Regarding recent research, ethically acceptable strategies to help avoid, or at least reduce, nocebo effects within clinical settings are depicted.
Keywords: Nocebo algesia, Hyperalgesia, clinical implications, Nocebo effects, Pain modulation, Pain treatment
Abstract
Introduction:
Nocebo-induced algesic responses occurring within clinical contexts present a challenge for health care practitioners working in the field of pain medicine.
Objectives:
Following the recent research on algesic nocebo effects, the scope of this review is to develop ethically acceptable strategies to help avoid, or at least reduce, nocebo responses within clinical settings.
Methods:
We reviewed relevant clinical studies that depict how patient-practitioner interactions may contribute to the reduction of nocebo responses.
Results:
A strong algesic nocebo effect may adversely impact a patient's condition by causing decreases in both the efficacy and effectiveness of interventions, as well as by promoting treatment nonadherence and discontinuation. These effects may be triggered through multiple channels and can lead to significant alterations in a patient's perception of pain, consequently producing a weakening of the specific positive effects of pharmacological, psychological, or physical pain-management interventions.
Conclusion:
To minimize nocebo effects in clinical settings, we identified and discussed five contextual aspects relevant to the treatment of patients with chronic pain: (1) negative patient–clinician communication and interaction during treatment; (2) emotional burden of patients during treatment with analgesic medication; (3) negative information provided via informational leaflets; (4) cued and contextual conditioning nocebo effects; and (5) patient's lack of positive information. Through an understanding of these elements, many preventive and ethically acceptable clinical actions can be taken to improve multidisciplinary pain treatment outcomes.
1. Introduction
Nocebo effects and responses—the adverse events related to negative expectations or anticipations—are often associated with the occurrence of both specific and unspecific adverse side effects4 as a consequence of reading a drug leaflet when taking a medication. Current research illustrates that nocebos produce behavioral, functional, and physiological changes.12 In particular, nocebo effects related to pain worsening or insufficient pain reduction in a medical context are of utmost importance for patients undergoing pain therapy.
In contrast to the definition of placebo analgesia,29 nocebo algesia occurs when changes in individual pain perception cause a lowering of the specific positive effects associated with a particular medical treatment or intervention.15 These effects have the potential to significantly decrease pharmacological treatment efficacy, as well as psychological and physical intervention effectiveness. This definition also includes nocebo algesic responses resulting from the absence of an expected entire effect of an analgesic. Colloca et al14 assumed that any medical treatment has 2 components: the specific effect of the administered medical treatment and the placebo effect, the additional effect that is shaped through the perception of the treatment being given. Effectiveness of the placebo relies on the “open administration” of treatments, during which patients are able to perceive the therapeutic actions taken. By suppressing this perception (“hidden administration”), medical treatments become less effective and the placebo component gets lost.
In clinical practice, 3 different kinds of algesic nocebo effects are possible. In one context, the patient's expected negative outcome in regard to a pain treatment reduces the specific positive outcomes associated with such intervention. Also, when a patient's overvalued expected positive outcome of a pain treatment is not confirmed after treatment exposure, this putative negative experience reduces the effectiveness of a subsequent pain treatment. Finally, patients can fail to expect a positive outcome from their treatment because of deficient information about the pain-reducing effects, which limits the occurrence of additional unspecific positive outcomes related to placebo-induced analgesia (Fig. 1).
Figure 1.
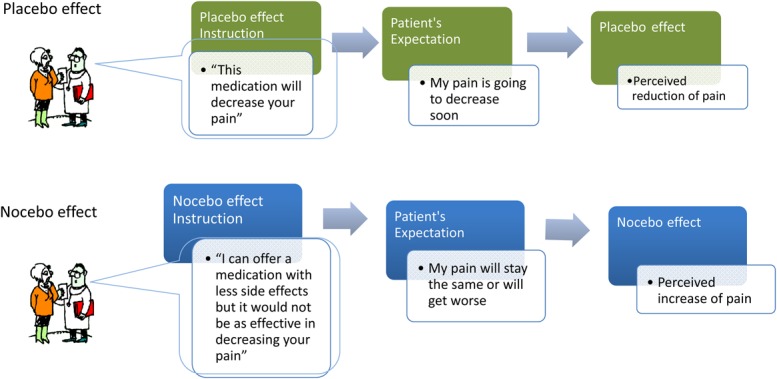
Placebo and nocebo effects influence pain outcomes. When an analgesic is given, information about its effects shapes the patient's expectation about its efficacy. Negative expectancies about the effect of an analgesic can reduce its efficacy (nocebo effect). When an analgesic is prescribed, it is useful to emphasize positive drug effects15 and to avoid overemphasizing side effects. Often, patient–clinician communication is characterized by unbalanced information that leans towards negative components. A patient wants the clinician to help relieve the pain and is willing to take the medication, but does not want to experience side effects. The clinician then finds an alternate solution. However, by prescribing a painkiller according to the WHO 3-Step Model for Pain Management (step 1), the health provider may accidentally minimize the medication's efficacy by providing additional unsought information regarding the level of action of the newly recommended drug.
