Abstract
Introduction
Spread through air spaces (STAS) is a recently recognized pattern of invasion in lung adenocarcinoma, however, it has not yet been characterized in squamous cell carcinoma (SCC).
Methods
We reviewed 445 resected stage I-III lung SCC and investigated the clinical significance of STAS. Cumulative incidence of recurrence (CIR) and lung cancer-specific death (CID) were evaluated by competing risks analyses and overall survival (OS) by Cox models.
Results
Of total 445 patients, 336 (76%) were >65 years old. Among 273 patients who died, 91 (33%) died of lung cancer whereas the remaining died of competing events or unknown cause. STAS was observed in 132 patients (30%) and the frequency increased with stage. The cumulative incidence of any, distant, and locoregional recurrence as well as lung cancer-specific death were significantly higher in patients with STAS compared to those without STAS, whereas there was no statistically significant difference in OS. In multivariable models for any recurrence and lung cancer-specific death, STAS was an independent predictor for both outcomes (p=0.034 and 0.016, respectively).
Conclusion
STAS was present in one third of resected lung SCC. In competing risks analysis in a cohort where three fourths of the patients were elderly, STAS was associated with lung cancer-specific outcomes. Our findings suggest that STAS is one of the most prognostically significant histologic findings in lung SCC.
Keywords: Lung, competing risks analysis, spread through air spaces, squamous cell carcinoma
INTRODUCTION
Despite extensive histologic studies,1–5 few clinically significant prognostic features have been discovered for lung squamous cell carcinoma (SCC). In the 2015 World Health Organization (WHO) Classification, SCC is classified into keratinizing, nonkeratinizing and basaloid subtypes.6, 7 However, this subtyping has been shown to have limited prognostic value or other clinical significance. Suggestions that basaloid SCC has a worse prognosis has not been validated in other studies.3, 4, 8–10 Recently, several studies demonstrated for lung SCC, the main histologic predictors of prognosis are single cell invasion and high grade tumor budding.1–5 Based on these observations Weichert et al developed a grading scheme for SCC based on the combination of the two most reliable and validated prognostic makers, i.e. tumor budding and nest size, and showed this to be a prognostic indicator for overall survival independent of age, sex and stage.4
Tumor spread through air space (STAS), a newly recognized form of invasion in lung adenocarcinoma has yet to be described in SCC. A growing number of independent studies have validated STAS to be a predictor of recurrence and survival in lung adenocarcinoma.11–15 Our group showed that STAS was an independent predictor of recurrence for patients with stage I lung adenocarcinoma who underwent limited surgical resection.11 Warth A. et al examined tumors of all stages and showed STAS was associated with both significantly reduced overall survival (OS) and disease-free survival (DFS) for all stages of adenocarcinoma.15 In addition, Warth A. et al found significantly increased STAS in more aggressive groups of papillary adenocarcinomas.16 STAS-like “aerogenous spread” was also described by Jin Y, et al in ROS1 rearranged lung adenocarcinomas associated with decreased E-cadherin expression and poor disease free survival.17 Shiono S et al, found in stage I adenocarcinomas, STAS correlated with overall survival and recurrence free survival both in univariate and multivariate analysis.14 Under the term free tumor clusters, Morimoto J et al demonstrated significantly reduced recurrence free survival in STAS positive stage I lung adenocarcinomas in addition to associations with aggressive clinical and pathologic characteristics.12 Collectively these studies provide convincing evidence that STAS is a clinically important in lung adenocarcinoma, but the presence, frequency, and prognostic value of STAS in lung SCC has not yet been defined.
We investigated comprehensive clinicopathologic analysis using a large cohort of patients with resected lung SCC with competing risks analysis to determine whether STAS is associated with tumor aggressiveness in lung SCC.
MATERIALS AND METHODS
Patient Cohorts
This retrospective study was approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center (MSK). We reviewed all patients with solitary lung SCC who underwent surgical resection at MSK between 1999 and 2009. Clinical data were collected from the prospectively maintained Thoracic Surgery Service lung carcinoma database. All tumors were classified according to the 2015 WHO Classification and were grouped into three histologic subtypes (keratinizing, nonkeratinizing, and basaloid squamous carcinoma) and staged according to the seventh edition of the TNM system.6, 18 Cases with induction therapy, positive surgical margin, and other disease progression were excluded. Other aspects of these cases have been reported previously.3, 19
Postoperative lung cancer surveillance was performed in accordance with National Comprehensive Cancer Network guidelines.20 Recurrences were confirmed by clinical, radiological, or pathological assessment, and were classified into locoregional (local + regional) and distant recurrence.21 Cause of death was classified into following 4 types; lung cancer-specific death, other cancer-specific death, noncancer-specific death, and unknown cause. Lung cancer-specific death was defined as death due to the progression of recurrent diseases of resected primary SCC. Other cancer-specific death was defined as death due to any malignant disease progression other than lung SCC. Death due to second primary lung cancer was included in other cancer-specific death. Noncancer-specific death was defined as death due to any other causes than malignant diseases.
The 445 lung SCC included are from our previously published study where squamous differentiation was confirmed in the poorly differentiated tumors using immunohistochemistry.19
Histologic Evaluation
Tumor slides selected in this cohort were reviewed by two pathologists (SH.L. and W.D.T.) who were blinded to patient clinical outcomes using an Olympus BX51 microscope (Olympus Optical Co. Ltd., Tokyo, Japan) with a standard 22-mm diameter eyepiece.
