Abstract
Eccentric contractions are skeletal muscle stretches with concurrent active force production; these contractions commonly occur during dynamic sports activities and can cause acute muscle injury. Recovery from this injury depends in part on pro-inflammatory processes, such as neutrophil infiltration at the injured site, which is affected by estrogen. The estrogen effect has been examined broadly, but without distinguishing between major compartments within muscle in which neutrophil infiltration can occur. Therefore, we compared neutrophil antigen expression in two compartments of eccentrically contracted muscle of ovariectomized mice with or without estrogen. To quantify neutrophil antigen expression, serial cross sections of muscle were immunolabeled with antibodies that recognize 7/4 or Ly6C/G, and then quantified using computer-assisted image analysis. At 48 h post-injury, estrogen-positive (E+) mice had more 7/4-positive and Ly6C/G-positive myofibers, increased 7/4 area percentage, and more 7/4-positive cells in the connective tissue. In addition, E+ mice showed more 7/4-positive myofibers that were Ly6C/G-negative and more Ly6C/G-positive myofibers that were 7/4-negative. These data suggest that in injured muscle, estrogen increases 7/4 antigen in connective tissue and myofibers and is associated with more Ly6C/G-positive myofibers when the 7/4 antigen is absent from these myofibers.
Keywords: eccentric contraction, immunohistochemistry, Ly6C/G, muscle recovery, myofiber, neutrophil infiltration
Muscle activity that involves stretching with concurrent active force commonly occurs during sports activities, such as downhill running, and daily activities, such as lifting and squatting. This muscle activity is known as an eccentric contraction (EC) and it can cause acute muscle injury.
Recovery from ECs depends in part on pro-inflammatory processes, such as neutrophil infiltration. Depending on the EC model, neutrophils are evident at the injured muscle site as early as 6 h and as late as 3 days after ECs (Koh et al. 2003, Pizza et al. 2002, 2005). Neutrophils are detected in injured muscle by immunohistochemistry using antibodies that recognize protein antigens, such as His48 (van Goor et al. 1991, Werner-Klein et al. 2008), Ly6G/C (Daley et al. 2008, Hestdal et al. 1991, Jutila et al. 1994) and 7/4 (Hirsch and Gordon 1983). Although the 7/4 antigen has been determined to be derived from the Ly6B gene located on mouse chromosome 15 (Rosas et al. 2010), the function of this and the other two antigens is unknown. It has been reported that Ly6G/C-positive neutrophils are likely to be more mature than Ly6G/C-negative neutrophils (Bertoncello et al. 1989, Hestdal et al. 1991). Therefore, detection of cells using multiple markers might aid in understanding the neutrophil-related events of muscle recovery.
Once neutrophils and other phagocytic leukocytes migrate from blood vessels and move within muscle connective tissue to invade damaged myofibers, these cells phagocytose damaged material, so that myofiber regeneration can occur. Although phagocytosis can occur during the first three days post injury, either the peak or the last stage of phagocytic leukocyte invasion of myofibers occurs by 48 h post injury.
The significance of the 48 h post injury time in relation to neutrophils is indicated by the up-regulation of genes, such as CCR2 and CXCL5, which are associated with the accumulation of neutrophils at an inflamed site (Maus et al. 2002, Rousselle et al. 2013). For example, Barash et al. (2004) found increased CCR2 mRNA expression (via microarray analysis) in injured tibialis anterior muscle at 48 h post ECs. Also, Sachidanandan et al. (2002) reported that CXCL5 mRNA expression peaked at 48 h post freeze injury of the tibialis anterior muscle. Therefore, in addition to 24 h post injury (Schneider et al. 2012), 48 h post injury is an important time to examine the estrogen effect on neutrophil infiltration into damaged myofibers and connective tissue.
Estrogen affects the presence of neutrophils in EC injury models. For example, estrogen attenuated an increase in His48-positive cells in injured muscle of ovariectomized rats 24 h after downhill running (Enns et al. 2008). In addition, the mean antigen area of 7/4-positive cells in muscle of ovariectomized mice treated with estrogen after 24 h of ECs was greater than that in muscle of ovariectomized mice treated with placebo (Schneider et al. 2012). These data suggest that estrogen helps modulate neutrophil infiltration during the recovery of EC-injured muscle.