In the placebo arms of randomized controlled clinical trials, reported adverse events matching those experienced by patients receiving active medications have been observed. Informing patients about the potential of experiencing high pain, as well as describing possible adverse events during the informed consent process,4 may elicit nocebo effects. Namely, paradoxical responses have been documented when patients are informed that a prescribed drug may cause a side effect opposite to the pharmacological properties of such drug.22,31,32,37 Negative clinical outcomes have also been associated with disclosures of serious illnesses and prognoses (eg, cancer),30 and with easy access to health information resources.24,48
In comparison to the analgesic placebo response, the algesic nocebo effect is less understood.44 Neurobiological studies have revealed great similarities between the molecular basis of drug action and the related placebo responses, suggesting that a placebo can partially replace the verum and enhance its effects.13 These findings remain to be proven for algesic nocebo effects. However, at this stage there exists an informative basis on the nocebo response that allows for the development of therapeutic actions aimed at preventing treatment failure and improving clinical outcomes in patients undergoing pain-management therapies. The educational objectives of this review are (1) to present the psychological mechanisms and trigger factors deriving from patient-clinician communication involved in eliciting algesic nocebo effects; (2) and to learn how to identify and more importantly, how to avoid negative information or lack of positive details in clinical contexts that increase the occurrence of algesic nocebo effects.
2. Patient-clinician communication: nocebo effects in randomized controlled or nonrandomized clinical trials
In 2012, Colloca and Finniss outlined that patient-clinician communication can negatively alter patient outcomes.12 The way of instructing patients about painful medical procedures, pain medication or other pain interventions influences patients expectations and thereby the response to the particular intervention.
For example, a pioneering study reported that of 15 patients receiving lumbar puncture who were told to expect a headache afterwards, 7 experienced headaches. By contrast, of the 13 patients who were not warned about the possibility to have headache, none experienced such side effect. The authors concluded that “patients should not be told to expect a headache, as this may be a self-fulfilling prophecy.”19
Also, in a randomized controlled study, the effects of verbal suggestion have been investigated during the administration of epidural anesthesia for labor pain. Women were informed about the procedure using 2 styles of framing: “We are going to give you a local anesthetic that will numb the area and you will be comfortable during the procedure” or “You are going to feel a big bee sting; this is the worst part of the procedure.” Women who were informed through the positive-framing technique reported significantly less pain than those informed through the negative-framing style for the same procedure (Fig. 2).50
Figure 2.
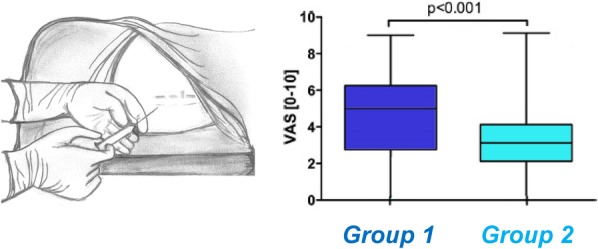
Interventional acute pain and framing effects. The manner information is presented during an epidural procedure that impacts pain perception in women experiencing labor pain associated with childbirth. Women were told what to expect about the procedure in 2 distinct ways. Group 1 was informed as follows: “You are going to feel a big bee sting; this is the worst part of the procedure”. Conversely, group 2 was given the following instruction: “We are going to give you a local anesthetic that will numb the area and you will be comfortable during the procedure.” The graph shows differences in pain ratings between both groups. Pain was assessed in a blinded fashion. Women in group 2 who were informed with a positive framing about the possibility to experience pain reported significantly less pain than those informed through the standard framing style for the same procedure. Data from Varelmann et al.50 Varelmann D, Pancaro C, Cappiello EC, Camann WR. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg 2010;110: 868–70. Promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Wolters Kluwer. Please contact healthpermissions@wolterskluwer.com for further information.