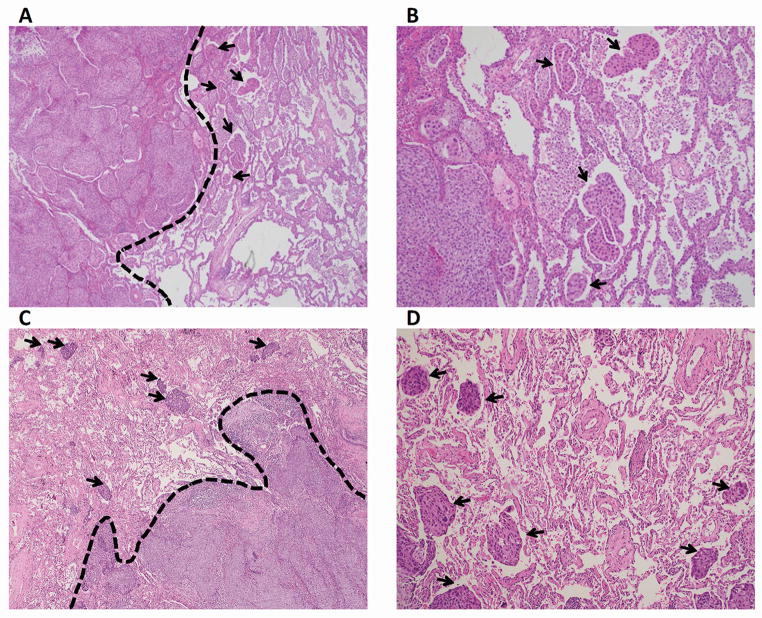
Tumor STAS was defined as small clusters of tumor cell nests within air spaces in the lung parenchyma beyond the edge of the main tumor. It was recorded as present if any STAS was identified and the extent of STAS was recorded as defined below. The edge of the main tumor was defined as the outer border of the tumor which is typically appreciated by low-power histologic examination (Figure 1A and C). Tumor STAS was considered present when tumor cell nests were identified beyond the edge of the main tumor even if it existed only in the first alveolar layer from the tumor edge. To avoid confusion with artificially detached or floating cells during tumor dissection, tumor cells were only considered if they appeared as detached small clusters within air spaces in a continuous manner from the edge of the tumor, and the distribution was consistent with the overall configuration of the circumferential tumor edge. Haphazardly distributed fragments of tumor with sharp jagged edges were regarded as artifacts. Recognition of STAS was straightforward in most cases because they had a solid growth pattern and the tumor edge had a relatively distinct border which often showed scarring and inflammation. Rarely squamous cell carcinomas spread along the surface of adjacent airspaces and may undermine overlying reactive pneumocytes. This differs from STAS in which the tumor cells are present within airspaces. There may be limited opportunity to search for STAS in tumors arising within a fibrotic underlying lung parenchyma and in cases where the submitted histologic sections did not sample the edge of the tumor including surrounding non-neoplastic lung.
Figure 1.
Morphologic Features of Tumor STAS (original magnification:× 00 in A and C; × 200 in B and D). A and C: Solid pattern STAS (arrows) identified within air spaces in the lung parenchyma beyond the edge (dashed line) of the main tumor, consisting of solid collections of tumor cells filling air spaces. B and D: Solid pattern STAS (arrows). STAS, spread through air spaces.
STAS was distinguished from alveolar macrophages by morphologic features where tumor cells showed a high N/C ratio and nuclear atypia, while macrophages showed small nuclei and cytoplasm that sometimes contained foamy vacuoles or carbon pigment. STAS was sometimes difficult to distinguish from tumor budding, which is defined as the presence of isolated single cancer cells or a cluster of cancer cells composed of fewer than five cells in the stroma at the outer edge of the tumor.3, 11, 13 However, tumor budding was observed within the tumor stroma at the invasive front of the tumor,3, 4 while STAS was observed within air spaces in the alveolar parenchyma beyond the edge of the tumor.
In addition, because lung specimens were not consistently inflated during processing, to account for artifactual collapse of alveolar spaces or atelectasis, the distance between tumor edge and the farthest STAS was measured in two ways: 1) by a ruler and reported in millimeters or 2) by the number of alveolar spaces between the tumor edge and the furthest STAS.
Necrosis in squamous cell carcinoma was distinguished from keratinization by complete loss of cellular morphology or layering of keratin and in some cases the presence of nuclear debris.
Immunohistochemistry
Ki-67 staining was performed on tissue microarray blocks as previously reported using standard avidin-biotin complex peroxidase technique (clone MIB-1, Immunotech, Westbrook ME, diluted at 1:100).3 Ki-67 proliferation index was recorded as the percentage of tumor cells with nuclear positive immunostaining in each tissue microarray core. The average percentage of the tumor cores was used as the Ki-67 proliferation index for each patient.3 Immunohistochemical staining to confirm squamous differentiation and exclude other histologic types was reported previously.19 A Ki-67 labeling index of ≥ 20% was classified as high and <20% was classified as low.3
Statistical Analysis
Associations between variables and the presence of STAS were analyzed using Fisher’s exact test (for categorical variables) and the Wilcoxon rank sum test (for continuous variables). The primary endpoint was cumulative incidence of recurrence (CIR) of any type (locoregional or distant) and cumulative incidence of death (CID) of lung cancer-specific cause. The hazard of recurrence was analyzed using competing risks methods. CIR and CID was estimated from the time of surgery using a cumulative incidence function that accounted for death without recurrence as a competing event in the first, and death due to non-cancer specific or unknown causes as competing events in the latter.22, 23 Patients alive and without documented recurrence at the last clinical contact were censored. As exploratory endpoints, we investigated the cumulative incidence of locoregional recurrence (distant recurrence as an additional competing risk), and cumulative incidence of distant recurrence (locoregional recurrence as additional competing risk). Differences in CIR or CID between groups were assessed using Gray’s test (univariable nonparametric analysis, stratified by p-stage) and Fine and Gray competing risk model.22, 24 We investigated the relationship between clinicopathologic variables and both CIR and CID overall and separately by surgery type (limited and lobectomy resection).
As a secondary outcome, OS was defined as the time from surgery to death or the last follow-up and estimated using the Kaplan–Meier method. Univariable and multivariable analyses of OS were performed using the Cox proportional hazards model. Multivariable models were constructed by starting with variables with p <0.1 from univariable analyses. All p values were based on two-tailed statistical analysis and p value less than 0.05 was considered statistically significance. Statistical analyses were conducted in R 3.0.1 (R Development Core Team) using the “cmprsk” and “maxstat” packages.
RESULTS
Patient Clinicopathologic Characteristics
Patient clinicopathologic characteristics are shown in Table 1. Of all 445 lung SCC patients, the stage distribution was: I/II/III; 249/131/65. The median age of the patients was 71.3 years (range, 38.5–88.4 years). Of the 445 patients total, 336 (76%) were > 65 years old. Two hundred and sixty-five (60%) patients were male. Ninety (20%) patients received adjuvant therapy. Most patients (434, 98%) were former or current smokers, reporting a median of 52 pack-year (range, 3–252). Sixty nine patients (16%) underwent sublobar resection and 376 (84%) underwent lobectomy. Median follow-up was 3.4 years (range, 0.01 – 13.0 years). Among 273 (61%) patients died during follow-up, one third (91, 33.3%) died of lung cancer-specific death, and the remainder died of competing events or unknown cause.
Table 1.
