0) or multilevel mixed-effects linear regression model (STATA12; StataCorp), as indicated. Values are reported as means ± SEM. The Shapiro-Wilk test of normality and F test to compare variances were applied to datasets when applicable.
Author Contributions
L.G., M.E.W., and C.W. conceived the project and designed the experiments. C.W., M.E.W., R.C., K.L., T.E.T., X.C., P.D.S., and C.L. performed the experiments and collected and analyzed the data. M.X., A.M.-F., and D.S. contributed to data analyses and interpretation. C.M.K. generated the original F12486.13 iPSC line. C.W., M.E.W., and L.G. wrote the manuscript.
Acknowledgments
We thank B. Conklin and M.A. Mandegar for the WTC11 line and the pUCM donor vector, T.C. Südhof and Y. Zhang for the mouse Ngn2 plasmid and differentiation protocol, S. Ordway and G. Howard for editorial assistance, and E. Nyguen for administrative assistance. This work was supported in part by NIH R01AG036884 and R01AG051390 and the Rainwater Foundation to L.G., NIH K08EY023610 to M.E.W., and NIH K01AG046374 to C.M.K.
Published: September 28, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.08.019.
Supplemental Information
References
- Abisambra J., Jinwal U.K., Miyata Y., Rogers J., Blair L., Li X., Seguin S.P., Wang L., Jin Y., Bacon J. Allosteric heat shock protein 70 inhibitors rapidly rescue synaptic plasticity deficits by reducing aberrant tau. Biol. Psychiatry. 2013;74:367–374. doi: 10.1016/j.biopsych.2013.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht H., Zbinden P., Rizzi A., Villetti G., Riccardi B., Puccini P., Catinella S., Imbimbo B.P. High throughput screening of beta-amyloid secretion inhibitors using homogenous time-resolved fluorescence. Comb. Chem. High Throughput Screen. 2004;7:745–756. doi: 10.2174/1386207043328256. [DOI] [PubMed] [Google Scholar]
- Amamoto R., Arlotta P. Development-inspired reprogramming of the mammalian central nervous system. Science. 2014;343:1239882. doi: 10.1126/science.1239882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andorfer C., Acker C.M., Kress Y., Hof P.R., Duff K., Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J. Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Zwilling Y., Bien-Ly N., Xu Q., Li G., Bernardo A., Yoon S.Y., Zwilling D., Yan T.X., Chen L., Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M., Litvan I., Houlden H., Adamson J., Dickson D., Perez-Tur J., Hardy J., Lynch T., Bigio E., Hutton M. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Barten D.M., Cadelina G.W., Hoque N., DeCarr L.B., Guss V.L., Yang L., Sankaranarayanan S., Wes P.D., Flynn M.E., Meredith J.E. Tau transgenic mice as models for cerebrospinal fluid tau biomarkers. J. Alzheimers Dis. 2011;24(Suppl 2):127–141. doi: 10.3233/JAD-2011-110161. [DOI] [PubMed] [Google Scholar]
- Braak H., Del Trecidi K. Neuroanatomy and pathology of sporadic Alzheimer's disease. Adv. Anat. Embryol. Cell Biol. 2015;215:1–162. [PubMed] [Google Scholar]
- Braak H., Thal D.R., Ghebremedhin E., Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Branca C., Wisely E.V., Hartman L.K., Caccamo A., Oddo S. Administration of a selective beta2 adrenergic receptor antagonist exacerbates neuropathology and cognitive deficits in a mouse model of Alzheimer's disease. Neurobiol. Aging. 2014;35:2726–2735. doi: 10.1016/j.neurobiolaging.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brideau C., Gunter B., Pikounis B., Liaw A. Improved statistical methods for hit selection in high-throughput screening. J. Biomol. Screen. 2003;8:634–647. doi: 10.1177/1087057103258285. [DOI] [PubMed] [Google Scholar]
- Brunden K.R., Trojanowski J.Q., Lee V.M. Evidence that non-fibrillar tau causes pathology linked to neurodegeneration and behavioral impairments. J. Alzheimers Dis. 2008;14:393–399. doi: 10.3233/jad-2008-14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K.R., Trojanowski J.Q., Lee V.M. Advances in tau-focused drug discovery for Alzheimer's disease and related tauopathies. Nat. Rev. Drug Discov. 2009;8:783–793. doi: 10.1038/nrd2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busskamp V., Lewis N.E., Guye P., Ng A.H., Shipman S.L., Byrne S.M., Sanjana N.E., Murn J., Li Y., Li S. Rapid neurogenesis through transcriptional activation in human stem cells. Mol. Syst. Biol. 2014;10:760. doi: 10.15252/msb.20145508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerbini T., Funahashi R., Luo Y., Liu C., Park K., Rao M., Malik N., Zou J. Transcription activator-like effector nuclease (TALEN)-mediated CLYBL targeting enables enhanced transgene expression and one-step generation of dual reporter human induced pluripotent stem cell (iPSC) and neural stem cell (NSC) lines. PLoS One. 2015;10:e0116032. doi: 10.1371/journal.pone.0116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda S., Ang C.E., Davila J., Pak C., Mall M., Lee Q.Y., Ahlenius H., Jung S.W., Sudhof T.C., Wernig M. Generation of induced neuronal cells by the single reprogramming factor ASCL1. Stem Cell Reports. 