Nocebo effects could be prompted by knowledge of adverse effects related to the use of pharmacological drugs, and could potentially last for long periods of time. A study performed by Mondaini et al37 investigated the sexual side effects associated with treatment of benign prostatic hyperplasia with finasteride (5 mg) by informing patients through 2 different disclosure styles. Randomization of patients into the 2 groups occurred after the treatment was described as having proven efficacy for managing benign prostatic hyperplasia. One of the groups was provided with information regarding uncommon but potential sexual side effects, specifically naming erectile dysfunction, decreased libido and problems with ejaculation, while the other group was not informed about such effects. At 6- and 12-month follow-ups, a significant difference between reported sexual side effects was observed between the 2 disclosure groups. Of the group informed about the sexual side effects, 43.6% reported sexual dysfunction compared to 15.3% in the noninformed group.
These effects are not limited to health care-delivery settings, but can also affect clinical research. In a randomized double-blind placebo-controlled trial, inclusion and description of potential side effects as a result of the administration of aspirin and/or sulfinpyrazone for unstable angina pectoris resulted in significant withdrawal from the study. More importantly, it was noted that the description of potential gastrointestinal side effects in the consent form correlated with an astonishing 6-fold increase of reported gastrointestinal side effects, which also led to consequent patient-initiated cessation of therapy.40
Informed consent practices may be inadvertently inducing nocebo effects by triggering negative expectancies through the explanation of possible adverse effects related to medication use. Thus, it calls for a need to balance the ethical principles of protecting the patient's autonomy and right-to-know, with the possibility of unintentionally triggering these nocebo effects and causing harm to the patient.52
One particular population who may be further at risk of experiencing hyperalgesic nocebo responses are patients suffering from chronic pain disorders, especially those who require long-term pharmacological interventions for the management of pain. A study found that by changing the label of rizatriptan—a 5HT1B/1D agonist prescribed for migraines—to placebo, efficacy was significantly decreased.26
Moreover, a recent review conducted by Dieppe et al20 argues how nocebo effects, which have been previously shown to increase both pain and anxiety, could influence the worsening of symptoms in patients suffering from osteoarthritis (OA), a population that already presents with high prevalence rates for both depression and anxiety.3,7,9 It has been shown that negative expectations for increased pain intensity produce anticipatory anxiety, which can itself lead to algesia and hyperalgesia, and that can also engage other pathways that aid in pain transmission, such as the cholecystokininergic pathway.
Reported side effects in the placebo arms of clinical trials support this theory. Some studies have suggested that these experienced side effects could be arising from the informed consent process, and thus could represent expectancy-induced nocebo effects. A systematic review that evaluated side effects of antimigraine medication in the placebo arms of randomized placebo-controlled clinical trials found a high level of reported adverse events that matched those observed with the use of the active drugs. The review, which looked at 8 trials using nonsteroidal anti-inflammatory drugs (NSAIDs), 56 using triptans, and 9 using anticonvulsants, suggests that the results are consistent with the expectancy mechanisms that explain the occurrence of nocebo and placebo effects. Anticonvulsant placebos, for example, led to the development of anorexia and memory difficulties, which are common side effects experienced with the use of this medication. More importantly, these were only observed in the anticonvulsant-placebo group, but not in the NSAID- or triptan-placebo groups. Additionally, when comparing the 3 types of placebos, anticonvulsant placebos led to a higher rate of adverse events when compared to NSAIDs and triptans, consequently leading to a higher number of adverse event-related withdraws from the study from that group when compared to the triptan and NSAID-placebo groups.2
Changes in behavior triggered by nocebo effects may pose a tremendous risk for patients whose health strongly depends on treatment adherence. A recent 2-year prospective clinical cohort study evaluated side effects, side effect expectations, quality of life, and adherence to treatment in a cohort of 111 patients with hormone-receptor-positive breast cancer who were indicated to start adjuvant endocrine treatment. The results showed that long-term side effects, as well as nonadherence, were strongly determined by negative patient expectancies prior to the initiation of the therapy. These negative expectations were associated with an increased risk for nocebo-induced side effects, treatment-specific side effects, as well as nonadherence, and thus should be regarded as factors of importance when trying to achieve positive clinical outcomes in this particular population. Based on these results, it is feasible to improve quality of life and adjuvant endocrine therapy (AET) treatment adherence by optimizing individual pretreatment expectations and minimizing the influence of nocebo effects.41