Association between tumor spread through air space (STAS) and clinicopathological variables
| STAS
|
|||||
|---|---|---|---|---|---|
| All Patients (n=445) | Absent (n=313) | Present (n=132) | |||
| Variables | n (%) | n (%) | n (%) | p | |
| Patient characteristics | |||||
| Age | ≤65 | 109 (24) | 70 (64) | 39 (36) | 0.12 |
| >65 | 336 (76) | 243 (72) | 93 (28) | ||
|
| |||||
| Sex | Female | 180 (40) | 134 (74) | 46 (26) | 0.14 |
| Male | 265 (60) | 179 (57) | 86 (65) | ||
|
| |||||
| Smoking | Never | 11 (2) | 8 (73) | 3 (27) | 1 |
| Former/current | 434 (98) | 305 (70) | 129 (30) | ||
|
| |||||
| Smoking pack-year | ≤90 | 365 (82) | 259 (71) | 106 (29) | 0.6 |
| >90 | 80 (18) | 54 (67) | 26 (33) | ||
|
| |||||
| Surgery | Lobectomy | 376 (84) | 263 (70) | 113 (30) | 0.8 |
| Sublobar resection | 69 (16) | 50 (72) | 19 (28) | ||
|
| |||||
| Adjuvant therapy | No | 355 (80) | 256 (72) | 99 (28) | 0.12 |
| Yes | 90 (20) | 57 (63) | 33 (37) | ||
|
| |||||
| Pathological factors | |||||
| T classification | T1 | 189 (42) | 146 (77) | 43 (23) | 0.012 |
| T2 | 197 (45) | 133 (67) | 64 (32) | ||
| T3 | 50 (11) | 30 (60) | 20 (40) | ||
| T4 | 9 (2) | 4 (44) | 5 (56) | ||
|
| |||||
| N classification | N0 | 314 (71) | 232 (74) | 82 (26) | 0.016 |
| N1 | 85 (19) | 56 (66) | 29 (34) | ||
| N2 | 46 (10) | 25 (54) | 21 (46) | ||
|
| |||||
| P-stage | I | 249 (56) | 191 (77) | 58 (23) | 0.002 |
| II | 131 (29) | 85 (65) | 46 (35) | ||
| III | 65 (15) | 37 (57) | 28 (43) | ||
|
| |||||
| Tumor size (cm) | 3.0 (2.0, 5.0)# | 3.0 (2.0, 5.0) # | 4.0 (3.0, 5.0) # | 0.001 | |
|
| |||||
| Tumor differentiation | Well | 51 (11) | 38 (12) | 13 (10) | 0.3 |
| Moderately | 176 (40) | 129 (41) | 47 (36) | ||
| Poorly | 218 (49) | 146 (47) | 72 (55) | ||
|
| |||||
| Subtype | Keratinizing | 253 (57) | 174 (69) | 79 (31) | 0.4 |
| Nonkeratinizing | 164 (37) | 121 (74) | 43 (26) | ||
| Basaloid | 28 (6) | 18 (64) | 10 (36) | ||
|
| |||||
| Tumor budding | Low (<10/HPF) | 375 (84) | 264(70) | 111 (30) | 1 |
| High (≥10/HPF) | 70 (16) | 49 (70) | 21 (30) | ||
|
| |||||
| Single cell invasion | Absent | 264 (59) | 183 (69) | 81 (31) | 0.6 |
| Present | 181 (41) | 130 (72) | 51 (28) | ||
|
| |||||
| Lymphatic invasion | Absent | 227 (51) | 183 (81) | 44 (19) | <0.001 |
| Present | 218 (49) | 130 (60) | 88 (40) | ||
|
| |||||
| Vascular invasion | Absent | 216 (49) | 166 (77) | 50 (23) | 0.004 |
| Present | 229 (51) | 147 (64) | 82 (36) | ||
|
| |||||
| Necrosis | Absent | 172 (39) | 135 (78) | 37 (22) | 0.003 |
| Present | 273 (61) | 178 (65) | 95 (35) | ||
|
| |||||
| Nuclear diameter | Small (≤4 lymphocytes) | 303 (68) | 223 (74) | 80 (26) | 0.034 |
| Large (>4 lymphocytes) | 142 (32) | 90 (63) | 52 (37) | ||
|
| |||||
| Nuclear atypia | Mild | 69 (16) | 53 (77) | 16 (23) | 0.052 |
| Moderate | 255 (57) | 185 (73) | 70 (27) | ||
| Severe | 121 (27) | 75 (62) | 46 (38) | ||
|
| |||||
| Mitotic count | Low (<15/10 HPFs) | 129 (29) | 102 (79) | 27 (21) | 0.012 |
| High (≥15/10 HPFs) | 316 (71) | 211 (67) | 105 (33) | ||
|
| |||||
| Ki-67 labeling index | Low (<20%) | 47 (11) | 41 (87) | 6 (13) | 0.007 |
| High (≥20%) | 398 (89) | 272 (68) | 126 (32) | ||
|
| |||||
| STAS | Absent | 313 (70) | |||
| Present | 132 (30) | ||||
Significant p values are shown in bold.
median(25th, 75 th percentile).
Incidence of STAS and Association between Clinicopathologic Variables and STAS
STAS was observed in 132 patients (30%). All cases showed a solid nest pattern (Figure 1). Associations between clinicopathologic factors and STAS are summarized in Table 1. Pathologic features characteristic of aggressive tumor behavior were more frequently identified in tumors with STAS compared to those without STAS: lymphatic invasion (40% vs. 19%; p <0.001), vascular invasion (36% vs. 23%; p = 0.004), tumor necrosis (35% vs. 22%; p = 0.003), larger tumor size (median, 4cm vs. 3 cm; p = 0.001), larger nuclear diameter (> 4 lymphocytes, 37% vs. 26%, p = 0.034), higher mitotic count (>15 mitoses/10 high power fields, 33% vs. 21%; p = 0.012), higher Ki-67 labeling index (≥20%, 32% vs. 13%; p = 0.007), and increasing incidence with higher stage tumor (p-stage I [23%] vs. stage II [35%] vs. stage III [43%]; p = 0.002).
Limited and Extensive STAS
The median distance between tumor surface and farthest STAS from the tumor edge was 1.4 mm (range, 0.3–4.2 mm) measured with a ruler (Supplementary Figure 1A) and 5 (range, 1–15) based on the number of alveolar spaces (Supplementary Figure 1B). Using 3 alveolar spaces as a potential cutoff,15 32 patients were identified as having limited STAS (solid cell nests ≤3 alveoli away from the tumor edge) whereas 100 were considered extensive (tumor cell nests >3 alveoli away from the tumor edge).
In univariable competing risk model for any recurrence and lung cancer-specific death (Table 2), subhazard ratio of limited STAS (reference, no STAS) was similar to that of extensive STAS (limited vs. extensive: recurrence, 2.23 vs. 1.70; lung cancer-specific death, 2.39 vs. 2.47, respectively). Therefore we decided not to use extent of STAS in further multivariable competing risk regression models for any recurrence and lung cancer-specific death.
Table 2.