2014;3:282–296. doi: 10.1016/j.stemcr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E.E., Wu J.W., Myeku N., Figueroa Y.H., Herman M., Marinec P.S., Gestwicki J.E., Dickey C.A., Yu W.H., Duff K.E. Methylthioninium chloride (methylene blue) induces autophagy and attenuates tauopathy in vitro and in vivo. Autophagy. 2012;8:609–622. doi: 10.4161/auto.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aiuto L., Zhi Y., Kumar Das D., Wilcox M.R., Johnson J.W., McClain L., MacDonald M.L., Di Maio R., Schurdak M.E., Piazza P. Large-scale generation of human iPSC-derived neural stem cells/early neural progenitor cells and their neuronal differentiation. Organogenesis. 2014;10:365–377. doi: 10.1080/15476278.2015.1011921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehdashti S.J., Zheng W., Gever J.R., Wilhelm R., Nguyen D.T., Sittampalam G., McKew J.C., Austin C.P., Prusiner S.B. A high-throughput screening assay for determining cellular levels of total tau protein. Curr. Alzheimer Res. 2013;10:679–687. doi: 10.2174/15672050113109990143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVos S.L., Goncharoff D.K., Chen G., Kebodeaux C.S., Yamada K., Stewart F.R., Schuler D.R., Maloney S.E., Wozniak D.F., Rigo F. Antisense reduction of tau in adult mice protects against seizures. J. Neurosci. 2013;33:12887–12897. doi: 10.1523/JNEUROSCI.2107-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey C.A., Ash P., Klosak N., Lee W.C., Petrucelli L., Hutton M., Eckman C.B. Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression. Mol. Neurodegener. 2006;1:6. doi: 10.1186/1750-1326-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatokun A.A., Liu J.O., Dawson V.L., Dawson T.M. Identification through high-throughput screening of 4′-methoxyflavone and 3′,4′-dimethoxyflavone as novel neuroprotective inhibitors of parthanatos. Br. J. Pharmacol. 2013;169:1263–1278. doi: 10.1111/bph.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman E.M., Vayuvegula B., Gupta S., van den Noort S. Norepinephrine inhibits gamma-interferon-induced major histocompatibility class II (Ia) antigen expression on cultured astrocytes via beta-2-adrenergic signal transduction mechanisms. Proc. Natl. Acad. Sci. USA. 1988;85:1292–1296. doi: 10.1073/pnas.85.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruninger F. Invited review: drug development for tauopathies. Neuropathol. Appl. Neurobiol. 2015;41:81–96. doi: 10.1111/nan.12192. [DOI] [PubMed] [Google Scholar]
- K Hancock M., Kopp L., Kaur N., Hanson B.J. A facile method for simultaneously measuring neuronal cell viability and neurite outgrowth. Curr. Chem. Genom. Transl. Med. 2015;9:6–16. doi: 10.2174/2213988501509010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill J.A., Robinette B.L., Freudenrich T., Mundy W.R. Use of high content image analyses to detect chemical-mediated effects on neurite sub-populations in primary rat cortical neurons. Neurotoxicology. 2013;34:61–73. doi: 10.1016/j.neuro.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Hester M.E., Murtha M.J., Song S., Rao M., Miranda C.J., Meyer K., Tian J.B., Boulting G., Schaffer D.V., Zhu M.X. Rapid and efficient generation of functional motor neurons from human pluripotent stem cells using gene delivered transcription factor codes. Mol. Ther. 2011;19:1905–1912. doi: 10.1038/mt.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M., Arai T., Masuda-Suzukake M., Nonaka T., Yamashita M., Akiyama H., Hasegawa M. Methylene blue reduced abnormal tau accumulation in P301L tau transgenic mice. PLoS One. 2012;7:e52389. doi: 10.1371/journal.pone.0052389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wolfing H., Chieng B.C., Christie M.J., Napier I.A. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Jain S., van Kesteren R.E., Heutink P. High content screening in neurodegenerative diseases. J. Vis. Exp. 2012:e3452. doi: 10.3791/3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordeli E., Lambert S., Bennett V. AnkyrinG. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J. Biol. Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- Lasagna-Reeves C.A., de Haro M., Hao S., Park J., Rousseaux M.W., Al-Ramahi I., Jafar-Nejad P., Vilanova-Velez L., See L., De Maio A. Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron. 2016;92:407–418. doi: 10.1016/j.neuron.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Jin M., Zhang D., Yang T., Koeglsperger T., Fu H., Selkoe D.J. Environmental novelty activates beta2-adrenergic signaling to prevent the impairment of hippocampal LTP by Abeta oligomers. Neuron. 2013;77:929–941. doi: 10.1016/j.neuron.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Maria E., Tabaton M., Vigo T., Abbruzzese G., Bellone E., Donati C., Frasson E., Marchese R., Montagna P., Munoz D.G. Corticobasal degeneration shares a common genetic background with progressive supranuclear palsy. Ann. Neurol. 2000;47:374–377. doi: 10.1002/1531-8249(200003)47:3<374::aid-ana15>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mather M., Harley C.W. The locus coeruleus: essential for maintaining cognitive function and the aging brain. Trends Cogn. Sci. 2016;20:214–226. doi: 10.1016/j.tics.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medda X., Mertens L., Versweyveld S., Diels A., Barnham L., Bretteville A., Buist A., Verheyen A., Royaux I., Ebneth A. Development of a scalable, high-throughput-compatible assay to detect Tau aggregates using iPSC-derived cortical neurons maintained in a three-dimensional culture format. J. Biomol. Screen. 2016;21:804–815. doi: 10.1177/1087057116638029. [DOI] [PubMed] [Google Scholar]
- Meredith J.E., Jr., Sankaranarayanan S., Guss V., Lanzetti A.J., Berisha F., Neely R.J., Slemmon J.R., Portelius E., Zetterberg H., Blennow K. Characterization of novel CSF Tau and ptau biomarkers for Alzheimer's disease. PLoS One. 2013;8:e76523. doi: 10.1371/journal.pone.0076523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S.W., Chen X., Tracy T.E., Li Y., Zhou Y., Wang C., Shirakawa K., Minami S.S., Defensor E., Mok S.A. Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits. Nat. Med. 2015;21:1154–1162. doi: 10.1038/nm.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka Y., Chan A.H., Judge L.M., Yoo J., Huang M., Nguyen T.D., Lizarraga P.P., So P.L., Conklin B.R. Isolation of single-base genome-edited human iPS cells without antibiotic selection. Nat. Methods. 2014;11:291–293. doi: 10.1038/nmeth.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M., Hamto P., Adame A., Devidze N., Masliah E., Mucke L. Age-appropriate cognition and subtle dopamine-independent motor deficits in aged tau knockout mice. Neurobiol. Aging. 2013;34:1523–1529. doi: 10.1016/j.neurobiolaging.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratore C.R., Srikanth P., Callahan D.G., Young-Pearse T.L. Comparison and optimization of hiPSC forebrain cortical differentiation protocols. PLoS One. 2014;9:e105807. doi: 10.1371/journal.pone.0105807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A.J., Kaleem M., Marlowe L., Pittman A.M., Lees A.J., Fung H.C., Duckworth J., Leung D., Gibson A., Morris C.M. The H1c haplotype at the MAPT locus is associated with Alzheimer's disease. Hum. Mol. Genet. 2005;14:2399–2404. doi: 10.1093/hmg/ddi241. [DOI] [PubMed] [Google Scholar]
- Myers A.J., Pittman A.M., Zhao A.S., Rohrer K., Kaleem M., Marlowe L., Lees A., Leung D., McKeith I.G., Perry R.H. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol. Dis. 2007;25:561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Nelson P.T., Alafuzoff I., Bigio E.H., Bouras C., Braak H., Cairns N.J., Castellani R.J., Crain B.J., Davies P., Del Tredici K. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J. Neuropathol. Exp. Neurol. 2012;71:362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Y., Zhao X., Bao G., Zou L., Teng L., Wang Z., Song M., Xiong J., Bai Y., Pei G. Activation of beta2-adrenergic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat. Med. 2006;12:1390–1396. doi: 10.1038/nm1485. [DOI] [PubMed] [Google Scholar]
- Nicholas C.R., Chen J., Tang Y., Southwell D.G., Chalmers N., Vogt D., Arnold C.M., Chen Y.J., Stanley E.G., Elefanty A.G. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573–586. doi: 10.1016/j.stem.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z.P., Yang N., Vierbuchen T., Ostermeier A., Fuentes D.R., Yang T.Q., Citri A., Sebastiano V., Marro S., Sudhof T.C. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F., Solfrizzi V., Seripa D., Imbimbo B.P., Lozupone M., Santamato A., Tortelli R., Galizia I., Prete C., Daniele A. Tau-based therapeutics for Alzheimer's disease: active and passive immunotherapy. Immunotherapy. 2016;8:1119–1134. doi: 10.2217/imt-2016-0019. [DOI] [PubMed] [Google Scholar]
