Abstract
Considerable evidence indicates that glucocorticoid hormones enhance the consolidation of memory for emotionally arousing events through interactions with the noradrenergic system of the basolateral complex of the amygdala (BLA). We previously reported that intra-BLA administration of a β-adrenoceptor agonist immediately after inhibitory avoidance training enhanced memory consolidation and increased hippocampal expression of the protein product of the immediate early gene activity-regulated cytoskeletal-associated protein (Arc). In the present experiments corticosterone (3 mg/kg i.p.) was administered to male Sprague-Dawley rats immediately after inhibitory avoidance training to examine effects on long-term memory, amygdala norepinephrine levels, and hippocampal Arc expression. Corticosterone increased amygdala norepinephrine levels 15 min after inhibitory avoidance training, as assessed by in vivo microdialysis, and enhanced memory tested at 48 h. Corticosterone treatment also increased expression of Arc protein in hippocampal synaptic tissue. The elevation in BLA norepinephrine appears to participate in corticosterone-influenced modulation of hippocampal Arc expression as intra-BLA blockade of β-adrenoceptors with propranolol (0.5 μg/ 0.2 μL) attenuated the corticosterone-induced synaptic Arc expression in the hippocampus. These finding indicate that noradrenergic activity at BLA β-adrenoceptors is involved in corticosterone-induced enhancement of memory consolidation and expression of the synaptic-plasticity-related protein Arc in the hippocampus.
Keywords: BLA, fear conditioning, local translation, noradrenaline, noradrenergic, avoidance learning, stress, immediate early gene, memory consolidation, synaptic plasticity
Introduction
Events associated with emotional arousal are typically well remembered. Such memory enhancement involves the release of the adrenal stress hormones epinephrine and glucocorticoids (Cahill & Alkire, 2003; McGaugh, 2004; Roozendaal, Quirarte, & McGaugh, 1997; Roozendaal, McEwen, & Chattarji, 2009; Van Stegeren, Wolf, Everaerd, Scheltens, Barkhof, & Rombouts, 2007). Systemic administration of either epinephrine or the glucocorticoid corticosterone enhances memory consolidation when given immediately after training on a variety of emotionally arousing learning tasks, including inhibitory avoidance, spatial water maze, conditioned taste aversion and object recognition training (Gold & vanBuskirk, 1975; Miranda, Quirarte, Rodriguez-Garcia, McGaugh, & Roozendaal, 2008; Okuda, Roozendaal, & McGaugh, 2004; Roozendaal, Portillo-Marquez, & McGaugh, 1996; Roozendaal, Hahn, Nathan, de Quervain, & McGaugh, 2004). Extensive evidence that blockade of β-adrenoceptors in the basolateral complex of the amygdala (BLA) prevents the memory-enhancing effects of peripherally administered stress hormones, indicates that noradrenergic activity in the BLA is critically involved in enabling stress hormone effects on memory consolidation (Liang, Juler, & McGaugh, 1986; Quirarte, Roozendaal & McGaugh, 1997; Roozendaal et al., 2009). There is also considerable evidence that posttraining infusions of norepinephrine or β-adrenoceptor agonists into the BLA enhance memory consolidation (Ferry & McGaugh, 1999; Gallagher, Kapp, Musty, & Driscoll, 1977; Hatfield & McGaugh, 1999; LaLumiere, Buen, & McGaugh, 2003; McIntyre, Miyashita, Setlow, Marjon, Steward, Guzowski, & McGaugh, 2005; Miranda, La Lumiere, Buen, Bermudez-Rattoni, & McGaugh, 2003; Roozendaal, Castello, Vedana, Barsegyan, & McGaugh, 2008). Moreover, inhibitory avoidance training increases norepinephrine levels in the BLA and the increase is positively correlated with subsequent memory of the training (McIntyre, Hatfield, & McGaugh, 2002). Systemically administered epinephrine also increases norepinephrine levels in the brain (Gold & van Buskirk, 1978) including the amygdala (Williams, Men, Clayton, & Gold, 1998). Although prior studies reported that glucocorticoids interact with the noradrenergic system of the BLA to influence memory consolidation, it is not known whether corticosterone influences norepinephrine levels in the BLA.
Stress hormone and training-induced amygdala activation modulates synaptic plasticity in brain areas engaged at the time of memory consolidation (McGaugh, McIntyre and Power, 2002). Noradrenergic activity in the BLA appears to be necessary for mediating stress effects on other brain regions involved in memory, as blockade of β-adrenoceptors in the BLA prevents inhibitory avoidance memory enhancement induced by a glucocorticoid receptor (GR) agonist administered directly into the hippocampus (Roozendaal, Nguyen, Power, & McGaugh, 1999). Similarly, both GR and β-adrenoceptor activation in the BLA influence hippocampal dentate gyrus long-term potentiation (LTP) (Akirav & Richter-Levin, 2002; Ikegaya, Nakanishi, Saito & Abe, 1997; Vouimba, Yaniv, & Richter-Levin, 2007). We previously reported that stimulation of β-adrenoceptors within the BLA after inhibitory avoidance training enhances memory and increases the expression of activity-regulated cytoskeletal-associated (Arc) protein levels in the dorsal hippocampus without influencing Arc mRNA levels (McIntyre et al., 2005). This finding suggests that amygdala modulation of Arc protein and synaptic plasticity in the hippocampus occurs at a posttranscriptional level.
Hippocampal expression of Arc is known to play a role in the maintenance of long-term plasticity and memory. Inhibition of Arc protein expression with infusions of antisense oligodeoxynucleotides (AS ODNs) into the dorsal hippocampus impairs the maintenance of LTP without affecting its induction (Guzowski, Lyford, Stevenson, Houston, McGaugh, Worley, & Barnes, 2000; Messaoudi, Kanhema, Soule, Tiron, Dagyte, da Silva, & Bramham, 2007). Similarly, intra-hippocampal infusions of AS ODNs impair long-term, but not short-term memory for spatial water-maze and inhibitory avoidance tasks (Guzowski et al., 2000; McIntyre et al., 2005). These findings are consistent with evidence of impaired long-term memory and abnormal synaptic plasticity in transgenic mice lacking the Arc gene (Plath, Ohana, Dammermann, Errington, Schmitz, Gross, Mao, Engelsberg, Mahike, Welzi, Kobalz, Stawrakakis, Fernandez, Walteriet, Bick-Sander, Therstappen, Cooke, Blanquet, Wurst, Salmen, Bosl, Lipp, Grant, Bliss, Wolfer, & Kuhl, 2006). The evidence that Arc is translated in synapses in vitro (Bloomer et al., 2008; Sanders et al, 2008; Waung et al, 2008; Yin et al, 2002), suggests that the influence of emotional arousal and stress hormones on memory consolidation involves an amygdala-mediated influence on local translation of synaptic proteins, such as Arc, in hippocampal synapses.