Univariable competing-risk regression model for any recurrence and lung cancer-specific death and Cox model for all-cause death
| Any recurrence | Lung cancer-specific death | All-cause death | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Variable | SHR | 95% CI | p | SHR | 95% CI | p | HR | 95% CI | p |
| Age: >65 vs ≤65 | 0.93 | 0.63, 1.37 | 0.7 | 0.84 | 0.53, 1.32 | 0.4 | 1.85 | 1.35, 2.53 | <0.001 |
|
| |||||||||
| Sex: female vs. male | 1.3 | 0.91, 1.86 | 0.15 | 1.59 | 1.02, 2.48 | 0.043 | 1.16 | 0.90, 1.48 | 0.2 |
|
| |||||||||
| Smoking pack-year: >90 vs ≤90 | 1.55 | 1.05, 2.29 | 0.027 | 1.46 | 0.90, 2.39 | 0.13 | 1.54 | 1.15, 2.06 | 0.004 |
|
| |||||||||
| Surgery: lobectomy vs. sublobar | 0.94 | 0.59, 1.50 | 0.8 | 1.10 | 0.64, 1.90 | 0.7 | 1.15 | 0.83, 1.60 | 0.4 |
|
| |||||||||
| Pathological stage (vs Stage I) resection | |||||||||
| Stage II | 1.72 | 1.18, 2.52 | 0.005 | 2.69 | 1.67, 4.34 | <0.001 | 1.37 | 1.05, 1.80 | 0.023 |
| Stage III | 2.1 | 1.31, 3.38 | 0.002 | 4.05 | 2.36, 6.93 | <0.001 | 2.00 | 1.43, 2.81 | <0.001 |
|
| |||||||||
| Tumor size: per 1 cm increase | 1.07 | 1.00, 1.15 | 0.062 | 1.14 | 1.06, 1.22 | <0.001 | 1.11 | 1.05, 1.17 | <0.001 |
|
| |||||||||
| Tumor differentiation (vs well) | |||||||||
| Moderately | 0.8 | 0.48, 1.34 | 0.4 | 0.92 | 0.47, 1.78 | 0.8 | 1.04 | 0.68, 1.57 | 0.9 |
| Poorly | 0.82 | 0.50, 1.35 | 0.4 | 1.09 | 0.58, 2.05 | 0.8 | 1.19 | 0.80, 1.77 | 0.4 |
|
| |||||||||
| Lymphatic invasion: present vs. absent | 1.74 | 1.23, 2.44 | 0.002 | 2.59 | 1.67, 4.02 | <0.001 | 1.53 | 1.20, 1.95 | 0.001 |
|
| |||||||||
| Vascular invasion: present vs. absent | 1.34 | 0.96, 1.88 | 0.086 | 1.75 | 1.15, 2.66 | 0.009 | 1.26 | 0.99, 1.61 | 0.057 |
|
| |||||||||
| Necrosis: present vs. absent | 1.51 | 1.05, 2.17 | 0.025 | 1.69 | 1.08, 2.66 | 0.023 | 1.00 | 0.78, 1.28 | 1 |
|
| |||||||||
| Nuclear size: large vs. small | 1.48 | 1.04, 2.09 | 0.028 | 1.51 | 0.99, 2.31 | 0.053 | 1.22 | 0.94, 1.57 | 0.13 |
|
| |||||||||
| Nuclear atypia (vs mild) | |||||||||
| Intermediate | 1.01 | 0.63, 1.61 | 1 | 1.13 | 0.63, 2.02 | 0.7 | 0.94 | 0.68, 1.31 | 0.7 |
| Severe | 1.34 | 0.81, 2.22 | 0.3 | 1.59 | 0.85, 2.97 | 0.14 | 1.19 | 0.82, 1.71 | 0.4 |
|
| |||||||||
| Mitotic count: high vs. low | 0.97 | 0.67, 1.40 | 0.9 | 1.04 | 0.67, 1.63 | 0.8 | 0.86 | 0.66, 1.11 | 0.2 |
|
| |||||||||
| Ki-67: ≥20% vs. <20% | 1.35 | 0.76, 2.40 | 0.3 | 2.28 | 0.92, 5.67 | 0.076 | 1.43 | 0.95, 2.16 | 0.091 |
|
| |||||||||
| Tumor budding: ≥10/HPF vs. <10/HPF | 1.58 | 1.02, 2.45 | 0.040 | 2.27 | 1.42, 3.64 | 0.001 | 2.02 | 1.49, 2.74 | <0.001 |
|
| |||||||||
| Single cell invasion: present vs. absent | 1.28 | 0.91, 1.80 | 0.2 | 1.58 | 1.05, 2.37 | 0.029 | 1.56 | 1.22, 1.98 | <0.001 |
|
| |||||||||
| STAS: present vs. absent | 1.82 | 1.29, 2.58 | 0.001 | 2.45 | 1.63, 3.69 | <0.001 | 1.25 | 0.97, 1.61 | 0.090 |
|
| |||||||||
| Distance of STAS (vs. no STAS) | |||||||||
| Limited STAS | 2.23 | 1.25, 4.01 | 0.007 | 2.39 | 1.15, 4.99 | 0.020 | 0.83 | 0.50, 1.37 | 0.5 |
| Extensive STAS | 1.70 | 1.16, 2.50 | 0.006 | 2.47 | 1.60, 3.81 | <0.001 | 1.42 | 1.08, 1.87 | 0.013 |
CI, confidence interval; HR, hazard ratio; SHR, sub-hazard ratio; STAS, spread through air spaces
Cumulative Incidence of Recurrence and lung cancer-specific death by Tumor STAS
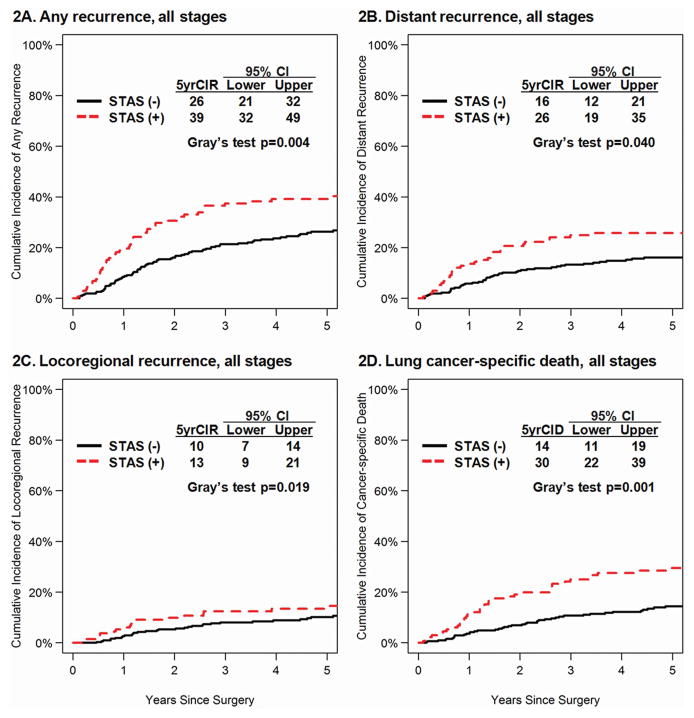
CIR and CID curves by STAS absent/present were shown in Figure 2 (all stages), Figure 3 (stage II-III), and Supplementary Figure 2 (stage I). Among all-stage 445 patients, patients with STAS had higher incidence of any recurrence (Figure 2A, p=0.004), distant recurrence (Figure 2B, p=0.040), locoregional recurrence (Figure 2C, p=0.019), and lung cancer-specific death (Figure 2D, p=0.001) compared to those without STAS.