Furthermore, an interventional single-cohort study conducted by Heisig et al25 in a cohort of 137 women with estrogen-receptor–positive breast cancer showed that enhanced information delivery about AET improved adherence rates at 3 months follow-up. Assessment of treatment adherence was based on a validated self-assessment questionnaire that asked participants how many tablets had they taken in the last 12 weeks. Specifically, patients were provided with more thorough information about endocrine therapy within 1 to 3 weeks after surgery, in addition to the usual clinical information given. Investigators measured knowledge and satisfaction with the supplementary information before and after the informing process, whereas knowledge and treatment adherence were assessed at 3 months after the start of hormone treatment. Patients who reported more satisfaction with the additional AET information provided, as well as with the additional information about potential adverse effects of the therapy, showed higher adherence at follow-up with 73.5% of patients reporting to have taken all tablets. Better learning and comprehension were also associated with better adherence at 3 months follow-up.25
The results from these studies bring attention to the important role that health care practitioners hold within patient–clinician interactions. Factors related to the information delivery processes such as the information delivery processes, framing techniques employed, and level of interpersonal engagement, could potentially act on a patient's suggestibility and treatment-outcome expectancies. Altogether, these contextual factors can create a multilayered conditioning framework that could adversely impact not only treatment effectiveness and incidence of side effects, but could also negatively affect a patient's quality of life and potentially impact decision-making processes related to current and future treatments. In particular, Colloca and Finniss12 suggested that it is important to (1) frame disclosures and informed consents to carefully balance truthful information and expectancy empowerment; (2) tailor the information delivery process to the needs of the patient and learn about individual expectancies; and (3) educate health providers and patients about the potential role of endogenous modulatory systems in clinical encounters.4,34
The implications for patients who depend on pharmacological management of pain are extremely relevant, especially if diminished medication efficacy results in a need for higher doses or prolonged pharmacological intervention, which could increase the risk for adverse effects or addictive behaviors. Thus, these effects not only pose a risk for individual patient cases, but could also form part of a larger public health concern. Conversely, the purposeful engagement and control of these endogenous modulatory mechanisms (both nocebo and placebo) could also present a feasible and noncostly method for reducing risk factors at a population level.
3. Translational and clinical implications: identification and avoidance of nocebo effects in practice
The studies described above suggest that the occurrence of algesic nocebo effects can negatively impact a patient's condition by causing significant decreases in treatment efficacy and by promoting discontinuation of pain treatments. Thus, nocebo effects have a strong impact within clinical practice.36,42 Algesic nocebo effects are especially related to 5 contextual aspects of the treatment of patients with chronic pain:
3.1. Negative patient–clinician communication during treatment
A negative patient–clinician relation can suppress the complete effectiveness of a pain treatment. Bingel et al8 showed how divergent expectancies, shaped through positive and negative instructions, alter the analgesic efficacy of a potent opioid (remifentanil) in healthy volunteers. In their study, which followed a within-subject design, investigators assessed the effects of remifentanil on constant heat pain in 3 experimental conditions: with no expectation of analgesia, with expectancy of a positive analgesic effect, and with negative expectancy (hyperalgesia or exacerbation of pain). Positive treatment expectancy enhanced the analgesic benefit of remifentanil, whereas negative treatment expectancy abolished remifentanil analgesia. These results underline that negative communication during treatment can significantly reduce pharmacological effectiveness. There are only very few studies investigating these effects in patients. One particularly interesting study was published by the work group of Kaptchuk,28,30 who investigated the influence of patient-practitioner communication on the placebo effect in irritable bowel syndrome. They compared the effects of 2 placebo acupuncture treatments, one being placebo acupuncture enhanced with positive personal interactions, while the other was limited to neutral and business-like interactions. The latter is equivalent to negative communication. Both groups were compared to a waiting list group. Interestingly, results showed that both of the placebo acupuncture treatments led to symptom improvement that surpassed the normal course of the disease, ie, the natural progression of symptoms observed in the waiting group. Moreover, the negative therapeutic relationship, which was conducted in the limited treatment group, showed much lower effects of placebo acupuncture in comparison to the treatment in which positive interaction with patients (enhanced group) was carried out. These results underline that the lack of communication during treatment can significantly reduce treatment effectiveness, which can be seen as a nocebo effect. In this study, the entire potential development of positive placebo effects was suppressed. From an ethical perspective, it is of utmost importance to withhold such negative effects from patients and to exploit the entire potential of strategies boosting the effectiveness of any treatment and intervention.