Earlier reports did not indicate whether estrogen modulation of neutrophil infiltration ass specific to connective tissue. Quantitation of His48-positive cells at 24 h post injury did not distinguish between cells in connective tissue and those within the myofibers (Enns et al. 2008). Also, the majority of 7/4-positive cells detected at 24 h post injury were localized in connective tissue, but not within myofibers (Schneider et al. 2012). This distinction is important to our understanding of the role that these cells play in muscle regeneration, because cells that are distributed only within the connective tissue may have a different function than those that invade damaged myofibers.
To expand our understanding of the response of neutrophils in an estrogen-rich environment during muscle recovery, we investigated the expression of two common markers of neutrophil infiltration, 7/4 and Ly6C/G antigens, in connective tissue and myofibers at 48 h after EC injury.
Material and methods
Animals and procedures
Our study was approved by the University of Wisconsin-Madison School of Medicine Institutional Animal Care and Use Committee. The animals were housed singly in cages in the animal care facility with a 12 h light/12 h dark cycle and water and food available ad libitum.
Forty-eight 6-7-week-old female C57BL/6 mice were purchased from Harlan Laboratories (Indianapolis, IN). The current study was part of an earlier study (Schneider et al. 2012) in which the mice were divided into five groups: gonad-intact female, estrogen positive (E+) ovariectomized, E+ and progesterone ovariectomized, progesterone-positive ovariectomized, and ovariectomized female + placebo. Because no significant difference was found in the study by Schneider et al. (2012), i.e., ovariectomized mice with endogenous or exogenous estrogen (some have progesterone) have a similar leukocyte response to muscle injury, the five groups of mice were collapsed into two groups, E+ and E-, based on the presence of estrogen, for the purpose of this study. The E+ group consisted of 27 mice with exogenous estrogen with or without exogenous progesterone or endogenous estrogen and progesterone. The E- group consisted of 19 ovariectomized mice that did not receive estrogen, but were treated with placebo or exogenous progesterone.
After 7 days of acclimation in the animal care facility, the ovariectomized mice underwent pellet insertion as described previously (Schneider et al. 2012). The pellets were 21-day release pellets (Innovative Research of America, Sarasota, FL) of three types: 17-β estradiol 0.05 mg, progesterone 15 mg or placebo 0.05 mg. The placebo pellet contained no hormone but had the same slow release matrix as in the estradiol and progesterone pellets. These doses were chosen because previous reports (Farr et al. 1995, Grasso and Reichert 1996) indicated they would produce blood estradiol and progesterone levels similar to the levels of gonad-intact female mice.
All intact and ovariectomized mice were anesthetized and placed in an apparatus that consisted of a platform in which the left hind paw was inserted into an “open shoe” that was attached to a servomotor (Aurora Scientific, Aurora, Ontario). The left hind limb was immobilized behind the knee. The EC muscle injury protocol consisted of three bouts of 150 trials (Schneider et al. 2012). Each trial consisted of a period of passive muscle tension, an isometric contraction that was induced by electrical stimulation by needle electrodes implanted percutaneously near the tibial nerve, followed by an eccentric contraction. The eccentric contraction occurred by moving the shoe using the servomotor. The shoe moved so that the plantar flexor muscles were stretched. Then the left hind limb paw and plantar flexor muscles were allowed to return passively to a normal position. The electrical stimulation and muscle stretching instruments were controlled by the computer software, SuperScope II program v (GW Instruments, Inc., Somerville, MA) using a Macintosh computer (Apple Computers, Inc., Cupertino, CA). In addition, the software recorded muscle torque in volts during each trial.
To test whether the injuries in E+ and E- groups were similar, we compared the time-to-fatigue of the plantar flexor muscles during the first bout of 150 trials. To determine time-to-fatigue, we first calculated the peak active volts of muscle torque for individual trials in the first bout. The peak active volts value was calculated as maximum volts minus the baseline volts value. The baseline volts value is a mean of 11 values representing passive muscle tension. Each peak active volts value was normalized by dividing the peak active value by the baseline value. The number of the trial with a normalized peak active volts value closest to 0.500 was recorded. The 0.500 value was designated as muscle fatigue. Thus, muscle fatigue was defined as active eccentric torque 50% less than its initial value. Initial value was defined as the active volt value at EC trial 2 (trial 1 was categorized as a pre-trial). Because each trial represented a specific time, we used the number of the fatigue trial to calculate the time at which fatigue occurred. The time-to-fatigue was calculated for each mouse, then group means were determined.