Figure 2.
CIR by STAS in All Stages. A, CIR for any recurrence of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 39% vs. 26%; p = 0.004). B, CIR for distant recurrence of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 26% vs. 16%; p = 0.040). C, CIR for locoregional recurrence of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 13% vs. 10%; p = 0.019). D, Cumulative incidence of Death (CID) for lung cancer-specific death of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 30% vs. 14%; p = 0.001). STAS, spread through air spaces; CIR, cumulative incidence of recurrence. CID, Cumulative incidence of death
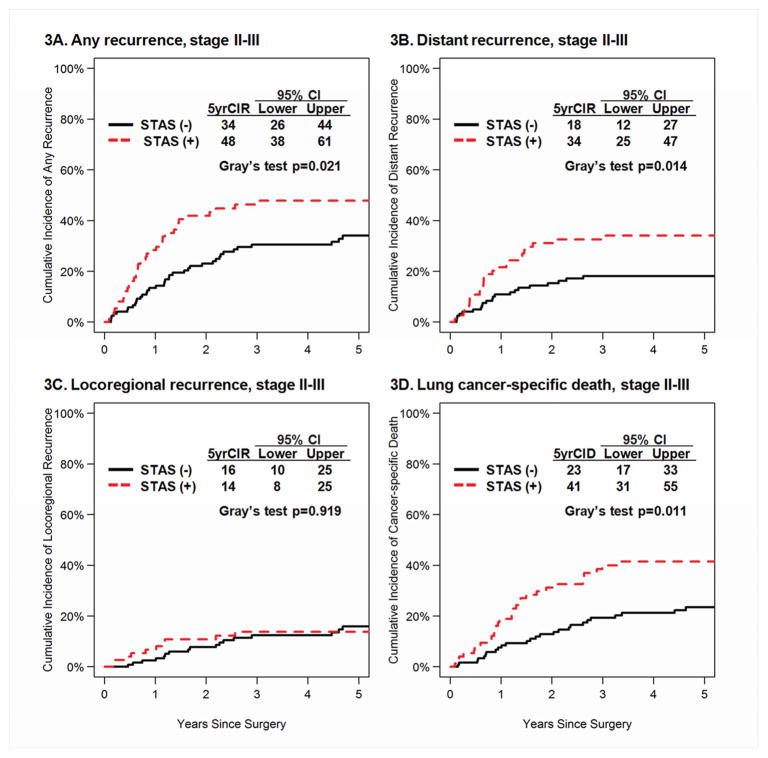
Figure 3.
CIR by STAS in Stages II & III Group. A, CIR for any recurrence of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 48% vs. 34%; p = 0.021). B, CIR for distant recurrence of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 34% vs.18%; p = 0.014). C, CIR for locoregional recurrence of patients with STAS-present tumors was not significantly higher than for patients with STAS-absent tumors (5-year CIR, 14% vs. 16%; p = 0.919). D, CID for lung cancer-specific death of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 41% vs. 23%; p = 0.011). STAS, spread through air spaces; CIR, cumulative incidence of recurrence. CID, cumulative incidence of death.
In stage II-III (Figure 2), patients with STAS had higher incidence of any and distant recurrence and lung cancer-specific death compared to those without STAS (p=0.021, p=0.014, and p=0.011, respectively) but there was no statistically significant difference in CIR for locoregional recurrence.
In stage I (Supplementary Figure 2), patients with STAS had higher incidence of locoregional recurrence and lung cancer-specific death compared to those without STAS (p=0.028 and p=0.027, respectively) but there were no statistically significant differences in CIR for any recurrence and distant recurrence.
Univariable and multivariable competing risk regression model for CIR, CID and Cox model for OS
Results from univariable and multivariable models are presented in Table 2 and Table 3, respectively. In multivariable competing risk regression model for any recurrence, smoking pack-year >90 and the presence of STAS were independent risk factors (p=0.043 and p=0.034, respectively); and for lung cancer-specific death, pathological stage II compared to I, pathological stage III compared to I, tumor budding ≥10 high power fields, and STAS were independent risk factors (p=0.025, p=0.009, p=0.044, and p=0.016, respectively). In multivariable Cox model for OS, higher age, Ki-67 ≥20%, and presence of single cell invasion were independent risk factors for worse OS (p<0.001, p=0.040, and p=0.016) but STAS was not (p=0.4).
Table 3.
Multivariable competing-risk regression model for any recurrence and lung cancer-specific death and Cox model for all-cause death
| Any recurrence | Lung cancer-specific death | All-cause death | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Variable | SHR | 95% CI | p | SHR | 95% CI | p | HR | 95% CI | p |
| Age: >65 vs ≤65 | -* | - | - | -* | - | - | 2.30 | 1.66, 3.20 | <0.001 |
|
| |||||||||
| Sex: female vs. male | -* | - | - | 1.42 | 0.89, 2.29 | 0.14 | -* | - | - |
|
| |||||||||
| Smoking pack-year: >90 vs ≤90 | 1.53 | 1.01, 2.32 | 0.043 | -* | - | - | 1.34 | 0.99, 1.82 | 0.060 |
|
| |||||||||
| Pathological stage (vs Stage I) | |||||||||
| Stage II | 1.59 | 0.98, 2.56 | 0.059 | 1.93 | 1.09, 3.43 | 0.025 | 1.11 | 0.79, 1.54 | 0.6 |
| Stage III | 1.65 | 0.90, 3.02 | 0.11 | 2.53 | 1.26, 5.08 | 0.009 | 1.49 | 0.99, 2.24 | 0.057 |
|
| |||||||||
| Tumor size: per 1 cm increase | 0.97 | 0.88, 1.07 | 0.6 | 1.01 | 0.92, 1.11 | 0.8 | 1.06 | 0.99, 1.13 | 0.094 |
|
| |||||||||
| Lymphatic invasion: present vs. absent | 1.25 | 0.80, 1.96 | 0.3 | 1.35 | 0.76, 2.39 | 0.3 | 1.25 | 0.94, 1.66 | 0.13 |
|
| |||||||||
| Vascular invasion: present vs. absent | 1.03 | 0.71, 1.49 | 0.9 | 1.10 | 0.67, 1.78 | 0.7 | 0.95 | 0.73, 1.24 | 0.7 |
|
| |||||||||
| Necrosis: present vs. absent | 1.43 | 0.99, 2.07 | 0.060 | 1.37 | 0.85, 2.22 | 0.2 | -* | - | - |
|
| |||||||||
| Nuclear size: large vs. small | 1.42 | 0.98, 2.04 | 0.063 | 1.37 | 0.87, 2.14 | 0.2 | -* | - | - |
|
| |||||||||
| Ki-67: ≥20% vs. <20% | -* | - | - | 1.90 | 0.72, 5.01 | 0.2 | 1.56 | 1.02, 2.40 | 0.040 |
|
| |||||||||
| Tumor budding: ≥10/HPF vs. <10/HPF | 1.35 | 0.84, 2.17 | 0.2 | 1.87 | 1.02, 3.43 | 0.044 | 1.56 | 1.09, 2.25 | 0.016 |
|
| |||||||||
| Single cell invasion: present vs. absent | -* | - | - | 0.97 | 0.56, 1.67 | 0.9 | 1.34 | 0.99, 1.81 | 0.057 |
|
| |||||||||
| STAS: present vs. absent | 1.50 | 1.03, 2.18 | 0.034 | 1.75 | 1.11, 2.75 | 0.016 | 1.12 | 0.85, 1.47 | 0.4 |
CI, confidence interval; HR, hazard ratio; SHR, sub-hazard ratio; STAS, spread through air spaces
variables with p-value >0.1 in univariable models were not analyzed in multivariable model
DISCUSSION
In this study, we demonstrated that STAS occurred in 30% of resected lung SCC and was a significant prognostic factor for distant and locoregional recurrence. In contrast to lung adenocarcinoma where STAS can manifest as micropapillary clusters, solid nests or single cells, all STAS lesions in lung SCC consist of solid tumor cell nests.11 Interestingly the proportion of STAS was similar (26–36%) in all squamous histologic subtypes. This observation contrasts with lung adenocarcinoma in which the presence of STAS was more frequent among more aggressive subtypes (papillary, micropapillary, solid) but less so among less aggressive subtypes (lepidic, acinar).11, 15 We found STAS in lung SCC was associated with p-stage, lymphatic and vascular invasion, necrosis, larger nuclear diameter, increased mitoses and high Ki-67 labeling index. The association with these characteristics of aggressive tumor behavior is similar to previous observations in lung adenocarcinoma where STAS correlated with lymphatic and vascular invasion, and p-stage.12–14, 25 These data show that multiple pathologic and clinical features of aggressiveness are associated with STAS in both SCC and adenocarcinoma.