Here, we examined glucocorticoid effects on amygdala norepinephrine levels and hippocampal Arc expression in regulating the consolidation of memory of inhibitory avoidance training. To investigate the effect of corticosterone on amygdala norepinephrine levels, rats were given systemic injections of a memory-enhancing dose of corticosterone immediately after training and norepinephrine levels in the amygdala were measured with in vivo microdialysis and high-performance liquid chromatography (HPLC). Western blot analysis and immunohistochemistry were used to determine whether memory-enhancing corticosterone treatment affects the expression of hippocampal Arc protein. The role of amygdala norepinephrine in mediating corticosterone-induced changes in hippocampal Arc expression was examined by blocking β-adrenoceptors with infusions of propranolol into the BLA immediately following post-training corticosterone treatment.
Materials and Methods
Subjects
Two-hundred-and-seventy-nine male Sprague-Dawley rats (250-275 g upon arrival), obtained from Charles River Breeding Laboratories (Wilmington, MA), were housed individually in a temperature-controlled (22° C) colony room, with food and water available ad libitum. Animals were maintained on a 12 h light – 12 h dark cycle (7:00 – 19:00 h, lights on) and kept in the animal colony for one week before commencement of surgical or behavioral procedures. All experimental procedures were in compliance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee (University of California, Irvine and University of Texas at Dallas),
Surgery
Rats used in the microdialysis experiment were anesthetized with sodium pentobarbital (50 mg/kg, i.p.), given atropine sulfate (0.4 mg/kg, i.p.) to maintain respiration, and a unilateral plastic guide cannula (CMA 12, Carnegie Medicin, North Chelmsford, MA, USA) for a microdialysis probe was implanted 1 mm above the left BLA [coordinates: anteroposterior (AP), -2.8 mm from Bregma; mediolateral (ML), +4.8 mm from midline; dorsoventral (DV), -6.6 mm below skull surface; incisor bar, -3.3 mm from interaural line (Paxinos & Watson, 2005)]. Animals for the intra-BLA infusion experiment were anesthetized with isoflurane (1% in O2) (Western Medical Supply) and given atropine sulfate. The skull was positioned in a stereotaxic frame (Stoelting, Wood Dale, Il) and two 15-mm-long guide cannulae (23 gauge; Small Parts, Miramar, Fl) were implanted bilaterally with the tips 2 mm above the BLA (AP, -2.7 mm; ML, ± 5.2 mm; DV, -6.4 mm). The guide cannulae were fixed in place with acrylic dental cement and two small anchoring screws. Stylets (15-mm long insect dissection pins) were inserted into each cannula to maintain patency. After surgery, rats were given 2.0 mL of saline to facilitate clearance of the drugs. Rats were allowed to recover for a minimum of 7 days before training.
Inhibitory Avoidance
In order to habituate rats to the experimental procedures, they were handled for 2 min per day for five consecutive days before training. They were then trained on an inhibitory avoidance task. The inhibitory avoidance apparatus consisted of a trough-shaped alley (91 cm long, 15 cm deep, 20 cm wide at the top and 6.4 cm wide at the floor) that was divided into two compartments, separated by a manually controlled sliding door that opened by retracting into the floor. The starting compartment (31 cm long) was white and illuminated, whereas the shock compartment (60 cm long) was made of two dark electrifiable metal plates and was not illuminated. The rats were placed in the light “safe” compartment and allowed to cross to the dark “shock” compartment. After a rat stepped completely into the dark compartment, the sliding door was closed and a single inescapable footshock (0.32 mA, 1 s) was delivered. The rat was removed from the dark compartment 15 s later and, after drug treatment, returned to the home cage. Some animals received a retention test 48 h after training. During the retention test, rats were returned to the light compartment of the inhibitory avoidance apparatus and the latency to reenter the dark compartment with all four paws (maximum latency 600 s) was measured. Memory of the training experience was inferred from longer crossing latencies on the retention test. No shock or drug was delivered during retention testing. Other rats were sacrificed, either 15 min, 30 min, 45 min, or 1 h after training and drug treatment, and brains were used for analysis of Arc protein expression.
Microdialysis and HPLC
Norepinephrine levels in the amygdala were measured with in vivo microdialysis combined with high-performance liquid chromatography (HPLC) with coulometric detection (ESA Coulochem II, Chelmsford, MA, USA). A dialysis probe (CMA 12, 1-mm membrane tip; Carnegie Medicin, North Chelmsford, MA, USA) was inserted through the guide cannula at least 2 h before baseline samples were collected. Rats were allowed to move around freely in a familiar Plexiglas holding cage with bedding covering the floor during the collection of 4 baseline samples. Artificial cerebrospinal fluid (in mM: NaCl, 128; KCl, 2.5; CaCl2, 1.3; MgCl2, 0.998; Na2HPO4, 1.3; and glucose, 1.0, brought to pH 6.5 with NaOH) was perfused through 2 m of PEEK tubing (CMA) and the probe at a rate of 1 μL/min. The probe recovery rate was determined in vitro by insertion of the microdialysis probe into a standard solution of norepinephrine. Both standard solution and dialysate were injected into the HPLC to determine the percentage of norepinephrine taken up by the probe. Samples collected from the microdialysis probe were injected automatically through HPLC equipment using an on-line injector (CMA/160) and were analyzed every 15 min. The HPLC system was optimized for detection of norepinephrine (E1 = ±100 mV, E2 = 190 mV, range 10 nA). The mobil phase [ESA Inc. MDTM consisting of 75 mM monobasic sodium dihydrogen phosphate, 1.7 mM 1-octanesulfonic acid sodium salt, 200 mL triethylamine (TEA), 25 mM EDTA and 10% acetonitrile in 200 mL of distilled, deionized water] was pumped (ESA/580) through the HPLC tubing at a flow rate of 0.5 mL/min.
After four stable baseline samples were collected, rats were taken from the holding cage and trained on the inhibitory avoidance task (footshock: 0.32 mA, 1 s). Immediately after training, rats were given a systemic injection of either vehicle or corticosterone (3 mg/kg, i.p.) and were then returned to their holding cages. Some rats were given injections of vehicle or corticosterone (3 mg/kg, i.p.) in the absence of training. Microdialysis sampling continued while animals were trained on the inhibitory avoidance task and for 2 h following the training procedure.
Drug treatment
The adrenocortical hormone corticosterone (3 mg/kg, i.p.; Sigma-Aldrich) or vehicle was injected immediately after inhibitory avoidance training in a volume of 2 mL/kg. Corticosterone was first dissolved in 100% ethanol and then diluted in 0.9% saline to reach its final concentration. The final concentration of ethanol was 5%. The vehicle solution contained 5% ethanol in saline only. This dose was selected on the basis of previous experiments (de Quervain, Roozendaal, & McGaugh, 1998; Hui, Figueroa, Poytress, Roozendaal, McGaugh & Weinberger, 2004; Roozendaal, Okuda, Van der Zee, & McGaugh, 2006)
In some rats, the β-adrenoceptor antagonist DL-propranolol (0.5 μg per 0.2 μL; Sigma-Aldrich) or vehicle was infused into the BLA after training and corticosterone injection. Propranolol was dissolved in a vehicle of 0.9% saline. The infusion needles were 25 gauge microinfusion needles that were attached to 10-μL Hamilton microsyringes by polyethylene tubing (PE-20). The drug solution was backfilled into the needle and the infusion was driven by a minipump (Harvard Instruments). The injection needle protruded 2.0 mm beyond the cannula tip and a 0.2-μL injection volume was infused over the course of 32 s. Propranolol or vehicle were infused unilaterally into either the left or right BLA (and vehicle in the other hemisphere; Fig. 5A). Drug and vehicle infusions were counterbalanced across hemispheres in a randomized fashion.