The EC protocol was initiated 14–16 days after pellet insertion; the day of pellet insertion was considered day 1. Approximately 48 h after EC, the mice were anesthetized and the plantar flexor muscle group dissected; the anesthetized mice then were euthanized by cervical dislocation. The uterine horn was dissected and weighed to determine ovarian hormone status.
Muscle processing
Isopentane cooled by liquid nitrogen was used to freeze the plantar flexor muscles. The muscles were kept frozen at -70° C. Cross sections of the muscles were cut at or near the mid-belly of the lateral gastrocnemius using a cryostat (Leica CM1850; Leica Microsystems Inc., Bannockburn, IL). The 10 μm thick cross sections were applied to poly-L-lysine slides and kept frozen at -70° C until they were used for immunohistochemistry.
Immunohistochemistry
The primary antibodies used to detect leukocytes in muscle cross sections were rat monoclonal anti-mouse 7/4 (1:20; AbD Serotec, Raleigh, NC) and rat monoclonal anti-mouse Ly-6C/G (clone RB6-8C5, 1:20; Invitrogen, Camarillo, CA).
Immunohistochemistry was performed as described previously (Schneider et al. 2012) except for the substrate reaction step. We applied Vector® VIP substrate solution (Vector® VIP Substrate Kit; Vector Laboratories, Burlingame, CA) to all sections for 3 min. Although the entire plantar flexor muscle group was harvested, the injury was evident primarily in the lateral gastrocnemius; therefore, only this area was used to compare E+ and E- groups quantitatively.
Analysis of 7/4-positive or Ly6C/G-positive myofibers
Although the Ly6C/G connective tissue immunohistochemistry did not meet the criteria for quantifying connective tissue-positive cells, we quantified Ly6C/G-positive myofibers in addition to 7/4-positive myofibers to examine markers that may reflect different maturity states within among the myofibers. Images of areas of interest on the computer screen were reviewed independently by two observers to quantify the number of 7/4-positive and Ly6C/G-positive myofibers. Positive myofibers were defined as fibers with distinct boundaries and that exhibited one of two criteria: 1) three or more 7/4-positive or Ly6C/G-positive cells approximately 3 μm in diameter and medium to strong intensity at the sarcolemma, or 2) one or more 7/4 or Ly6C/G-positive cells of medium to strong intensity within the sarcoplasm. Discrepancies in the identification of positive myofibers per area of interest between the two observers were resolved by consensus. A mean of 7/4-positive and Ly6C/G-positive myofibers per group then was determined.
Analysis of Ly6C/G status of 7/4-positive myofibers
The Ly6C/G status (positive or negative) of 7/4-positive myofibers also was determined. Two observers independently assessed the Ly6C/G status of the 7/4-positive myofibers, i.e., whether 7/4-positive myofibers were Ly6C/G-positive or -negative. This analysis was completed for the 22 E+ mice and 11 E- mice that were observed to have 7/4-positive myofibers. Discrepancies between the two observers were resolved by consensus. Finally, to determine the number of 7/4-positive/Ly6C/G-negative myofibers per mouse, the number of 7/4-positive/Ly6C/G-positive myofibers was subtracted from the total number of 7/4-positive myofibers, then the mean difference was calculated for the E+ and E- groups.
Tissue analysis of connective tissue compartment
We quantified 7/4-positive and Ly6C/G-positive cells within an area of interest of the lateral gastrocnemius. Within this area of interest, two compartments were assessed individually: the large connective tissue compartment surrounding bundles of myofibers and the compartment within myofibers.
