Abstract
With the widespread use of 2-deoxy-2-(18F) fluoro-D-glucose positron emission tomography-computed tomography (FDG PET/CT) in oncologic imaging, it has become increasingly important for physicians who interpret FDG PET/CT scans to confidently recognize the spectrum of incidentally encountered benign and malignant findings. The adrenal glands represent an interesting nexus of multiple rare and common benign intrinsic tumors as well as metastases from a variety of primary malignancies. Given the breadth of adrenal gland pathology, careful description of the FDG PET/CT appearance of these pathologies is of value to help reduce misinterpretation. In this manuscript, we retrospectively and systematically review the FDG PET/CT imaging characteristics of benign adrenal myelolipomas in a small consecutive patient series. The myelolipomas in this series demonstrated differing degrees of macroscopic fat visible on CT, with generally mild FDG uptake fusing to the nonfatty portions of the lesions. At imaging follow-up, all of the myelolipomas in this series remained unchanged in appearance, helping to confirm their benign nature. The typical appearance of a myelolipoma on FDG PET/CT is a fat-containing adrenal mass with low-level FDG uptake in the nonfatty aspects of the mass, and such a lesion requires no further imaging workup.
Keywords: Adrenal mass, 2-deoxy-2-(18F) fluoro-D-glucose, myelolipoma, standardized uptake value
Introduction
Positron emission tomography (PET) with 2-deoxy-2-(18F) fluoro-D-glucose (FDG) has become a powerful tool in oncology imaging, particularly when the functional information available from PET is combined with the anatomic information from computed tomography (CT).[1,2,3] The widespread use of FDG PET/CT for cancer staging, re-staging, and surveillance has necessitated an understanding of the spectrum of benign and malignant incidental findings visible on the acquired CT images[4,5,6] and the management recommendations that should be made based on those findings. The adrenal glands are a particularly important site to distinguish benign incidental findings (e.g., adrenocortical adenomas, myelolipomas, and cystic lymphangiomas,)[7,8] from metastatic disease (including from primary lung cancer, melanoma, etc.) and primary adrenal cortical carcinomas.
Toward the goal of improving diagnostic confidence when encountering incidental adrenal masses on PET/CT, we describe in this manuscript, the spectrum of appearances of benign adrenal myelolipomas on FDG PET/CT. Multiple case reports have noted a variety of appearances of these lesions. For example, Ludwig, et al. described significant but heterogeneous hypermetabolism is a very large (15 cm) adrenal myelolipoma that was surgically resected.[9] Gemmel et al. evaluated a patient with bilateral predominantly fat-density myelolipomas and described no significant FDG uptake within either mass.[10] Of particular interest, Althoen, et al. presented a case of lung cancer with a metastases within a preexisting myelolipoma.[11]
However, despite these interesting case reports, the literature lacks a systematic approach to describing the typical FDG PET/CT appearance of adrenal myelolipomas. We have retrospectively reviewed recent cases at our institution to carry out such a study, and we present the results herein.
Materials and Methods
This retrospective study was approved by our hospital's Institutional Review Board. The archive of prior PET examinations (including metabolic cardiac and oncology studies) was queried using the keyword “myelolipoma” over the date range from January 1, 2006 to January 1, 2016. A total of 24 scans from 19 patients were retrieved by the search. Out of the 19 patients, 11 (representing a total of 14 scans) had definitive findings of macroscopic fat on the CT portion of the included PET/CT examinations after central review by two experienced radiologists (MSJ and LBS), while the remaining eight patients had indeterminate findings and were excluded from the remainder of the analysis. However, one patient was excluded as the PET/CT had been performed at an outside institution and height and weight were not available to calculate uptake parameters. All patients had been imaged on either a GE Discovery DRX PET/CT scanner (General Electric, Waukesha, WI, USA) or a Siemens Biograph mCT PET/CT scanner (Siemens, Erlangen, Germany) operating in three-dimensional emission mode with CT attenuation correction.