Figure 5.
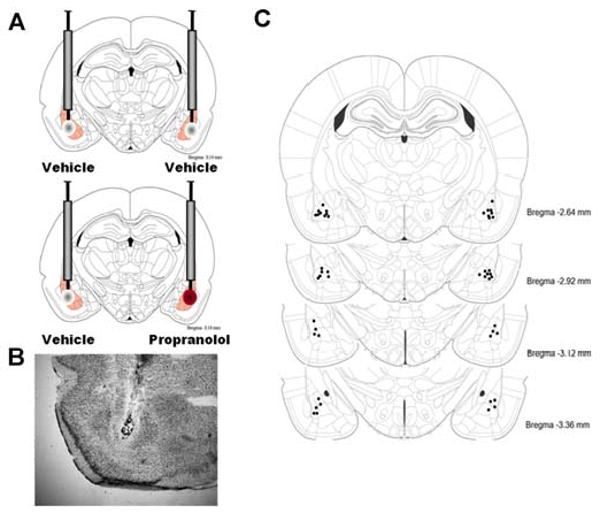
Intra-basolateral amygdala infusions of propranolol or vehicle. A. Rats were infused with vehicle into the BLA of one hemisphere and propranolol into the BLA of the other hemisphere immediately after inhibitory avoidance training. The side of the vehicle/drug infusion was counter-balanced. B. Representative location of cannulae and needle tips within the BLA C. Injection needle tips in the BLA of all rats included in the experiment. Adapted from Paxinos & Watson (2005).
Tissue Preparation
Rats were deeply anesthetized with isoflurane (Western Medical Supply) 15 min, 30 min, 45 min, or 1 h after training and drug treatment and brains were rapidly removed and flash frozen by submersion for 2 min in a beaker filled with 2-methylbutane sitting in a dry-ice ethanol bath. Cage control animals given no training were processed identically. The brains from rats given intra-BLA infusions were cut horizontally just above the rhinal fissure and several 40-μm thick sections were taken with a cryostat, mounted on glass slides and stained with thionin. Brain sections were analyzed under a light microscope to identify the location of infusion. Only brains with needle tracks terminating in the BLA were used for analysis. For Arc comparisons, a cryostat was used to make three 500-μm-thick coronal sections from frozen brain tissue at the level of the hippocampus (-2.3 to -4.0 mm from Bregma) and tissue punches were taken from the Ammon's horn and dentate gyrus regions of the dorsal hippocampus using a tissue punch kit (1.22 mm in diameter), and pooled into one sample with each hemisphere collected separately. The tissue punches were stored at -80° C for later Western blot analysis. For immunohistochemistry, a cryostat was used to take eight 20-μm thick sections of frozen brain tissue at the level of the dorsal hippocampus in between the 500-μm-thick sections, for a total of 24 sections, and mounted on Superfrost (Fisher Scientific) glass slides so that a brain from each treatment group was on each slide with its position on the slide counterbalanced between treatment groups.
Synaptoneurosome Preparation
The tissue punches from one hemisphere were homogenized with a nylon pestle, 16 strokes, in 70 μL homogenization buffer solution [in mM: NaCl, 124; KCl, 5; CaCl2·2 H2O, 0.1; MgCl2·6 H2O, 3.2; NaHCO3, 26; glucose, 10; pH 7.4, containing 20% protease inhibitor cocktail (Sigma), and 10% protease inhibitor cocktail II (Sigma)]. After homogenization, the total volume was brought to 500 μL with the homogenization buffer. The homogenate was backfilled into a 1 mL syringe then filtered through 3 layers of 100 micron nylon mesh (Small Parts) inside a 13 mm syringe filter holder (Pall Life Sciences). The filtered solution was backfilled again into a 1 mL syringe and filtered through a 5-μm pore nitrocellulose filter (Sigma-Aldrich) inside a 13 mm syringe filter holder. The final filtered solution was centrifuged at 10,000 × g for 10 min at 4° C. The supernatant was removed and the pellet was resuspended in 50 μL cold homogenization buffer.
Protein assay and Immunoblotting
For whole dorsal hippocampal homogenate fractions, the frozen tissue punches were sonicated in a buffer containing 0.1 M phosphate buffer, pH 7.4 [containing 10% glycerol, 20% protease inhibitor cocktail (Sigma), and 10% protease inhibitor cocktail II (Sigma)]. Total protein concentrations from both whole hippocampal homogenate and synaptoneurosome fractions were determined using a Qubit fluorometer and Qubit protein assay kit (Invitrogen). Homogenate and synaptoneurosome fractions containing approximately 15 μg total protein were heated in a sample buffer with a reducing agent (Invitrogen), loaded and then run on 4-12% Bis-Tris MIDI gels (Invitrogen). Tissue from each condition was loaded onto each gel. The gels were then electroblotted to a nitrocellulose membrane using an iBlot dry-blotting system (Invitrogen). Membranes were then washed in Tris-buffered saline (TBS: 150 mM NaCl/100 mM Tris base, pH 7.5) and incubated with primary antibodies diluted in blocking solution (5% Carnation nonfat dry milk in TBS-Tween) overnight at 4°C. The primary antibodies were anti-Arc (rabbit polyclonal; 1:5000, Synaptic Systems) and anti-actin (rabbit; 1:3000, Sigma). To verify the separation of synapses from cell somas in the synaptoneurosome preparation, blots were also probed with the primary antibody for the nuclear protein, anti-acetyl-H3 (rabbit; 1:3000, Millipore). To measure the efficiency of the synaptoneurosome procedure, blots were also probed with the primary antibody for the synaptic protein, anti-PSD-95 (rabbit; 1:500; Pierce) in order to determine the amount of synaptic enrichment. On the next day, the membranes were incubated with a secondary HRP-linked antibody (goat anti-rabbit; 1:4000, Millipore) for 1 h. Immunoreactivity was detected using chemiluminescence (ECL Western Blot Kit; Pierce). Invitrogen markers were run on all gels to determine the relative mobility of the immunoreactive bands. For densitometric quantification of these results, the films were scanned and converted into TIF files for analysis with NIH Image J software.
Immunohistochemistry
The brain slices were fixed in 2% paraformaldehyde, washed in TBS, and quenched in 2% hydrogen peroxide. They were then blocked in NEN-PerkinElmer blocking agent and incubated overnight with Arc primary antibody (1:2000, Synaptic Systems). The next day, the slides were incubated with the secondary HRP-conjugated antibody (goat anti-rabbit, 1:3000, Millipore). The antibodies were detected with a cyanine-3 substrate kit (CY3 Direct FISH; PerkinElmer Life Sciences) and counterstained with DAPI (Molecular Probes, 1:500) as a nuclear stain. Images were acquired on a Zeiss Axio Imager fluorescence microscope.