3.2. Emotional burden of patients during analgesic medication
Patients' emotional burden while going through pain-management treatments can interfere with the intervention's positive effects, and thus can adversely impact clinical outcomes. The described core psychological mechanisms of expectancy and learning interact with further cognitive-affective processes such as emotions and motivation (eg, anxiety, desire for relief), somatic focus and awareness, or cognitions and attitudes towards the treatment.9,21,23,33,45,46,51 The development of nocebo responsiveness and the actual nocebo effect in a person is the result of the complex interaction between factors that can be traced back to the individual learning history around analgesic drugs or treatments, as well as current contextual factors referring to the analgesic or placebo treatment.39
3.3. Negative information provided via leaflets
The power of instructions is one of the important results that placebo research has revealed. Negative information, which can be provided through medication leaflets, can cause a negative effect on mood that leads to decreased willingness to take medication.
When an analgesic is given, the current information about its effects shapes the current expectation about its efficacy. One important point for the prescribing physician is to emphasize positive drug effects and to avoid overemphasizing side effects. Because of limited contact hours in clinical settings, it is highly probable that the focus of an interaction between the patient and the therapist lies on informing patients about side effects, rather than on providing information on positive drug effects. It is, therefore, important to explain the positive drug effects along with the mechanisms of drug action. Interpersonal interactions, rather than providing written material only, are especially helpful27 and support the patient in accepting the medication and benefiting from it.
Expectation of treatment side effects is consistently linked with the occurrence of those symptoms. Patient expectations, including those generated by the informed consent process, can have a large influence on the side effects that patients feel after starting a new medical treatment.43 Such symptoms may be the result of the nocebo effect, as the expectation of side effects may lead to them being experienced. A review of clinical drug trials showed that about one in 5 placebo-treated participants spontaneously reported side effects,47 and almost 1 in 10 placebo users withdrew from treatment because of side effects.35 Investigations show that the side effects reported by the placebo group are usually the same side effects as those that are experienced by participants who are receiving the active treatment.35,38 Nocebo interventions can thus have a robust negative effect and can increase pain.50
3.4. Cued and contextual conditioning nocebo effects
Based on the model of classical conditioning, the algesic nocebo effect is viewed as a learnt response, which is triggered by the exposure to a painful stimulus previously associated with a cue or a certain context. According to the traditional stimulus substitution model,1,45 the repeated association of an initially neutral conditioning stimulus (CS, eg, the sight of the physician's or physiotherapist's white coat, the clinician's office, the smell of a treatment) with the unconditioned stimulus (eg, painful interventions such as invasive nerve blockade or chiropractic intervention) leads to a conditioned response (increased pain response: algesic nocebo effect) when the CS is presented (eg, the patient's contact with the physician, the patient's entrance in the office of the clinician; the patient's act of taking a treatment). Conditioned nocebo effects have negative effects based on the associations with previously experienced painful interventions.