STAS is an insidious pattern of tumor invasion that is not visible to pathologists on gross exam. Furthermore, STAS is easily overlooked because pathologists are not trained to look for STAS routinely. In addition if histologic sections do not include surrounding lung parenchyma it is impossible to look for STAS. Optimal processing of gross specimens for STAS requires histologic sampling to include the circumferential tumor edge as well as adjacent lung parenchyma.
Identification of STAS raises important issues in the differential diagnosis. The presence of tumor cells within airspaces beyond the edge of the main tumor differs from the previously reported predominant alveolar space filling pattern identified within the main tumor that has been reported to be associated with favorable prognosis.26, 27 However, this pattern has not been recognized in other recent studies.3, 4 The distinction from alveolar macrophages is less of a challenge than in adenocarcinoma since in SCC the alveolar tumor cells consist of solid nests. The cohesiveness of the solid nests of tumor cells along with the cytologic, particularly nuclear atypia are morphologically different from alveolar macrophages which are typically not cohesive and the cytoplasm contains foamy material or carbon pigment. Rarely one might need to perform immunohistochemistry for epithelial (i.e. AE1/AE3) and macrophage (i.e. CD68) markers to answer this question. The main challenge is to distinguish STAS from artifactual floaters. In STAS the tumor cell nests have smooth borders and are seen in a continuous pattern from the edge of the tumor to the most distant location. Artifacts are more likely to show jagged edges and random distribution on the slides, such as isolated fragments of tumor on the tissue section.
It has been hypothesized that STAS is an ex vivo artifact caused by spreading through a knife surface (STAKS).28 The importance of distinguishing this from artifacts is appreciated by the authors who have published on this topic and criteria are proposed on how to make this distinction.13, 15, 25 STAS is morphologically different from tissue floaters and contaminant or extraneous tissues that can lead to diagnostic errors.29–31 Stedman’s Medical Dictionary defines artifact as “anything, especially in a histologic specimen or a graphic record, that is caused by the technique used and is not a natural occurrence, but is merely incidental”.32 The first part of this definition is the basis of the STAKs hypothesis which claims the tumor cells are spread by the force of a knife during lung resection specimen prosection.28 However, uncommonly surgical specimens may be encountered in a setting apart from removal of the main tumor; we have seen STAS as the predominant histologic pattern in lung adenocarcinoma specimens where no knife cut across the main tumor, suggesting this is not an ex vivo artifact.33 Another important component of the definition of an artifact is the point that artifacts are merely incidental findings. Therefore if STAS were just an artifact, it should have no clinical significance. However, as demonstrated in the current study of SCC and in the growing number of lung adenocarcinoma studies STAS has consistently been shown to be a predictor of recurrence and survival.11–17 It is argued that poorly differentiated tumor cells have a tendency for dissociation explaining why STAS is associated with poor prognosis. However, in our study of lung SCC not only was STAS a predictor of cancer specific outcomes, but it was independent of grade and multiple other histologic predictors of prognosis. While STAS is seen more frequently in adenocarcinomas with micropapillary and solid patterns12, 15, 16 data suggests that STAS increases the risk of recurrence independent of the micropapillary and solid patterns.12, 25 The risk of recurrence in limited resections for lung adenocarcinoma was 5.2 for STAS compared to 1.3 and 1.3 for micropapillary and solid patterns, respectively.25 Perhaps one of the most important questions is whether pathologists should completely ignore the presence of STAS, particularly in the margins of limited resections. Based upon the clinical evidence in both lung adenocarcinoma and SCC, this does not seem wise.
Our study of lung SCC showed that STAS was an independent predictor of both recurrence and cancer-specific death, but not of OS by multivariable analyses adjusting for p-stage and other pathological prognostic variables. We found a strong correlation between STAS and high-grade morphologic patterns such as nuclear size, nuclear atypia, mitotic count and Ki-67 labeling index, suggesting STAS is associated with tumor proliferation. Furthermore, our data showed a strong correlation of STAS with aggressive tumor behavior such as lymphatic and vascular invasion. The finding that STAS correlates with aggressive histologic features and clinical behavior is consistent with the concept that STAS represents air space invasion by the tumor cells, a pattern of invasion distinct from lymphovascular invasion and stroma infiltration. Histologic features such as lymphatic and vascular invasion are routinely reported as recommended by major organizations such as the College of American Pathologists.34 Like other studies,1–5 the current cohort showed tumor budding and single cell invasion were of prognostic importance. However, we found STAS to be independent of both lymphatic/vascular invasion and tumor budding and single cell invasion in predicting both recurrence free survival and cancer-specific death.