Statistical Analysis
Inhibitory avoidance retention latencies were analyzed with two-sample t-tests to make pair-wise comparisons between the vehicle-injected and corticosterone-injected groups. Norepinephrine levels in the microdialysis samples were normalized by comparing each sample to the individual rat's average level of norepinephrine in the four samples taken before training (baseline). Differences in the mean percentage of baseline norepinephrine between and within groups were analyzed using a repeated-measures ANOVA with Fischer's post-hoc tests. A Pearson correlation test was used to evaluate the relationship between norepinephrine values during training and retention latencies in individual rats. For Western blot densitometry results, Arc was normalized to actin and this ratio was then normalized to that from a cage control sample run on the same gel to account for film variation. Finally, those ratios were expressed as a percentage of the corresponding vehicle control group. For rats receiving BLA infusions, the western blot densitometry results were expressed as a ratio of Arc to actin and were then expressed as a ratio of the drug-infused hemisphere to the vehicle-infused hemisphere (or a ratio of the left hemisphere to the right hemisphere if the rat received a bilateral vehicle infusion). Finally, those ratios were expressed as a percentage of the bilateral vehicle control group. The percentages were then compared using a Student's t-test to make pair-wise comparisons between drug groups. A probability level of p<.05 was considered significant. Data are presented as mean ± SEM.
Results
Immediate post-training systemic injections of corticosterone enhance inhibitory avoidance retention latencies and increase training-induced norepinephrine levels in the BLA
Systemic injections of vehicle or corticosterone (3 mg/kg, i.p.) were given immediately after inhibitory avoidance training. Training latencies did not differ between the two treatment groups: 11.9 s ± 3.0 s for the vehicle group and 20 s ± 5.9 s for the corticosterone (t(15)=-1.09; p=.30). A two sample t-test for 48-h retention latencies revealed a significant drug effect (t(15)=-3.44; p<.01). Mean retention latencies of the vehicle-treated group (n= 7) were 29.6 ± 7.1 s and those of the corticosterone group (n= 10) were 312.6 ± 68.0 s (Fig. 1).
Figure 1.
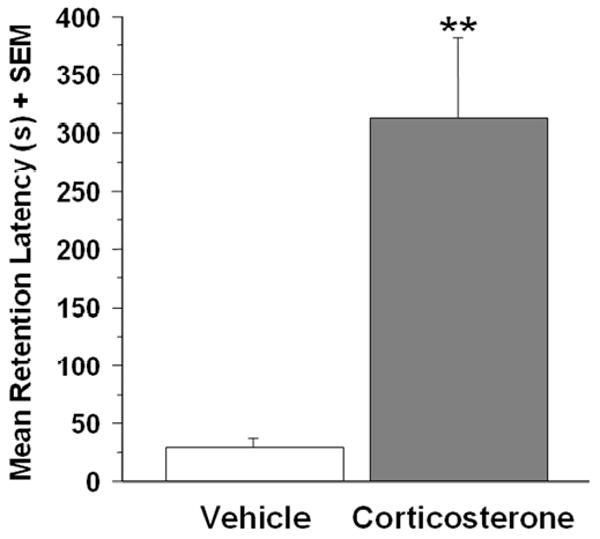
Effect of immediate posttraining corticosterone (3 mg/kg, i.p.) treatment on 48-h inhibitory avoidance memory retention latencies. Corticosterone-treated rats (n=10) had significantly longer retention latencies than did the vehicle treated rats (n=7;**p<.01). Results represent mean + SEM for latency in seconds.
To examine whether post-training corticosterone administration increased norepinephrine levels in the BLA, microdialysis was performed in some of the vehicle- and corticosterone-treated rats used in the behavioral experiment above. Norepinephrine levels were measured during and after inhibitory avoidance training and corticosterone injection (Fig. 2A). A repeated-measures ANOVA for norepinephrine levels (percentage of baseline) in 15-min samples taken from rats trained on the inhibitory avoidance task revealed a significant drug effect (F1,10=2.72;p<.01). Fisher's post-hoc tests indicated that norepinephrine levels in the corticosterone (n=6) group were significantly higher than those of vehicle-treated (n=5) animals in the first 15-min sample following training (p<.001) and were positively correlated with 48-h retention latencies (r=0.8; p<.01). No significant differences in norepinephrine levels were observed between vehicle (n=4) or corticosterone (n=4) treated animals in the absence of inhibitory avoidance training (F1,7=1.24;p=.27; Fig. 2B).
Figure 2.
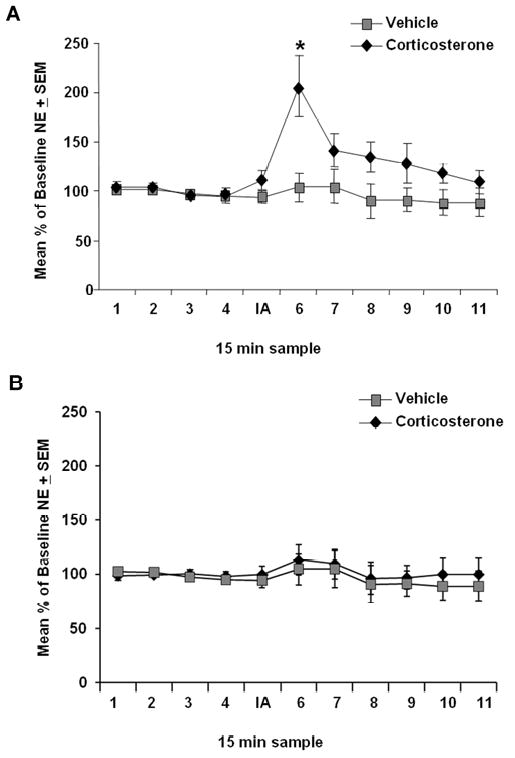
Effect of immediate post-training corticosterone treatment on norepinephrine (NE) levels in the BLA. Microdialysis samples were collected every 15 minutes. Norepinephrine levels (mean ± SEM) are expressed as a percentage change from average baseline levels. (A) Corticosterone treatment (n=6; 3 mg/kg, i.p.) significantly increased norepinephrine release in the amygdala of animals trained on an inhibitory avoidance task compared with vehicle-injected animals (n=5; *p<.05). (B) No difference was observed in corticosterone- or vehicle-injected animals (n=4; n=4 respectively) that were not trained on the task
Systemic corticosterone administration after inhibitory avoidance training increases Arc protein expression in hippocampal synaptic fractions
Hippocampal Arc protein expression has been shown to peak in the hippocampus one hour after spatial exploration in a novel context (Ramirez-Amaya, Vazdarjanova, Mikhael, Rosi, Worley, & Barnes, 2005). To determine the effect of corticosterone administration after inhibitory avoidance training on synaptic Arc protein expression, separate groups of rats were trained on the inhibitory avoidance task and sacrificed 15 min, 30 min, 45 min, or 1 h after immediate post-training injections of vehicle or corticosterone (3 mg/kg). Figure 3 shows Western blot data expressing Arc protein levels in synaptoneurosome fractions from the dorsal hippocampus for the different time points. A two sample t-test did not reveal a significant difference in Arc protein expression between the vehicle- and corticosterone-injected animals at 15 min (vehicle, n=7, corticosterone, n=8; t(13)=-.98; p=.35) or at 30 min (vehicle, n=6, corticosterone, n=8; t(12)=.17; p=.87) after training and injections. However, a two sample t-test did reveal a significant difference in Arc protein expression between the vehicle- and corticosterone-injected animals at 45 min (vehicle, n=4, corticosterone, n=7; t(9)=-2.28; p<.05) and 1 h after training (vehicle, n=7, corticosterone, n=8; t(13) = -2.30; p<.05). Therefore, all subsequent experiments examined Arc protein expression in the hippocampus 1 h after training and injections. Increased Arc protein immunoreactivity was observed in dorsal hippocampal slices taken from corticosterone-injected rats as compared to vehicle-injected animals (Fig. 4A). Although there was a significant corticosterone-induced increase in Arc protein expression in synaptoneurosome fractions, a two sample t-test did not reveal a significant difference in Arc expression in whole dorsal hippocampus homogenate from the vehicle and corticosterone-injected animals (vehicle, n=18, corticosterone, n=22) (t(38) = -1.53;p=.14). Figure 4B and 4D shows Western blot data expressing Arc protein levels in the hippocampus for synaptoneurosome fractions collected 1 h after training. There was a significant effect of corticosterone on Arc protein expression when paired with training, but corticosterone administered to non-trained rats did not increase Arc expression above the levels seen in the vehicle-injected group in synaptoneurosome fractions (vehicle, n=7, corticosterone, n=8; t(13) = 0.09;p=.93; Fig. 4C). The nuclear protein acetyl-H3 was present in the whole homogenate, but not in synaptoneurosomes, indicating that there was no nuclear contamination of the synaptic fractions (Fig. 4E). Moreover, there was a 5-fold enrichment of the synaptic protein, PSD-95, in the synaptoneurosome fractions compared to the whole dorsal hippocampus homogenate (Fig. 4F).