For the three patients with multiple scans demonstrating definitive myelolipomas, only the first scan was included in the analysis to provide a more accurate cross-section of findings and eliminate the bias introduced by including the same tumor in multiple times. The 10 FDG PET/CT scans were analyzed on a Siemens syngo.via Workstation (Siemens, Erlangen, Germany). The lesions were all measured in the longest dimension. Spherical volumes of interest (VOIs) were manually placed in the fatty components of the masses as well as in the soft tissue density components, and lean-body-mass corrected maximum standardized uptake values (SUVmax) were calculated. The VOIs were placed so as to avoid any activity from adjacent structures. Although SUVmax can be subject to inherent noise limitations given that it is a single-voxel metric, many of the lesions in this study were small (limiting the application of peak SUV measurements) and subject to edge partial volume effects, potentially limiting accurate determination of average SUV from whole lesion VOIs. Average SUV for a standard 3-cm diameter sphere in the right lobe of the liver was also determined to assess the normal biodistribution on the PET scans and assure that they were quantifiable. The two centrally reviewing radiologists (MSJ and LBS) also arrived at a consensus approximation of the percent fat in each mass.
The Student's t-test was utilized to evaluate for a statistically significant difference between the measured SUVmax in the soft tissue and fatty portions of the lesions.
Results
For the 10 patients in the study, 7 (70%) were male and 3 (30%) were female. The average age was 69 years (range 56–80 years). One patient underwent FDG PET/CT for myocardial viability evaluation (and thus had PET/CT images only of the lower chest and upper abdomen) and the remaining patients were all being staged or restaged for known cancer diagnoses and had whole-body skull base to mid-thigh PET/CT acquisitions.
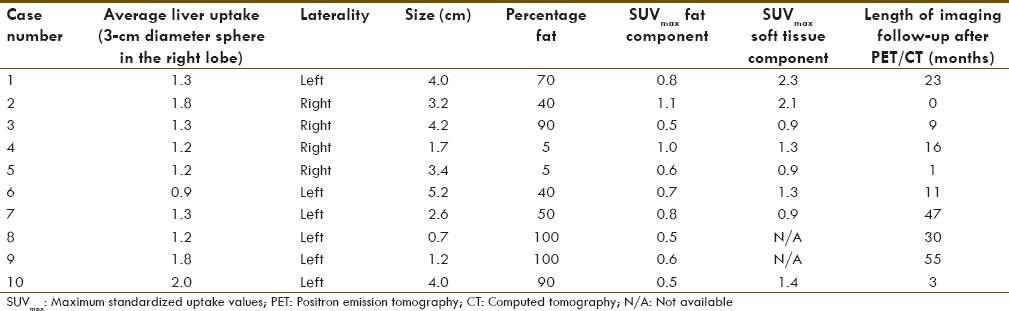
Table 1 summarizes the findings from each of the 10 tumors included in the final analysis including laterality, size, estimated percentage of fat composition, SUVmax in the fatty component of each lesion, SUVmax in the soft tissue component of each lesion, and length of imaging follow-up available subsequent to the FDG PET/CTs included in this analysis. Average liver uptake in each patient is also included in Table 1. Six of the 10 tumors (60%) were left-sided. The average tumor size was 3.0 cm with a range from 0.7 to 5.2 cm. There was also a broad range of estimated percentage fat content in the tumors, from almost none (approximately 5%) to entirely visually composed of fat (100%).
Table 1.
Selected parameters from the myelolipomas included in this study
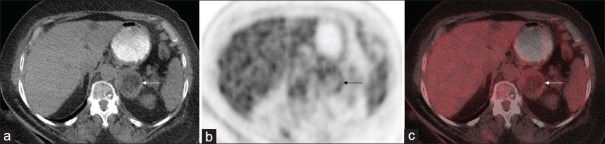
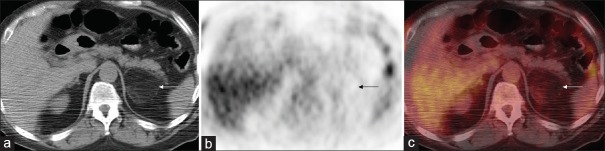
Of note, the fat components of the myelolipomas generally demonstrated lower uptake of FDG than the soft tissue components. The areas of fat had average SUVmax of 0.7 with a range from 0.5 to 1.1. The soft tissue areas of the masses had average SUVmax of 1.4 with a range from 0.9 to 2.3. The difference in uptake between the fat and soft tissue components was statistically significant (P = 0.0023). Figure 1 (case 7 from Table 1) and Figure 2 (case 11 from Table 1) demonstrate the visually different uptake between the fat and soft tissue portions of the masses that was confirmed by the above quantitation.