The reason why we found STAS was significantly associated only with cancer specific outcomes but not OS in our cohort is probably because the majority of patients were elderly with high risk of competing events or death from causes other than lung cancer. More than three fourths of patients (76%) were elderly (>65 years). We found of the 273 patients who died during follow up, only one third died of lung cancer whereas the remainder died of competing events or unknown cause. Our statistical method of competing risk analysis makes adjustments for this.22, 23 We also demonstrated that STAS was independently associated with high risk of recurrence in patients undergoing lobectomy but not sublobar resection. This differs from lung adenocarcinoma where we showed that STAS was associated with high risk of recurrence in patients undergoing sublobar resection.11 A likely explanation is that in our SCC cohort, few patients underwent sublobar resection in contrast to our lung adenocarcinoma series, limiting the power of statistical analysis of this patient subgroup.
In conclusion, we found STAS was present in one third of resected lung SCC. It was associated with aggressive clinical pathologic factors but it was found to be an independent predictor of lung cancer-specific outcomes and it appears to be one of the most prognostically significant histologic findings in lung SCC. Our findings of the clinical significance of STAS in lung SCC are against the concept that it is an artifact that should be ignored by pathologists. These results should be validated in independent cohorts, and interobserver agreement of recognizing STAS should be evaluated. Since we found STAS to show greater prognostic significance than lymphatic, vascular and visceral pleural invasion, all histologic features recommended to be recorded in pathology reports for lung cancer specimens, in the future, STAS may be appropriate to add to this list.
Supplementary Material
Distance of Tumor STAS from Edge of Main Tumor. A, Distance between tumor surface and farthest STAS away from the tumor edge was measured by a ruler with a median of 1.4 mm (range 0.3–4.2 mm). B, Distance between tumor surface and farthest STAS away from the tumor edge was measured by number of alveolar spaces with a median of 5 (range 1–15).
CIR by STAS in Stage I Group. A, CIR for any recurrence of patients with STAS-present tumors was not significantly higher than for patients with STAS-absent tumors (5-year CIR, 28% vs. 22%; p = 0.094). B, CIR for distant recurrence of patients with STAS-present tumors was not significantly higher than for patients with STAS-absent tumors (5-year CIR, 15% vs. 15%; p = 0.847). C, CIR for locoregional recurrence of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 13% vs. 7%; p = 0.028). D, CID for lung cancer-specific death of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 14% vs. 9%; p = 0.027). STAS, spread through air spaces; CIR, cumulative incidence of recurrence. Cumulative incidence of death.
CID by STAS in Stage I Group. CID for lung cancer-specific death of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors with 10 years follow up. CID: Cumulative incidence of death.
Acknowledgments
We thank Alex Torres of the MSK Thoracic Surgery Service for editorial assistance.
Grant numbers and sources of support: This authors’ laboratory work was supported in part by grants from the National Institutes of Health (U54 CA137788, P30 CA008748, and P50 CA086438-13), the U.S. Department of Defense (LC110202), the DallePezze Foundation and the Derfner Foundation.
Footnotes
Disclosure/conflict of interest
The authors declare no conflict of interest.
DISCLOSURES: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masuda R, Kijima H, Imamura N, Aruga N, Nakamura Y, Masuda D, Takeichi H, Kato N, Nakagawa T, Tanaka M, Inokuchi S, Iwazaki M. Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. MolMedRep. 2012;6:937–43. doi: 10.3892/mmr.2012.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taira T, Ishii G, Nagai K, Yoh K, Takahashi Y, Matsumura Y, Kojima M, Ohmatsu H, Goto K, Niho S, Takashima H, Inoue H, Ohe Y, Ochiai A. Characterization of the immunophenotype of the tumor budding and its prognostic implications in squamous cell carcinoma of the lung. Lung Cancer. 2012;76:423–30. doi: 10.1016/j.lungcan.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Kadota K, Nitadori J, Woo KM, Sima CS, Finley DJ, Rusch VW, Adusumilli PS, Travis WD. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol. 2014;9:1126–39. doi: 10.1097/JTO.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weichert W, Kossakowski C, Harms A, Schirmacher P, Muley T, Dienemann H, Warth A. Proposal of a prognostically relevant grading scheme for pulmonary squamous cell carcinoma. Eur Respir J. 2015 doi: 10.1183/13993003.00937-2015. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Shen H, Qiu C, Zhang T, Hu P, Qu X, Liu Q, Du J. Invasion Types Are Associated With Poor Prognosis in Lung Squamous Carcinoma Patients. Medicine (Baltimore) 2015;94:e1634. doi: 10.1097/MD.0000000000001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: International Agency for Research on Cancer; 2015. [DOI] [PubMed] [Google Scholar]
- 7.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243–60. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 8.Moro D, Brichon PY, Brambilla E, Veale D, Labat F, Brambilla C. Basaloid bronchial carcinoma. A histologic group with a poor prognosis. Cancer. 1994;73:2734–9. doi: 10.1002/1097-0142(19940601)73:11<2734::aid-cncr2820731114>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim DJ, Kim KD, Shin DH, Ro JY, Chung KY. Basaloid carcinoma of the lung: a really dismal histologic variant? AnnThoracSurg. 2003;76:1833–7. doi: 10.1016/s0003-4975(03)01296-7. [DOI] [PubMed] [Google Scholar]
- 10.Moro-Sibilot D, Lantuejoul S, Diab S, Moulai N, Aubert A, Timsit JF, Brambilla C, Brichon PY, Brambilla E. Lung carcinomas with a basaloid pattern: a study of 90 cases focusing on their poor prognosis. EurRespirJ. 2008;31:854–9. doi: 10.1183/09031936.00058507. [DOI] [PubMed] [Google Scholar]
- 11.Kadota K, Nitadori JI, Sima CS, Ujiie H, Rizk NP, Jones DR, Adusumilli PS, Travis WD. Tumor Spread Through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences Following Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol. 2015;10:806–14. doi: 10.1097/JTO.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto J, Nakajima T, Suzuki H, Nagato K, Iwata T, Yoshida S, Fukuyo M, Ota S, Nakatani Y, Yoshino I. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J Thorac Cardiovasc Surg. 2016;152:64–72. e1. doi: 10.1016/j.jtcvs.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 13.Onozato ML, Kovach AE, Yeap BY, Morales-Oyarvide V, Klepeis VE, Tammireddy S, Heist RS, Mark EJ, Dias-Santagata D, Iafrate AJ, Yagi Y, Mino-Kenudson M. Tumor Islands in Resected Early-stage Lung Adenocarcinomas are Associated With Unique Clinicopathologic and Molecular Characteristics and Worse Prognosis. AmJ SurgPathol. 2013;37:287–94. doi: 10.1097/PAS.0b013e31826885fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiono S, Yanagawa N. Spread through air spaces is a predictive factor of recurrence and a prognostic factor in stage I lung adenocarcinoma. Interact Cardiovasc Thorac Surg. 2016 doi: 10.1093/icvts/ivw211. [DOI] [PubMed] [Google Scholar]