Figure 3.
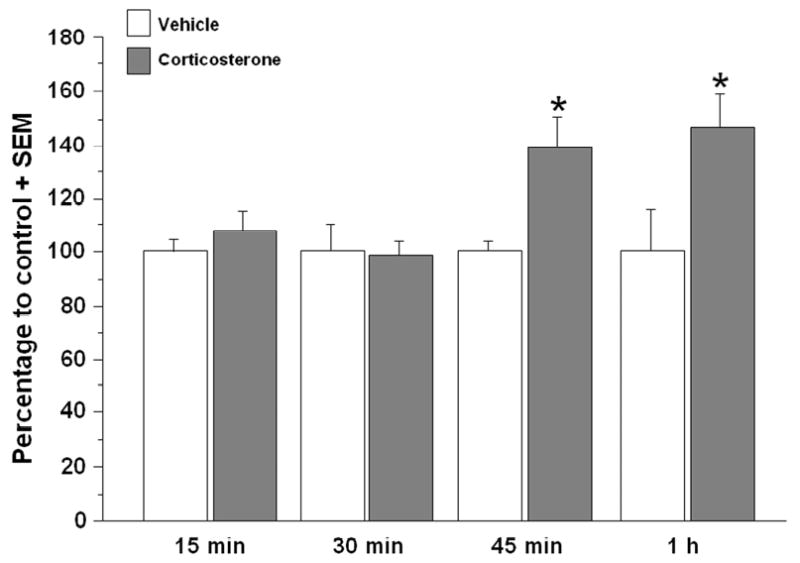
Western blot analysis at different time points following training for the effect of immediate post-training systemic corticosterone on Arc protein expression in synaptoneurosome fractions of the dorsal hippocampus. Memory-enhancing corticosterone (3 mg/kg) does not appear to increase Arc protein expression at 15 min or 30 min after training and injection. There is a significant increase with corticosterone injections in Arc protein expression at 45 min, and a slightly larger increase at 1 h after training and injection (*p<.05). Densitometry values for Arc are normalized to actin and to cage control and then presented as a percentage of the control vehicle group. Data are presented as mean + SEM.
Figure 4.
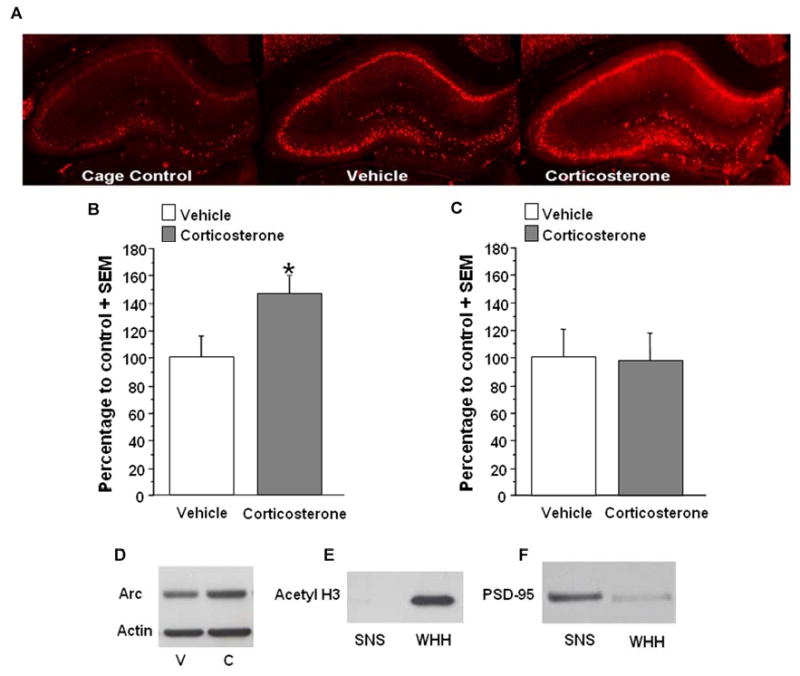
A. Immunohistochemical analysis of the effect of systemic corticosterone on Arc protein in the hippocampus of animals trained on an inhibitory avoidance task. Memory-enhancing injections of corticosterone (3 mg/kg, i.p.) appear to increase Arc immunoreactivity in the dorsal hippocampus, particularly in the dendrites of the CA1 region and dentate gyrus. B. Western blot quantification of corticosterone effect on Arc protein expression in synaptoneurosome fractions of the hippocampus. In animals trained on an inhibitory avoidance task, memory-enhancing injections of corticosterone (3 mg/kg, i.p.) significantly increase Arc expression in synaptoneurosome fractions (corticosterone, n=8; vehicle, n=7; *p<.05) C. Corticosterone treatment (3 mg/kg) did not significantly affect Arc protein expression in synaptoneurosome fractions (corticosterone, n=8; vehicle, n=7) taken from animals that were not trained. D. Western blot from synaptoneurosome fractions of rats receiving either vehicle (V) or corticosterone (C) injections. E. Western blot for acetyl H3. Acetyl H3 is present in the whole hippocampal homogenate (WHH) but not in synaptoneurosome fractions (SNS). F. Western blot for PSD-95. There is a 5-fold enrichment of PSD-95 in synaptoneurosome (SNS) fractions compared to whole hippocampal homogenate (WHH). Densitometry values for Arc are normalized to actin and to cage control and then presented as a percentage of the control vehicle group. Data are presented as mean + SEM.