To quantify positive cells in the connective tissue, antibody-stained sections were examined using computer-assisted image analysis. Because 7/4-positive and Ly6C/G-positive cells normally do not exist in uninjured muscle, we set a criterion for quantifying these cells. This criterion was: the presence of at least three cells stained with moderate intensity in the connective tissue within the lateral gastrocnemius of at least 70% of the mice in each group. The 7/4, but not the Ly6C/G connective tissue immunohistochemistry, met this criterion (anti-Ly6C/G immunostaining was located mainly within myofibers). The 7/4 connective tissue immunostaining was quantified as described previously (Schneider et al. 2012), except for two changes. The 7/4 immunohistochemistry often resulted in lighter background staining in individual myofibers, especially those myofibers that were not invaded by neutrophils. To avoid counting these myofibers, the upper pixel limit of a single object was set at 495 μm2 in the Image-Pro® Plus version 5.2 software. This setting meant that each single object larger than 495 μm2 was not counted as a positive cell. This approach seemed to ensure that the software only counted the positive cells, which were smaller in area and more intensely stained than these lighter stained myofibers.
To count only 7/4-positive connective tissue cells, labeling within myofibers was excluded by manually erasing the 7/4-positive myofibers from the area of interest. After the manual erasing was checked by a second observer, three variables were assessed for each area of interest: 1) 7/4 area percentage, 2) mean 7/4 antigen area, and 3) 7/4 number. A mean of each variable was calculated per group.
The 7/4 leukocyte area percentage (percent leukocyte) is the percentage of 7/4 antigen within the area of interest. To calculate this value, the 7/4 antibody-labeled pixels were converted to area. The 7/4 area then was divided by the total adjusted area of interest, which was the total area of interest area minus the erased areas that were identified as debris, blood vessels, muscle spindles, and/or gaps, and multiplied by 100.
The mean 7/4 antigen area is an indication of the average size of 7/4-positive cells within an area of interest. This statistic was calculated by the Image-Pro® Plus software.
The third statistic was 7/4 number. The 7/4 number represents the number of 7/4-positive cells within an area of interest and was determined by the Image-Pro® Plus software.
Statistical analysis
Data are presented as means ± SE and were analyzed using SPSS versions 19 and 22 (SPSS Inc., Chicago, IL) Mann-Whitney U test. Values for p ≤ 0.05 were considered significant.
Results
Time-to-fatigue
There was no significant difference in time-to-fatigue of the EC-injured muscles in the E+ and E- mice (p = 0.396).
Anti-7/4 and anti-Ly6C/G immunohistochemistry
In the EC-injured muscles, anti-7/4 immunostaining was of medium intensity and located in connective tissue and myofibers; anti-Ly6C/G immunohistochemistry was less intense and located mainly within myofibers (Fig. 1).
Fig. 1.
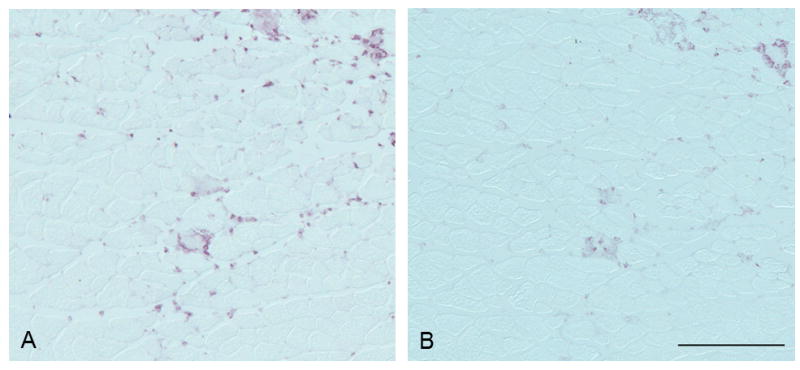
Anti-7/4 immunostaining (A) showed medium intensity and was observed in connective tissue and myofibers. Anti-Ly6C/G immunostaining (B) was less intense and was located mainly within myofibers. Bar = 200 μm.
Myofiber immunohistochemistry
The 7/4-positive myofiber count from one mouse of each group was more than three standard deviations from the mean; therefore, these two mice were removed from the analysis, which left 27 mice in the E+ group and 19 mice in the E- group. In the Ly6C/G myofiber infiltration analysis, one mouse of the E+ group could not be assessed owing to unidentifiable myofiber boundaries; therefore, this animal also was excluded from analysis. The E+ mice had significantly more 7/4-positive and Ly6C/G-positive myofibers than the E- mice (7/4, p = 0.015, Ly6C/G, p = 0.029; Fig. 2).
Fig. 2.