These effects have been investigated in several studies showing that prior conditioning is relevant in the development of nocebo-induced hyperalgesia and that the duration of the conditioning linearly influences the perpetuation of the nocebo effect.10,11,16–18 It was shown that nocebo algesic effects were the same, independently whether the effects were elicited via verbal suggestions alone or via conditioning paradigms.17 These results are of utmost importance especially in the treatment of individuals who cannot express their pain experience because of impairment or undeveloped verbal communication skills (eg, infants, immigrants, patients with communication disorders due to stroke and dementia).49
For example, the exposure to repetitive painful procedures can lead to anticipatory pain behaviors and conditioned nocebo hyperalgesia in hospitalized full-term infants of diabetic mothers.49 These infants underwent many venipunctures for measuring blood glucose concentrations. When compared to normal infants, infants of diabetic mothers presented anticipatory pain behaviors when their skin was just cleaned before injection. It is evident that the exposure to skin cleaning became a CS triggering defensive behaviors for inducing pain responses in the absence of a painful stimulation, which is suggestive of conditioned nocebo effects. These kinds of findings emphasize a need for treating pain adequately in early life as well as in noncommunicative patients.
3.5. Lack of information: unintentional “hidden” administration of analgesic treatment
Hidden administration of medication occurs when patients are unaware of the details related to an ongoing pharmacological treatment and can lead to a dramatic reduction in the efficacy and effectiveness of analgesic medication and intervention. Hence, this clinical context promotes the occurrence of algesic nocebo effects. Analgesics given in a covert fashion (eg, through a computer-controlled infusion pump or as part of a cocktail of drugs)5,14 show worse outcomes compared to analgesics that are openly administered and of which patients are fully aware of (Fig. 3).
Figure 3.
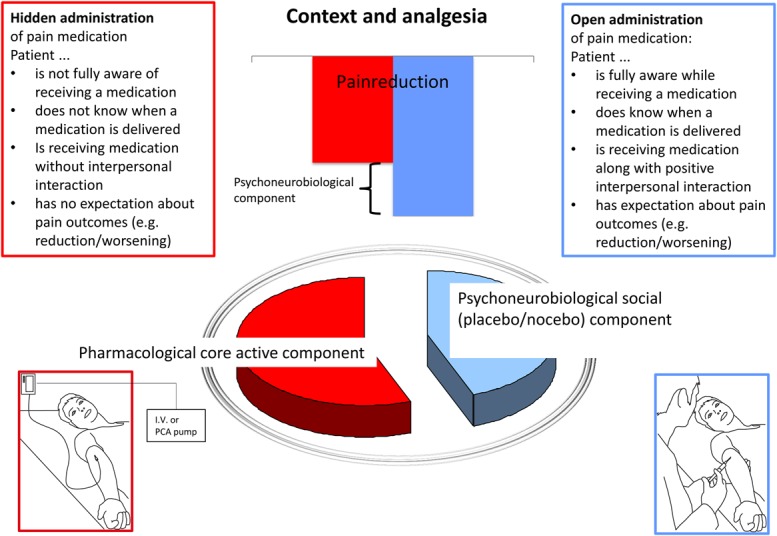
Graphical abstract. The “Open-Hidden” research paradigm developed by Benedetti et al6 and Colloca et al14 is not just an alternative study design but a model that can summarize circumstances of the daily clinical practice impacting the development of the patient's expectancies. A hidden (covert) administration consists of giving a medication without specifying the exact time of administration, for example, throughout a computer-managed infusion pump. An open (overt) administration of a medication takes place in full view and it is perceived by the patient. The latter produces better results than the former type of administration. An administered medication can be perceived by seeing, feeling, smelling, and/or tasting it. The higher the patient's treatment awareness is, the higher is the potential to create positive placebo effects. Thus, any medication can be given in a context in which the patient's expectancies can be either empowered or silenced resulting in turn in better or worse pain outcomes.
In their already mentioned study, Bingel et al8 also investigated the effects of hidden medication (analgesics). The subjects were told that their medication was stopped'; however, they received analgesics covertly. This condition of “no expectation of analgesia” led to significantly decreased pain reduction than the “positive expectation” condition. However, the “negative expectation” condition resulted in the worst outcome. Another study conducted on patients with headache provided either rizatriptan or a placebo with varying instructions, and showed that labeling a verum medication as placebo leads to a significant reduction of its efficacy.26
In both outpatient and inpatient practice, unintentional hidden administration of a treatment may be responsible for lack of full analgesic effectiveness. In the complex hospital setting, most patients cannot identify their pain medication in their unlabeled pillbox or in other forms of applications. They neither know what is included within an infusion, nor can they see the infusion bags. Furthermore, at times the treatment room becomes overwhelming for patients and causes the perception of receiving the medication to disappear among all the hospital setting elements. Because of restricted time management in hospitals, there is little time for communication with the patient, which causes physicians or nurses to not be able to give proper explanation about the analgesics to be given to patients. This unintentional “hidden” administration of the medication results in a significant lack of positive medication effects, and thus becomes a relevant nocebo-induced algesic effect.