Noradrenergic blockade in the BLA attenuates the corticosterone-induced hippocampal Arc protein expression
There is extensive evidence that glucocorticoid effects on memory consolidation enhancement require noradrenergic activity within the BLA (Ferry and McGaugh, 2000; Quirarte et al. 1997; Roozendaal, Quirarte, & McGaugh, 2002; Roozendaal et al., 2006a; Roozendaal, Okuda, de Quervain, & McGaugh, 2006). Moreover, noradrenergic activity in the BLA is required to mediate inhibitory avoidance memory enhancement induced by direct infusions of a GR agonist into the hippocampus (Roozendaal et al., 1999a). To examine whether the corticosterone-induced increase in hippocampal synaptic Arc expression after inhibitory avoidance requires concurrent noradrenergic activity in the BLA, the β-adrenoceptor antagonist propranolol (0.5 μg in 0.2 μL) or vehicle were infused unilaterally into either the left or right BLA (and vehicle into the other hemisphere), immediately after inhibitory avoidance training and systemic administration of corticosterone (3 mg/kg) (Fig. 5A-C). Arc protein expression was reduced in synaptoneurosome fractions taken from the dorsal hippocampus that was ipsilateral to the intra-BLA propranolol infusion site. The percentage of Arc protein levels of rats given injections of corticosterone followed by intra-BLA infusions of propranolol was significantly lower than that of rats given injections of corticosterone followed by bilateral vehicle infusions (propranolol/vehicle, n=10, vehicle/vehicle, n=7; t(15) = 2.57;p<.05; Fig. 6A and B).
Figure 6.
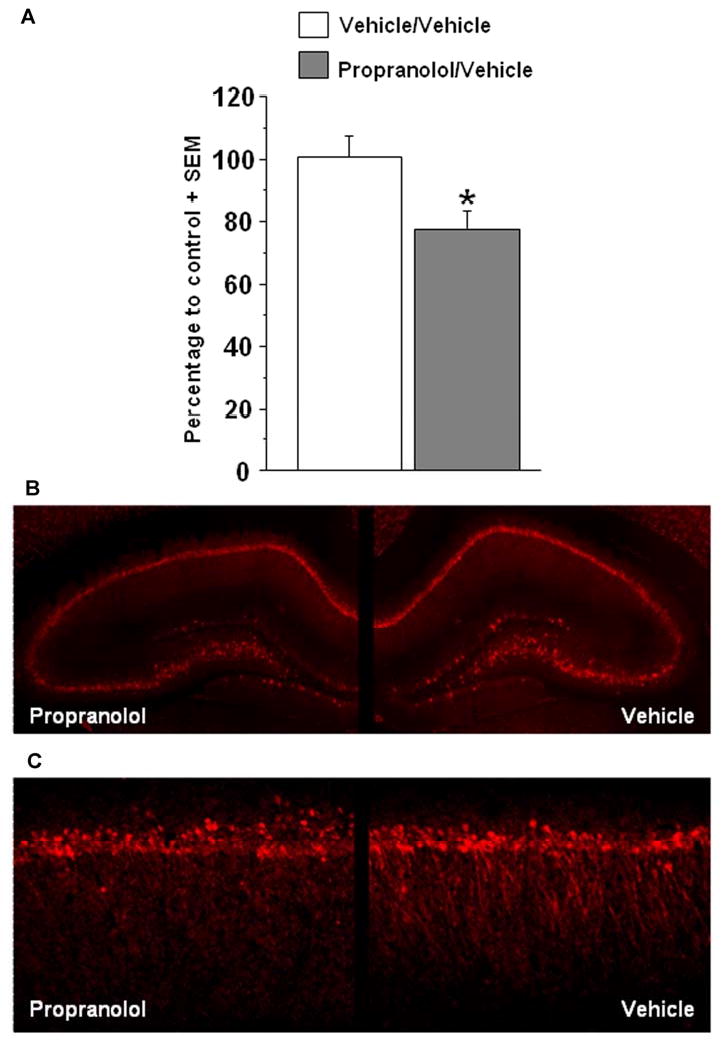
Effect of post-training intra-BLA infusions of propranolol (0.5 μg/ 0.2 μL) on corticosterone-induced Arc expression in hippocampal synaptoneurosome fractions. A. Western blot densitometry values for Arc were normalized to actin, expressed as a ratio of the drug-infused hemisphere to the vehicle-infused hemisphere (or ratio of left-right hemisphere for bilateral, vehicle-infused rats), and are finally expressed as a percentage of the bilateral control group. For propranolol-treated rats (n=10), the percentage of Arc expression was significantly lower in hippocampal synaptoneurosome fractions than in synaptoneurosome fractions taken from bilateral vehicle-treated rats (n=7; *p<.05). B. In the top panel, immunohistochemistry illustrates a moderate reduction in Arc staining in the hippocampus ipsilateral to the intra-BLA propranolol infusion. Here, the reduction appears to be greater in the dentate gyrus and CA3 regions, however this pattern was not reliably observed. C. 20× magnification of the CA1 region of the dorsal hippocampus reveals a reduction in Arc staining in the dendritic regions of the hippocampus ipsilateral to the intra-BLA propranolol infusion. Data are presented as mean + SEM.
Discussion
The major findings of the present experiments are that a memory-enhancing dose of corticosterone administered systemically after inhibitory avoidance training, 1) increased norepinephrine levels in the amygdala and, 2) increased the expression of the plasticity-associated protein Arc in hippocampal synapses. Additionally, intra-BLA infusions of the β-adrenoceptor antagonist propranolol attenuated the corticosterone effect on hippocampal Arc expression when administered at a dose that blocks corticosterone-induced enhancement of memory. These results support the hypothesis that glucocorticoids interact with noradrenergic mechanisms in the BLA to influence hippocampal synaptic plasticity and memory consolidation.
The present microdialysis data are consistent with previous evidence that norepinephrine release in the BLA is increased following inhibitory avoidance training. Inhibitory avoidance training with a relatively high footshock produces a 200-300 percent increase in norepinephrine (Hatfield, Spanis, & McGaugh, 1999; McIntyre et al, 2002; Quirarte, Galvez, Roozendaal, & McGaugh, 1998). In the current experiments, a lower footshock intensity was used so that the control animals would not demonstrate either evidence of long-term memory or any substantial increase in BLA norepinephrine release. However, importantly, the training paired with injections of corticosterone resulted in a 200 percent increase in norepinephrine levels as well as a robust enhancement of inhibitory avoidance memory. In fact, the increase in amygdala norepinephrine during the 15 min after training was significantly correlated with 48 h retention latency. It is possible that the corticosterone injection increased the synthesis or release of norepinephrine by binding to glucocorticoid receptors (GR) in the nucleus of the solitary tract (NTS), locus coeruleus or amygdala. In support of the notion that GR activation enhances the synthesis and/or release of norepinephrine, intra-BLA infusions of a β-adrenoceptor antagonist block the memory-enhancing effects of an intra-NTS GR agonist infusion (Roozendaal, Williams, & McGaugh, 1999). However, in another report, administration of corticosterone following training on an object recognition task did not alter phosphorylated tyrosine hydroxylase immunoreactivity in the BLA (Roozendaal et al, 2006a), suggesting that corticosterone may not enhance memory consolidation through the modulation of local norepinephrine synthesis in the BLA. Thus, the observed elevation may instead be due to an effect of corticosterone on the reuptake of norepinephrine, such as that observed in the medial prefrontal cortex (Grundemann, Schechinger, Rappold, & Schomig., 1999).