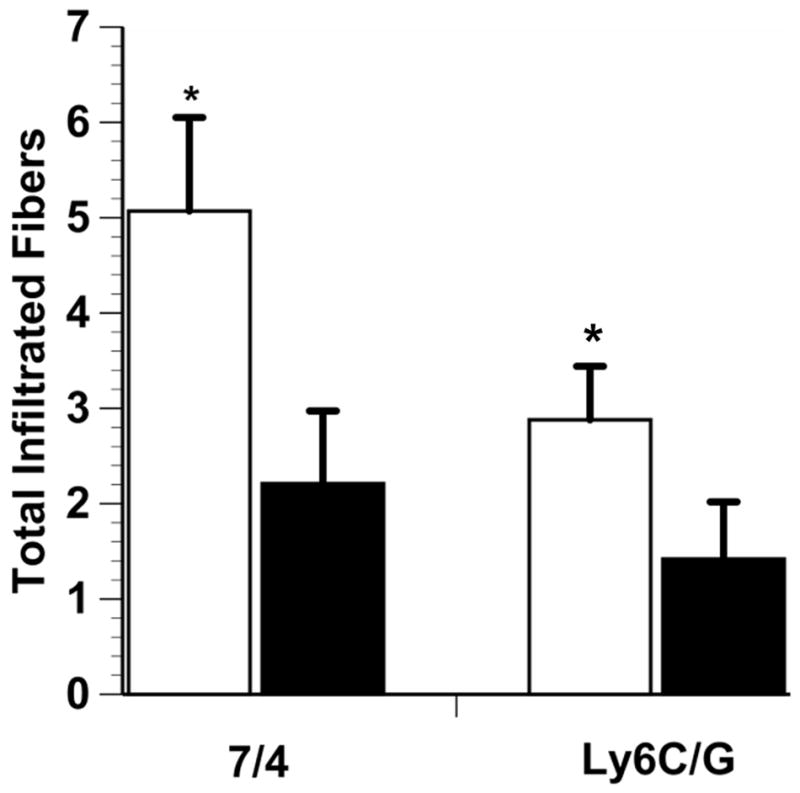
7/4-Positive and Ly6C/G-positive myofibers in injured lateral gastrocnemius muscle. White bars, E+ mice; black bars, E-mice. E+ mice had a significantly larger number of 7/4-positive and Ly6G-positive myofibers than E- mice (*p < 0.05). Total infiltrated fiber is the absolute number of stained fibers in the analyzed area of interest and data are means ± SE.
Overall, the majority (78.7%) of 7/4-positive myofibers were also Ly6C/G positive. The E+ mice group had significantly more 7/4-positive/Ly6C/G-negative myofibers than the E- mice (p = 0.013). Also, the E+ group had significantly more Ly6C/G-positive/7/4-negative myofibers than the E- mice (p = 0.002).
Connective tissue immunohistochemistry
In general, 7/4-positive cells were more abundant in the injured muscles of E+ mice than in the injured muscles of E- mice (Fig. 3).The 7/4 area percentage was significantly greater in the E+ group than in the E- group (p = 0.016) (Fig. 4). The E+ group also had significantly more 7/4-positive cells in the connective tissue than the E- group (p = 0.021) (Fig. 4); however, the mean 7/4 antigen area between the two groups was not significantly different (p = 0.051).
Fig. 3.