Figure 1.
(a) Axial noncontrast attenuation-correction computed tomography, (b) axial positron emission tomography, and (c) fused positron emission tomography/computed tomography images from a patient with a left adrenal myelolipoma (white and black arrows) that demonstrates higher 2-deoxy-2-(18F) fluoro-D-glucose uptake in the soft-tissue-density portions of the lesion than in the fat-density portions
Figure 2.
(a) Axial noncontrast attenuation-correction computed tomography, (b) axial positron emission tomography, and (c) fused positron emission tomography/computed tomography images from another patient with a left adrenal myelolipoma (white and black arrows) that again shows the subtle differential uptake between the soft tissue and fat components of the mass
Nine out of 10 (90%) patients had an imaging follow-up after the FDG PET/CT scans included in this analysis. The average time of follow-up was 20 months with a range from 0 to 55 months. Of those patients with imaging follow-up, 3/10 (30%) had subsequent PET/CTs, and the remaining 7/10 (70%) had a follow-up with CT. Importantly, none of the myelolipomas changed in size or morphology during the follow-up period.
Discussion
Physicians who interpret nuclear medicine studies must be familiar with incidental findings on the CT portions of fusion modality studies such as FDG PET/CT. While the adrenal glands can harbor both benign and malignant masses, they are particularly common sites for “incidentalomas.”[4] There is value in understanding the spectrum of findings in such lesions so that patients undergoing staging examinations are not improperly diagnosed as having metastatic disease and also so that unnecessary further workup of definitively benign lesions is not recommended.
Our retrospective study indicates that adrenal myelolipomas have relatively low-level FDG uptake in the nonfat-density portions of the lesions, with negligible/background levels of uptake in the fat-density portions. Imaging follow-up of these lesions demonstrated no changes over time. A myelolipoma exhibiting the FDG PET/CT imaging characteristics described in this manuscript can be confidently described as a benign lesion. Given the case report of intense uptake from a lung metastasis within an adrenal myelolipoma,[11] we would caution that deviation from the described imaging features (e.g., intense or heterogeneous FDG uptake in the nonfat-density aspects of a myelolipoma or significant morphologic change on follow-up imaging) should be interpreted with caution. Such findings could indicate the presence of a collision tumor (i.e., a tumor composed of two different adjacent histologies,[12] such as a myelolipoma with a metastatic lesion or even a myelolipoma with a co-existing adrenal cortical carcinoma[13]) or another fat-containing retroperitoneal mass (e.g., retroperitoneal liposarcoma) that abuts or involves the adrenal gland.
The most important limitations of this study are its small size and retrospective nature. Myelolipomas are an uncommon benign tumor (0.08%–0.2% incidence at autopsy),[7] limiting the ability to assess these lesions in large series. Furthermore, given the benignity of these lesions, prospective evaluation of their characteristics with a high radiation dose modality such as FDG PET/CT would not be practical. Additional limitations for this study arise from the small size of a fraction of the lesions, limiting assessment of the percent fat in the mass and making it difficult to reliably measure the SUVmax parameters. For small lesions, the partial volume effects inherent in PET imaging also hinder accurate measurement of uptake. The use of SUVmax as a quantitative parameter can be subject to significant limitations from noise, however SUVmax remains the most commonly employed metric for quantitative assessment in PET and was thus chosen for lesion evaluation in this study. Finally, any adrenal myelolipomas that lacked macroscopic fat on CT would not have been captured by the search that was utilized, and the appearance of such lesions was not assessed in this study.