In this sense, open administration of medication is crucial when looking to prevent algesic nocebo effects in inpatients, and this is possible by: (1) purposefully structuring the context in which medication is provided to patients, (2) emphasizing relevant and positive information, (3) labeling the medication so that patients have awareness as to what they are receiving (Table 1).
Table 1.
Specific strategies to help structure the patient-clinician communication and avoid nocebo effects

4. Conclusive remarks
In conclusion, this review discussed pain-related factors in clinical practice and settings that can favor the occurrence of nocebo effects and negatively impact pain treatment effectiveness/efficacy. Nonetheless, an adequate and positive patient–clinician communication and interaction could help balance the unfavorable effects that less controllable factors, such as prior negative therapeutic experiences, may elicit in a patient's health and outcomes. Clinical aspects of daily practice can be optimized through an understanding of the elements responsible for the nocebo phenomenon to develop ethically acceptable strategies aimed at improving multidisciplinary pain therapeutic approaches.
Disclosures
Supported by the Department of Anesthesiology, the University Medical Center Hamburg-Eppendorf (UKE) and the Deutsche Forschungsgemeinschaft DFG (FOR35 1328/1 to RK [Kl 1350/3-1]) and the University of Maryland, Baltimore (LC) and the National Institute of Dental and Craniofacial Research (NIDCR, R01DE025946, LC).
The finding agencies have no roles in the study.
The authors have no conflicts of interest to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
References
- [1].Ader R, Mercurio MG, Walton J, James D, Davis M, Ojha V, Kimball AB, Fiorentino D. Conditioned pharmacotherapeutic effects: a preliminary study. Psychosom Med 2010;72:192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Amanzio M, Corazzini LL, Vase L, Benedetti F. A systematic review of adverse events in placebo groups of anti-migraine clinical trials. PAIN 2009;146:261–9. [DOI] [PubMed] [Google Scholar]
- [3].Axford J, Butt A, Heron C, Hammond J, Morgan J, Alavi A, Bolton J, Bland M. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin Rheumatol 2010;29:1277–83. [DOI] [PubMed] [Google Scholar]
- [4].Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA 2002;287:622–7. [DOI] [PubMed] [Google Scholar]
- [5].Benedetti F. Placebo analgesia. Neurol Sci 2006;27:s100–s2. [DOI] [PubMed] [Google Scholar]
- [6].Benedetti F, Amanzio M, Maggi G. Potentiation of placebo analgesia by proglumide. Lancet 1995;346:1231. [DOI] [PubMed] [Google Scholar]
- [7].Benedetti F, Lanotte M, Lopiano L, Colloca L. When words are painful: unraveling the mechanisms of the nocebo effect. Neuroscience 2007;147:260–71. [DOI] [PubMed] [Google Scholar]
- [8].Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 2011;3:70ra14. [DOI] [PubMed] [Google Scholar]
- [9].Colloca L, Benedetti F. Nocebo hyperalgesia: how anxiety is turned into pain. Curr Opin Anaesthesiol 2007;20:435–9. [DOI] [PubMed] [Google Scholar]
- [10].Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. PAIN 2009;144:28–34. [DOI] [PubMed] [Google Scholar]
- [11].Colloca L, Benedetti F. How prior experience shapes placebo analgesia. PAIN 2006;124:126–33. [DOI] [PubMed] [Google Scholar]
- [12].Colloca L, Finniss D. Nocebo effects, patient-clinician communication, and therapeutic outcomes. JAMA 2012;307:567–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Colloca L, Klinger R, Flor H, Bingel U. Placebo analgesia: psychological and neurobiological mechanisms. PAIN 2013;154:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson's disease. Lancet Neurol 2004;3:679–84. [DOI] [PubMed] [Google Scholar]