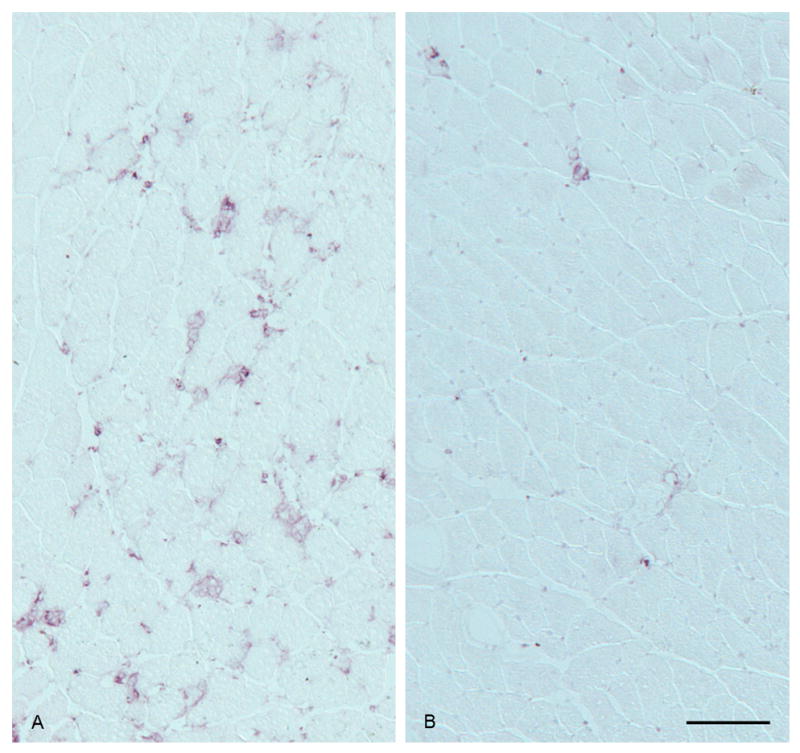
7/4-Positive cells were more abundant in the injured muscle of an E+ mouse (A) than in the injured muscle of an E- mouse (B). Bar = 100 μm.
Fig. 4.
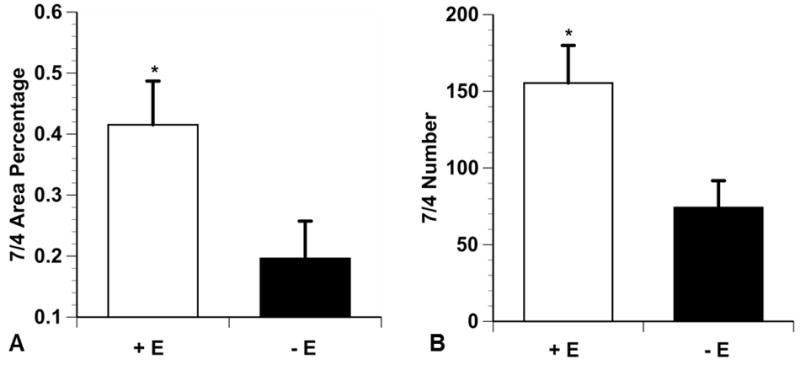
7/4 Connective tissue immunohistochemistry. The 7/4 percentage of area and number in connective tissue of injured lateral gastrocnemius muscle of E+ mice (A) were significantly greater than for E- mice (B) (p < 0.05). Data are means ± SE.
Discussion
We found that E+ mice had an increased number of 7/4-positive and Ly6C/G-positive myofibers, greater 7/4 area percentage in the connective tissue, and more 7/4-positive cells in the connective tissue area than E- mice at 48 h post EC injury. In addition, E+ mice had more 7/4-positive myofibers that also were Ly6C/G-negative. Our investigation appears to be the first to use a mouse muscle injury model to show that estrogen modulates 7/4 and Ly6C/G antigen expression in two compartments of injured muscle at 48 h post injury.
Our findings that E+ mice had more 7/4-positive cells in myofibers and greater 7/4 area percentage and 7/4 number within the connective tissue of EC-injured muscle than E- mice at 48 h post injury suggest that estrogen may up-regulate 7/4 antigen expression. In an earlier study, subcutaneous injections of 17 α-ethynyl estradiol increased 7/4 (Ly6B) mRNA (accession number M30689) expression in the female rat uterus (Naciff et al. 2002), which may explain the increased 7/4 antigen that we found. Estrogen, therefore, may stimulate neutrophils to transcribe the epitope of 7/4 antigen in EC injured muscle.
Alternatively, 7/4 antigen expression may be increased in the injured muscle of E+ mice because of a prolonged presence of neutrophils. Molloy et al. (2003) showed that a physiological dose of estrogen delayed neutrophil apoptosis in vitro. Therefore, the increased 7/4 antigen expression that we observed in the injured muscle of E+ mice may have been due to estrogen-prevented neutrophil apoptosis. This also might explain the finding that E+ mice had significantly more Ly6C/G-positive myofibers than E- mice. Ly6C/G has its greatest expression in the most mature granulocytes without colony forming potential in the bone marrow (Bertoncello et al. 1989, Hestdal et al. 1991). Thus, the extended presence of neutrophils due to an estrogen rich environment that prevents neutrophil apoptosis may ensure that more neutrophils reach maturity, which explains the larger number of Ly6C/G-positive myofibers in the E+ mice.