Conclusions
Adrenal myelolipomas are an uncommon but still encountered incidental benign mass on FDG PET/CT that have an appearance of mild homogeneous FDG uptake in their soft tissue density components and lack significant uptake in their fatty components. In oncology patients, we believe that deviation from these imaging characteristics (e.g., focal intense FDG uptake within the mass) should prompt concern for a collision tumor or other nonbenign process.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Faulhaber P, Nelson A, Mehta L, O’Donnell J. 24. The fusion of anatomic and physiologic tomographic images to enhance accurate interpretation. Clin Positron Imaging. 2000;3:178. doi: 10.1016/s1095-0397(00)00090-x. [DOI] [PubMed] [Google Scholar]
- 2.Pannu HK, Bristow RE, Cohade C, Fishman EK, Wahl RL. PET-CT in recurrent ovarian cancer: Initial observations. Radiographics. 2004;24:209–23. doi: 10.1148/rg.241035078. [DOI] [PubMed] [Google Scholar]
- 3.Jeong HS, Baek CH, Son YI, Ki Chung M, Kyung Lee D, Young Choi J, et al. Use of integrated 18F-FDG PET/CT to improve the accuracy of initial cervical nodal evaluation in patients with head and neck squamous cell carcinoma. Head Neck. 2007;29:203–10. doi: 10.1002/hed.20504. [DOI] [PubMed] [Google Scholar]
- 4.Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: White paper of the ACR incidental findings committee. J Am Coll Radiol. 2010;7:754–73. doi: 10.1016/j.jacr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Patel MD, Ascher SM, Paspulati RM, Shanbhogue AK, Siegelman ES, Stein MW, et al. Managing incidental findings on abdominal and pelvic CT and MRI, Part 1: White paper of the ACR Incidental Findings Committee II on adnexal findings. J Am Coll Radiol. 2013;10:675–81. doi: 10.1016/j.jacr.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 6.Khosa F, Krinsky G, Macari M, Yucel EK, Berland LL. Managing incidental findings on abdominal and pelvic CT and MRI, Part 2: White paper of the ACR Incidental Findings Committee II on vascular findings. J Am Coll Radiol. 2013;10:789–94. doi: 10.1016/j.jacr.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Lattin GE, Jr, Sturgill ED, Tujo CA, Marko J, Sanchez-Maldonado KW, Craig WD, et al. From the radiologic pathology archives: Adrenal tumors and tumor-like conditions in the adult: Radiologic-pathologic correlation. Radiographics. 2014;34:805–29. doi: 10.1148/rg.343130127. [DOI] [PubMed] [Google Scholar]
- 8.Rowe SP, Bishop JA, Prescott JD, Salvatori R, Fishman EK. CT appearance of adrenal cystic lymphangioma: Radiologic-pathologic correlation. AJR Am J Roentgenol. 2016;206:81–5. doi: 10.2214/AJR.15.14786. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig V, Rice MH, Martin WH, Kelley MC, Delbeke D. 2-Deoxy-2-[18F] fluoro-D-glucose positron emission tomography uptake in a giant adrenal myelolipoma. Mol Imaging Biol. 2002;4:355–8. doi: 10.1016/s1536-1632(02)00018-5. [DOI] [PubMed] [Google Scholar]
- 10.Gemmel F, Bruinsma H, Oomen P, Collins J. PET/CT incidental detection of bilateral adrenal myelolipomas in a patient with a huge maxillary sinus carcinoma. Clin Nucl Med. 2010;35:132–3. doi: 10.1097/RLU.0b013e3181c7c007. [DOI] [PubMed] [Google Scholar]
- 11.Althoen MC, Siegel A, Tsapakos MJ, Seltzer MA. Lung cancer metastasis to an adrenal myelolipoma detected by PET/CT. Clin Nucl Med. 2011;36:922–4. doi: 10.1097/RLU.0b013e318217ae93. [DOI] [PubMed] [Google Scholar]
- 12.Katabathina VS, Flaherty E, Kaza R, Ojili V, Chintapalli KN, Prasad SR. Adrenal collision tumors and their mimics: Multimodality imaging findings. Cancer Imaging. 2013;13:602–10. doi: 10.1102/1470-7330.2013.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Fisher C, Thway K. “Dominant” myelolipoma encasing adrenal cortical carcinoma: An unusual variation of myelolipoma occurring as a synchronous and predominant neoplasm. Int J Surg Pathol. 2014;22:731–5. doi: 10.1177/1066896914532538. [DOI] [PubMed] [Google Scholar]