- 15.Warth A, Muley T, Kossakowski CA, Goeppert B, Schirmacher P, Dienemann H, Weichert W. Prognostic Impact of Intra-alveolar Tumor Spread in Pulmonary Adenocarcinoma. The American journal of surgical pathology. 2015;39:793–801. doi: 10.1097/PAS.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 16.Warth A, Muley T, Harms A, Hoffmann H, Dienemann H, Schirmacher P, Weichert W. Clinical Relevance of Different Papillary Growth Patterns of Pulmonary Adenocarcinoma. The American journal of surgical pathology. 2016 doi: 10.1097/PAS.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 17.Jin Y, Sun PL, Park SY, Kim H, Park E, Kim G, Cho S, Kim K, Lee CT, Chung JH. Frequent aerogenous spread with decreased E-cadherin expression of ROS1-rearranged lung cancer predicts poor disease-free survival. Lung Cancer. 2015 doi: 10.1016/j.lungcan.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7. Chicago: American Joint Committee on Cancer; 2010. [Google Scholar]
- 19.Kadota K, Nitadori J, Rekhtman N, Jones DR, Adusumilli PS, Travis WD. Reevaluation and reclassification of resected lung carcinomas originally diagnosed as squamous cell carcinoma using immunohistochemical analysis. The American journal of surgical pathology. 2015:39. doi: 10.1097/PAS.0000000000000439. Epub Ahead. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network. Guidelines for surveillance following therapy for NSCLC. National Comprehensive Cancer Network; 2016. [Accessed 07/30/16, 2016]. [Google Scholar]
- 21.Donington J, Ferguson M, Mazzone P, Handy J, Jr, Schuchert M, Fernando H, Loo B, Jr, Lanuti M, de Hoyos A, Detterbeck F, Pennathur A, Howington J, Landreneau R, Silvestri G. American College of Chest Physicians and Society of Thoracic Surgeons consensus statement for evaluation and management for high-risk patients with stage I non-small cell lung cancer. Chest. 2012;142:1620–35. doi: 10.1378/chest.12-0790. [DOI] [PubMed] [Google Scholar]
- 22.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18:2301–8. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chappell R. Competing risk analyses: how are they different and why should you care? ClinCancer Res. 2012;18:2127–9. doi: 10.1158/1078-0432.CCR-12-0455. [DOI] [PubMed] [Google Scholar]
- 24.Lunn M. Applying k-sample tests to conditional probabilities for competing risks in a clinical trial. Biometrics. 1998;54:1662–72. [PubMed] [Google Scholar]
- 25.Kadota K, Eguchi T, Rizk NP, Woo KM, Sima CS, Park BJ, Jones DR, Travis WD, Adusumilli PS. Lung adenocarcinoma: Presence of Spread of Tumor through Alveolar Spaces (STAS), Micropapillary and Solid Patterns Determines Outcomes. J Thoracic Oncol. 2015;10:S280. [Google Scholar]
- 26.Funai K, Yokose T, Ishii G, Araki K, Yoshida J, Nishimura M, Nagai K, Nishiwaki Y, Ochiai A. Clinicopathologic characteristics of peripheral squamous cell carcinoma of the lung. AmJSurgPathol. 2003;27:978–84. doi: 10.1097/00000478-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe Y, Yokose T, Sakuma Y, Hasegawa C, Saito H, Yamada K, Ito H, Tsuboi M, Nakayama H, Kameda Y. Alveolar space filling ratio as a favorable prognostic factor in small peripheral squamous cell carcinoma of the lung. Lung Cancer. 2011;73:217–21. doi: 10.1016/j.lungcan.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Thunnissen E, Blaauwgeers HJ, de Cuba EM, Yick CY, Flieder DB. Ex Vivo Artifacts and Histopathologic Pitfalls in the Lung. Arch Pathol Lab Med. 2016;140:212–20. doi: 10.5858/arpa.2015-0292-OA. [DOI] [PubMed] [Google Scholar]
- 29.Gephardt GN, Zarbo RJ. Extraneous tissue in surgical pathology: a College of American Pathologists Q-Probes study of 275 laboratories. Arch Pathol Lab Med. 1996;120:1009–14. [PubMed] [Google Scholar]
- 30.Platt E, Sommer P, McDonald L, Bennett A, Hunt J. Tissue floaters and contaminants in the histology laboratory. Arch Pathol Lab Med. 2009;133:973–8. doi: 10.5858/133.6.973. [DOI] [PubMed] [Google Scholar]
- 31.Layfield LJ, Witt BL, Metzger KG, Anderson GM. Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol. 2011;136:767–72. doi: 10.1309/AJCP4FFSBPHAU8IU. [DOI] [PubMed] [Google Scholar]
- 32.Stedmans Medical Dictionary. 23. Baltimore: The Williams and Wilkins Co; 1976. [Google Scholar]
- 33.Lu S, Rekhtman N, Eguchi T, Jones DR, Adusumilli PS, Travis WD. Cases Demonstrating Spread Through Air Spaces (STAS) Reflects Invasive Growth and Not an Artifact. Journal of Thoracic Oncology 2016 WCLC Abstract. 2016 in press. [Google Scholar]
- 34.Protocol for the Examination of Specimens from Patients with Primary Non–Small Cell Carcinoma, Small Cell Carcinoma, or Carcinoid Tumor of the Lung. [Accessed September 18, 2016, 2016];College of American Pathologists. 2016 at http://www.cap.org/web/home/resources/cancerreporting-tools/cancer-protocol-templates.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distance of Tumor STAS from Edge of Main Tumor. A, Distance between tumor surface and farthest STAS away from the tumor edge was measured by a ruler with a median of 1.4 mm (range 0.3–4.2 mm). B, Distance between tumor surface and farthest STAS away from the tumor edge was measured by number of alveolar spaces with a median of 5 (range 1–15).
CIR by STAS in Stage I Group. A, CIR for any recurrence of patients with STAS-present tumors was not significantly higher than for patients with STAS-absent tumors (5-year CIR, 28% vs. 22%; p = 0.094). B, CIR for distant recurrence of patients with STAS-present tumors was not significantly higher than for patients with STAS-absent tumors (5-year CIR, 15% vs. 15%; p = 0.847). C, CIR for locoregional recurrence of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 13% vs. 7%; p = 0.028). D, CID for lung cancer-specific death of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors (5-year CIR, 14% vs. 9%; p = 0.027). STAS, spread through air spaces; CIR, cumulative incidence of recurrence. Cumulative incidence of death.
CID by STAS in Stage I Group. CID for lung cancer-specific death of patients with STAS-present tumors was significantly higher than for patients with STAS-absent tumors with 10 years follow up. CID: Cumulative incidence of death.