By contrast to our findings, other investigators of EC injury have reported that estrogen attenuated neutrophil infiltration in injured muscle (Enns et al. 2008, Tiidus et al. 2005). Another possible explanation for the discrepancy is the difference in species (mouse vs. rat) (Schneider et al. 2012). Another explanation may be that neutrophils were identified using detection of different neutrophil antigens. In the rat studies, neutrophils were detected using the expression of His48 (Enns et al. 2008, Tiidus 2001, 2005), which may be a different protein with a different function than the 7/4 protein (Rosas et al. 2010, van Goor et al. 1991, Werner-Klein et al. 2008). Therefore, estrogen may increase 7/4 antigen expression and decrease His48 antigen expression.
Our study had two major challenges or limitations. One challenge was to ensure that estrogen was not affecting the severity of the initial injury. To overcome this, and to ensure that the muscle injury for the E+ and E- groups was similar, the time-to-fatigue was calculated and compared. Because there was no significant difference (p = 0.396) in the time to reach muscle fatigue between the two groups, we concluded that muscle injury was similar in the two groups. Therefore, the differences we observed in neutrophil infiltration were unlikely to be related to a different injury responses by the two groups. Another limitation is our investigation of only the 48 h time due to limited resources. Examination at the start of muscle regeneration would have enriched and benefited our study.
To build upon the data of this current investigation, future studies should focus on the amount of 7/4 antigen expression, and the relation between post-injury time and factors that stimulate the migration of 7/4-positive cells from connective tissue to the damaged myofibers. Another important area for future study is to address whether the up-regulation of the 7/4 antigen by estrogen influences the duration of muscle repair.
At 48 h after an exercise-related muscle injury, estrogen positively modulates the 7/4 antigen in neutrophils in connective tissue and myofibers. Therefore, in this model of muscle injury, estrogen or estrogen-like compounds may ensure a robust response by neutrophils.
Acknowledgments
This study was supported by a research grant from the National Institute of Nursing Research NIH R01 NR005258-04 (B. S. Schneider). We thank Kirsten Speck for her editing assistance and graph preparation.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Barash IA, Mathew L, Ryan AF, Chen J, Lieber RL. Rapid muscle-specific gene expression changes after a single bout of eccentric contractions in the mouse. Am J Physiol Cell Physiol. 2004;286:C355–C364. doi: 10.1152/ajpcell.00211.2003. [DOI] [PubMed] [Google Scholar]
- Bertoncello I, Bartelmez SH, Bradley TR, Hodgson GS. Changes in cell surface antigen expression during murine bone marrow cell regeneration in vivo and proliferation in vitro. Immunol Cell Biol. 1989;67:127–133. doi: 10.1038/icb.1989.18. [DOI] [PubMed] [Google Scholar]
- Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leuk Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol. 2008;194:81–93. doi: 10.1111/j.1748-1716.2008.01861.x. [DOI] [PubMed] [Google Scholar]
- Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol Behav. 1995;58:715–723. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- Grasso P, Reichert LE., Jr In vivo effects of follicle-stimulating hormone-related synthetic peptides on the mouse estrous cycle. Endocrinology. 1996;137:5370–5375. doi: 10.1210/endo.137.12.8940359. [DOI] [PubMed] [Google Scholar]
- Henderson RB, Hobbs JA, Mathies M, Hogg N. Rapid recruitment of inflammatory monocytes is independent of neutrophil migration. Blood. 2003;102:328–335. doi: 10.1182/blood-2002-10-3228. [DOI] [PubMed] [Google Scholar]
- Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- Jutila DB, Kurk S, Jutila MA. Differences in the expression of Ly-6C on neutrophils and monocytes following PI-PLC hydrolysis and cellular activation. Immunol Lett. 1994;41:49–57. doi: 10.1016/0165-2478(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Peterson JM, Pizza FX, Brooks SV. Passive stretches protect skeletal muscle of adult and old mice from lengthening contraction-induced injury. J Gerontol A Biol Sci Med Sci. 2003;58:592–597. doi: 10.1093/gerona/58.7.b592. [DOI] [PubMed] [Google Scholar]