Our previous research demonstrated that immediate post-training intra-BLA infusions of clenbuterol, a β-adrenoceptor agonist, modulate hippocampal Arc expression (McIntyre et al., 2005). The present finding that memory-enhancing corticosterone treatment increases norepinephrine levels in the amygdala suggests that corticosterone may influence hippocampal Arc expression through stimulation of β-adrenoceptors in the BLA. However, there is also a high density of GRs in the hippocampus and it is known that both stress and corticosterone influence hippocampal synaptic plasticity (Diamond, Park, & Woodsen, 2002; Korz & Frey, 2003; Pu, Krugers, & Jöels, 2007; Yamada, McEwen, & Pavlides, 2003). To determine whether activation of BLA β-adrenoceptors is involved in corticosterone-enhanced hippocampal Arc expression, we administered the memory-enhancing dose of corticosterone together with intra-BLA infusions of vehicle or the β-adrenoceptor antagonist propranolol immediately after inhibitory avoidance training. The dose of propranolol used in the present experiments blocks the memory-enhancing effect of systemic administration of stress hormones, without otherwise impairing memory (Ferry, Roozendaal, & McGaugh, 1999; Quirarte et al, 1997; Roozendaal et al, 1999a; Roozendaal et al, 2004; Roozendaal et al, 2006a; Roozendaal et al, 2006b). In the present experiment each rat served as its own control: vehicle was infused into the BLA of one hemisphere and propranolol into the BLA of the other hemisphere, and Arc expression was compared in the dorsal hippocampus across the two hemispheres. This paradigm is useful in studies examining immediate early gene expression, as variability across animals may obscure subtle differences (McIntyre et al., 2005). Arc protein expression was consistently lower in synaptoneurosomes from the dorsal hippocampus ipsilateral to the BLA propranolol infusion than in the side ipsilateral to the vehicle infusion. The percentage of Arc expression to the control was significantly lower in rats that received intra-BLA infusions of propranolol than rats that received infusions of vehicle. These findings suggest that stimulation of β-adrenoceptors in the amygdala is required for the full effect of corticosterone on the expression of hippocampal synaptic-associated proteins and provide further support for a general role for the amygdala in the modulation of synaptic plasticity in efferent brain regions. Consistent with the view that arousal-induced β-adrenoceptor activation of the amygdala is required for hippocampal Arc expression, administration of corticosterone in the absence of inhibitory avoidance training did not enhance hippocampal Arc expression.
According to the time course of Arc expression in hippocampal synaptoneurosomes, protein levels peak at roughly the same time after training and corticosterone treatment as that seen in the whole hippocampus after exploration of a novel context (Ramirez-Amaya et al., 2005). No difference in hippocampal Arc expression was observed at the 15-min time point despite the elevation in amygdala norepinephrine levels at that time. This is consistent with our previous finding that clenbuterol infusions into the BLA increased hippocampal Arc levels 45 min later. The time required before measureable differences in Arc protein levels were detected suggests that newly synthesized proteins contributed to the observed elevation.
A great deal of attention has been paid to the transcription of Arc because it is dynamically regulated, and the transcript appears in stimulated dendrites (Lyford, Yamagata, Kaufman, Barnes, Sanders, Copeland, Gilbert, Jenkins, Lanahan, & Worley, 1995; Steward, Wallace, Lyford, & Worley, 1998). Training in many types of memory tasks increases Arc mRNA and protein levels in the hippocampus (Guzowski, Setlow, Wagner, & McGaugh, 2001; Huff, Frank, Wright-Hardesty, Sprunger, Matus-Amat, Higgins, & Rudy, 2006; Kelly & Deadwyler, 2002; McIntyre et al, 2005; Montag-Sallaz & Montag, 2003; Soule, Penke, Kanhema, Alme, Laroche, & Bramham, 2008). Substantial in vitro findings indicate that the Arc transcript in the postsynaptic density is translated locally and can be initiated by electrical stimulation or application of various neuromodulators (Bloomer et al., 2008; Sanders et al., 2008; Waung et al., 2008; Yin et al., 2002). Whereas research findings indicate a role for Arc transcription in vivo and synaptic translation in vitro, a role for local translation in vivo has not been established.
As Arc is translated in synapses in vitro, we hypothesize that the effect of emotional arousal and stress hormones on memory consolidation involves an amygdala-mediated influence on local translation of synaptic proteins, such as Arc, in hippocampal synapses. Whereas Arc expression appears to be greater throughout the dorsal hippocampus of corticosterone-treated rats vs. controls, a significant difference was measured only in tissue prepared for synaptoneurosome assay. The absence of a significant effect in the whole cell preparation may be due to variability across individual animals and “noise” within the whole dorsal hippocampus homogenates. However, the significant effect observed in tissue enriched with synapses indicates that corticosterone-induced differences in Arc expression are present at the synapse.
The findings presented here support the hypothesis that Arc translation can be regulated at hippocampal synapses in vivo. However a direct observation of local protein synthesis is not presently accessible in the intact brain of a behaving animal and, thus, alternative explanations such as stress-induced modulation of transport or turnover of Arc protein cannot be excluded. It is unlikely that Arc is the only plasticity-related protein to be modulated by stress hormones. We previously reported that stimulation of BLA β-adrenoceptors did not affect hippocampal cFos expression, suggesting that the BLA modulation of hippocampal plasticity may involve a specific subset of immediate early genes or proteins (McIntyre et al., 2005). Transcripts for these proteins may be transported in the same granule or these proteins may share the same translational repressor, for example. Perhaps amygdala modulated hippocampal plasticity proteins preferentially influence proteins with internal ribosomal entry sites (Pinkstaff, Chappell, Mauro, Edelman, & Krushel, 2001). These possibilities remain to be examined.