- Pascual J., Grijalba B., Garcia-Sevilla J.A., Zarranz J.J., Pazos A. Loss of high-affinity alpha 2-adrenoceptors in Alzheimer's disease: an autoradiographic study in frontal cortex and hippocampus. Neurosci. Lett. 1992;142:36–40. doi: 10.1016/0304-3940(92)90614-d. [DOI] [PubMed] [Google Scholar]
- Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Roberson E.D., Halabisky B., Yoo J.W., Yao J., Chin J., Yan F., Wu T., Hamto P., Devidze N., Yu G.Q. Amyloid-beta/Fyn-induced synaptic, network, and cognitive impairments depend on tau levels in multiple mouse models of Alzheimer's disease. J. Neurosci. 2011;31:700–711. doi: 10.1523/JNEUROSCI.4152-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., Guimaraes A., DeTure M., Ramsden M., McGowan E. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlachetzki J.C., Fiebich B.L., Haake E., de Oliveira A.C., Candelario-Jalil E., Heneka M.T., Hull M. Norepinephrine enhances the LPS-induced expression of COX-2 and secretion of PGE2 in primary rat microglia. J. Neuroinflammation. 2010;7:2. doi: 10.1186/1742-2094-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda Y., Yoshikawa M., Almeida O.F., Sumioka A., Maeda S., Osada H., Kondoh Y., Saito A., Miyasaka T., Kimura T. Toxic tau oligomer formation blocked by capping of cysteine residues with 1,2-dihydroxybenzene groups. Nat. Commun. 2015;6:10216. doi: 10.1038/ncomms10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones T.L., Kopeikina K.J., Koffie R.M., de Calignon A., Hyman B.T. Are tangles as toxic as they look? J. Mol. Neurosci. 2011;45:438–444. doi: 10.1007/s12031-011-9566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spittaels K., Van den Haute C., Van Dorpe J., Bruynseels K., Vandezande K., Laenen I., Geerts H., Mercken M., Sciot R., Van Lommel A. Prominent axonopathy in the brain and spinal cord of transgenic mice overexpressing four-repeat human tau protein. Am. J. Pathol. 1999;155:2153–2165. doi: 10.1016/S0002-9440(10)65533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow A., Van der Jeugd A., Zheng F., Ahmed T., Balschun D., Petrova O., Drexler D., Zhou L., Rune G., Mandelkow E. Tau-induced defects in synaptic plasticity, learning, and memory are reversible in transgenic mice after switching off the toxic Tau mutant. J. Neurosci. 2011;31:2511–2525. doi: 10.1523/JNEUROSCI.5245-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C., Hasko G., Zingarelli B., Nemeth Z.H., Salzman A.L., Kvetan V., Pastores S.M., Vizi E.S. Isoproterenol regulates tumour necrosis factor, interleukin-10, interleukin-6 and nitric oxide production and protects against the development of vascular hyporeactivity in endotoxaemia. Immunology. 1997;90:95–100. doi: 10.1046/j.1365-2567.1997.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberti D., Rizzini C., Spano P.F., Memo M. Characterization of tau proteins in human neuroblastoma SH-SY5Y cell line. Neurosci. Lett. 1997;235:149–153. doi: 10.1016/s0304-3940(97)00715-5. [DOI] [PubMed] [Google Scholar]
- Vossel K.A., Zhang K., Brodbeck J., Daub A.C., Sharma P., Finkbeiner S., Cui B., Mucke L. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016;17:5–21. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- Wisely E.V., Xiang Y.K., Oddo S. Genetic suppression of beta2-adrenergic receptors ameliorates tau pathology in a mouse model of tauopathies. Hum. Mol. Genet. 2014;23:4024–4034. doi: 10.1093/hmg/ddu116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C.W., Wszolek M.F., Shulman J.M., Salvaterra P.M., Lewis J., Hutton M., Feany M.B. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Yu J.T., Tan L., Ou J.R., Zhu J.X., Liu K., Song J.H., Sun Y.P. Polymorphisms at the beta2-adrenergic receptor gene influence Alzheimer's disease susceptibility. Brain Res. 2008;1210:216–222. doi: 10.1016/j.brainres.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Zhang J.H., Chung T.D.Y., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- Zhang X.D., Yang X.C., Chung N., Gates A., Stec E., Kunapuli P., Holder D.J., Ferrer M., Espeseth A.S. Robust statistical methods for hit selection in RNA interference high-throughput screening experiments. Pharmacogenomics. 2006;7:299–309. doi: 10.2217/14622416.7.3.299. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Pak C., Han Y., Ahlenius H., Zhang Z., Chanda S., Marro S., Patzke C., Acuna C., Covy J. Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron. 2013;78:785–798. doi: 10.1016/j.neuron.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.