- Maus U, von Grote K, Kuziel WA, Mack M, Miller EJ, Cihak J, Stangassinger M, Maus R, Schlondorff D, Seeger W, Lohmeyer J. The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Resp Crit Care Med. 2002;166:268–273. doi: 10.1164/rccm.2112012. [DOI] [PubMed] [Google Scholar]
- Mircescu MM, Lipuma L, van Rooijen N, Pamer EG, Hohl TM. Essential Role for Neutrophils but not Alveolar Macrophages at Early Time Points following Aspergillus fumigatus Infection. J Infect Dis 2009. 2009;200:647–56. doi: 10.1086/600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD, Worthen GS. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Invest. 2012;122:974–986. doi: 10.1172/JCI60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy EJ, O’Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, Watson RW. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood. 2003;102:2653–2659. doi: 10.1182/blood-2003-02-0649. [DOI] [PubMed] [Google Scholar]
- Naciff JM, Jump ML, Torontali SM, Carr GJ, Tiesman JP, Overmann GJ, Daston GP. Gene expression profile induced by 17alpha-ethynyl estradiol, bisphenol A, and genistein in the developing female reproductive system of the rat. Toxicol Sci. 2002;68:184–199. doi: 10.1093/toxsci/68.1.184. [DOI] [PubMed] [Google Scholar]
- Pizza FX, Koh TJ, McGregor SJ, Brooks SV. Muscle inflammatory cells after passive stretches, isometric contractions, and lengthening contractions. J Appl Physiol. 2002;92:1873–1878. doi: 10.1152/japplphysiol.01055.2001. [DOI] [PubMed] [Google Scholar]
- Pizza FX, Peterson JM, Baas JH, Koh TJ. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol (Lond) 2005;562:899–913. doi: 10.1113/jphysiol.2004.073965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas M, Thomas B, Stacey M, Gordon S, Taylor PR. The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B. J Leuk Biol. 2010;88:169–180. doi: 10.1189/jlb.0809548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle A, Qadri F, Leukel L, Yilmaz R, Fontaine JF, Sihn G, Bader M, Ahluwalia A, Duchene J. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123:1343–1347. doi: 10.1172/JCI66580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandan C, Sambasivan R, Dhawan J. Tristetraprolin and LPS-inducible CXC chemokine are rapidly induced in presumptive satellite cells in response to skeletal muscle injury. J Cell Sci. 2002;115:2701–2712. doi: 10.1242/jcs.115.13.2701. [DOI] [PubMed] [Google Scholar]
- Schneider BS, Vigil SA, Moonie S. Body weight and leukocyte infiltration after an acute exercise-related muscle injury in ovariectomized mice treated with estrogen and progesterone. Gen Comp Endocrinol. 2012;176:144–150. doi: 10.1016/j.ygcen.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiidus PM. Oestrogen and sex influence on muscle damage and inflammation: evidence from animal models. Curr Opin Clin Nutr Metab Care. 2001;4:509–513. doi: 10.1097/00075197-200111000-00008. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Deller M, Liu XL. Oestrogen influence on myogenic satellite cells following downhill running in male rats: a preliminary study. Acta Physiol Scand. 2005;184:67–72. doi: 10.1111/j.1365-201X.2005.01427.x. [DOI] [PubMed] [Google Scholar]
- van Goor H, Fidler V, Weening JJ, Grond J. Determinants of focal and segmental glomerulosclerosis in the rat after renal ablation. Evidence for involvement of macrophages and lipids. Lab Invest. 1991;64:754–765. [PubMed] [Google Scholar]
- Warren GL, Summan M, Gao X, Chapman R, Hulderman T, Simeonova PP. Mechanisms of skeletal muscle injury and repair revealed by gene expression studies in mouse models. J Physiol. 2007;582:825–841. doi: 10.1113/jphysiol.2007.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Klein M, Goggel R, Westhof A, Erb KJ. Development and characterisation of a novel and rapid lung eosinophil influx model in the rat. Pulm Pharmacol Ther. 2008;21:648–656. doi: 10.1016/j.pupt.2008.03.002. [DOI] [PubMed] [Google Scholar]