The results of the present experiments demonstrate that systemic corticosterone administration immediately after training on an inhibitory avoidance task increases amygdala norepinephrine levels, enhances long-term memory, and increases expression of the plasticity-associated protein Arc in hippocampal synapses. These findings are consistent with the view that emotional arousal and stress hormones modulate memory through an interaction with β-adrenoceptors in the BLA, which subsequently influences plasticity at efferent synapses. Memory influenced by BLA actions likely involves many brain regions, proteins and signaling cascades, but here we have detected a change in the plasticity-related protein Arc in hippocampal synapses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akirav I, Richter-Levin G. Mechanisms of amygdala modulation of hippocampal plasticity. Journal of Neuroscience. 2002;22:9912–9921. doi: 10.1523/JNEUROSCI.22-22-09912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer WAC, VanDongen HMA, VanDongen AMJ. Arc/Arg3.1 translation is controlled by convergent N-methyl-D-aspartate and Gs-coupled receptor signaling pathways. Journal of Biological Chemistry. 2007;283:582–592. doi: 10.1074/jbc.M702451200. [DOI] [PubMed] [Google Scholar]
- Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. Neurobiology of Learning and Memory. 2003;79:194–198. doi: 10.1016/s1074-7427(02)00036-9. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Park CR, Woodson JC. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2002;14:281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Clebuterol administration into the basolateral amygdala post-training enhances retention in an inhibitory avoidance task. Neurobiology of Learning and Memory. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala. Biological Psychiatry. 1999;46:1140–1152. doi: 10.1016/s0006-3223(99)00157-2. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta pharmacologica Sinica. 2000;21:481–493. [PubMed] [Google Scholar]
- Gallagher M, Kapp BS, Musty RE, Driscoll PA. Memory formation: evidence for a specific neurochemical system in the amygdala. Science. 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Facilitation of time-dependent memory processes with posttrial epinephrine injections. Behavioral Biology. 1975;13:145–153. doi: 10.1016/s0091-6773(75)91784-8. [DOI] [PubMed] [Google Scholar]
- Gold PE, van Buskirk R. Posttraining brain norepinephrine concentrations: Correlation with retention performance of avoidance training and with peripheral epinephrine modulation of memory processing. Behavioral Biology. 1978;25:509–520. doi: 10.1016/s0091-6773(78)91614-0. [DOI] [PubMed] [Google Scholar]
- Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nature Neuroscience. 1998;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent Arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. Journal of Neuroscience. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of immediate-early genes Arc, c-fos, and zif268. Journal of Neuroscience. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield T, McGaugh JL. Norepinephrine infused into the basolateral amygdala posttraining enhances retention in a spatial water maze task. Neurobiology of Learning and Memory. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Research. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. Journal of Neuroscience. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui GK, Figuerora IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiology of Learning & Memory. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Nakanishi K, Saito H, Abe K. Amygdala beta-noradrenergic influence on hippocampal long-term potentiation in vivo. Neuroreport. 1997;8:3143–3146. doi: 10.1097/00001756-199709290-00027. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Acquisition of a novel behavior induces higher levels of Arc mRNA than does overtrained performance. Neuroscience. 2002;110:617–626. doi: 10.1016/s0306-4522(01)00605-4. [DOI] [PubMed] [Google Scholar]
- Korz V, Frey JU. Stress-related modulation of hippocampal long-term potentiation in rats: Involvement of adrenal steroid receptors. Journal of Neuroscience. 2003;23:7281–7287. doi: 10.1523/JNEUROSCI.23-19-07281.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. Journal of Neuroscience. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KC, Juler RG, McGaugh JL. Modulating effects of post-training epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Research. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufman WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- McGaugh JL, McIntyre CK, Power AE. Amygdala modulation of memory consolidation: interaction with other brain systems. Neurobiology of Learning & Memory. 2002;78:539–552. doi: 10.1006/nlme.2002.4082. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. European Journal of Neuroscience. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proceedings of the National Academy of Sciences. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg 3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. Journal of Neuroscience. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MI, La Lumiere RT, Buen TV, Bermudez-Rattoni F, McGaugh JL. Blockade of noradrenergic receptors in the basolateral amygdala impairs taste memory. European Journal of Neuroscience. 2003;18:2605–2610. doi: 10.1046/j.1460-9568.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Quirarte GL, Rodriguez-Garcia G, McGaugh JL, Roozendaal B. Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learning & Memory. 2008;15:468–476. doi: 10.1101/lm.964708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag-Sallaz M, Montag D. Learning-induced Arc/Arg3.1 mRNA expression in the mouse brain. Learning & Memory. 2003;10:99–107. doi: 10.1101/lm.53403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proceedings of the National Academy of Sciences. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th. San Diego: Academic Press; 2005. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahike C, Welzi H, Kobalz U, Stawrakakis A, Fernandez E, Walteriet R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SGN, Bliss TVP, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Pinkstaff JK, Chappell SA, Mauro VP, Edelman GM, Krushel LA. Internal initiation of translation of five dendritically localized neuronal mRNAs. Proceedings of the National Academy of Sciences. 2001;98:2770–2775. doi: 10.1073/pnas.051623398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Z, Krugers HJ, Jöels M. Corticosterone time-dependently modulates beta-adrenergic effects on long-term potentiation in the hippocampal dentate gyrus. Learning & Memory. 2007;14:359–367. doi: 10.1101/lm.527207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Roozendaal B, McGaugh JL. Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opiod peptidergic drugs. Brain Research. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and proteína expresión : evidence for selective, network-specific reactivation. Journal of Neuroscience. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behavioral Neuroscience. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Stress-activated hormonal systems and the regulation of memory storage. Annals of the New York Academy of Sciences. 1997;821:247–258. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power A, Mc Gaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proceedings of the National Academy of Sciences. 1999a;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Williams CL, McGaugh JL. Glucocorticoid receptor activation in the rat nucleus of the solitary tract facilitates memory consolidation: involvement of the basolateral amygdala. European Journal of Neuroscience. 1999b;11:1317–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor-cAMP/PKA system in influencing memory consolidation. European Journal of Neuroscience. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. Journal of Neuroscience. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proceedings of the National Academy of Sciences. 2006a;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006b;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiology of Learning & Memory. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Differential effects of neonatal norepinephrine lesions on immediate early gene expression in developing and adult rat brain. Neuroscience. 2008;157:821–832. doi: 10.1016/j.neuroscience.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule J, Penke Z, Kanhema T, Alme MN, Laroche S, Bramham CR. Object-place recognition learning triggers rapid induction of plasticity-related immediate early genes and synaptic proteins in the rat dentate gyrus. Neural Plasticity. 2008;2008:269097. doi: 10.1155/2008/269097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Van Stegeren AH, Wolf OT, Everaerd W, Scheltens P, Barkhof P, Rombouts SA. Endogenous cortisol level interacts with norardrenergic activation in the human amygdala. Neurobiology of Learning and Memory. 2007;87:57–66. doi: 10.1016/j.nlm.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Vouimba RM, Yaniv D, Richter-Levin G. Glucocorticoid receptors and beta-adrenoceptors in the basolateral amygdala modulate synaptic plasticity in hippocampal dentate gyrus, but not in area CA1. Neuropharmacology. 2007;52:244–252. doi: 10.1016/j.neuropharm.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg 3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behavioral Neuroscience. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- Yamada K, McEwen BS, Pavlides C. Site and time dependent effects of acute stress on hippocampal LTP in freely behaving rats. Experimental Brain Research. 2003;152:52–59. doi: 10.1007/s00221-003-1519-0. [DOI] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proceedings of the National Academy of Sciences. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]