- [15].Colloca L, Miller FG. The nocebo effect and its relevance for clinical practice. Psychosomatic Med 2011;73:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. PAIN 2010;151:430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. PAIN 2008;136:211–8. [DOI] [PubMed] [Google Scholar]
- [18].Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, Valeriani M. Learning potentiates neurophysiological and behavioral placebo analgesic responses. PAIN 2008;139:306–14. [DOI] [PubMed] [Google Scholar]
- [19].Daniels A, Sallie R. Headache, lumbar puncture, and expectation. Lancet 1981;317:1003. [DOI] [PubMed] [Google Scholar]
- [20].Dieppe P, Goldingay S, Greville-Harris M. The power and value of placebo and nocebo in painful osteoarthritis. Osteoarthritis Cartilage 2016;24:1850–57. [DOI] [PubMed] [Google Scholar]
- [21].Finniss DG, Benedetti F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. PAIN 2005;114:3–6. [DOI] [PubMed] [Google Scholar]
- [22].Flaten MA, Simonsen T, Olsen H. Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosom Med 1999;61:250–5. [DOI] [PubMed] [Google Scholar]
- [23].Geers AL, Helfer SG, Weiland PE, Kosbab K. Expectations and placebo response: a laboratory investigation into the role of somatic focus. J Behav Med 2006;29:171–8. [DOI] [PubMed] [Google Scholar]
- [24].Grimes DA, Schulz KF. Nonspecific side effects of oral contraceptives: nocebo or noise? Contraception 2011;83:5–9. [DOI] [PubMed] [Google Scholar]
- [25].Heisig SR, Shedden-Mora MC, Blanckenburg P, Schuricht F, Rief W, Albert US, Nestoriuc Y. Informing women with breast cancer about endocrine therapy: effects on knowledge and adherence. Psychooncology 2015;24:130–7. [DOI] [PubMed] [Google Scholar]
- [26].Kam-Hansen S, Jakubowski M, Kelley JM, Kirsch I, Hoaglin DC, Kaptchuk TJ, Burstein R. Altered placebo and drug labeling changes the outcome of episodic migraine attacks. Sci Transl Med 2014;6:218ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kaptchuk TJ, Kelley JM, Conboy LA, Davis RB, Kerr CE, Jacobson EE, Kirsch I, Schyner RN, Nam BH, Nguyen LT, Park M, Rivers AL, McManus C, Kokkotou E, Drossman DA, Goldman P, Lembo AJ. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008;336:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kelley JM, Lembo AJ, Ablon JS, Villanueva JJ, Conboy LA, Levy R, Marci CD, Kerr C, Kirsch I, Jacobson EE. Patient and practitioner influences on the placebo effect in irritable bowel syndrome. Psychosomatic Med 2009;71:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Klinger R, Colloca L, Bingel U, Flor H. Placebo analgesia: clinical applications. PAIN 2014;155:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lamont EB, Christakis NA. Complexities in prognostication in advanced cancer: “To help them live their lives the way they want to”. JAMA 2003;290:98–104. [DOI] [PubMed] [Google Scholar]
- [31].Lancman ME, Asconape JJ, Craven WJ, Howard G, Penry JK. Predictive value of induction of psychogenic seizures by suggestion. Ann Neurol 1994;35:359–61. [DOI] [PubMed] [Google Scholar]
- [32].Luparello TJ, Leist N, Lourie CH, Sweet P. The interaction of psychologic stimuli and pharmacologic agents on airway reactivity in asthmatic subjects. Psychosom Med 1970;32:509–13. [DOI] [PubMed] [Google Scholar]
- [33].Lyby PS, Forsberg JT, Asli O, Flaten MA. Induced fear reduces the effectiveness of a placebo intervention on pain. PAIN 2012;153:1114–21. [DOI] [PubMed] [Google Scholar]
- [34].Miller FG, Colloca L. The placebo phenomenon and medical ethics: Rethinking the relationship between informed consent and risk-benefit assessment. Theor Med Bioeth 2011;32:229–43. [DOI] [PubMed] [Google Scholar]
- [35].Mitsikostas D, Chalarakis N, Mantonakis L, Delicha EM, Sfikakis P. Nocebo in fibromyalgia: meta-analysis of placebo-controlled clinical trials and implications for practice. Eur J Neurol 2012;19:672–80. [DOI] [PubMed] [Google Scholar]
- [36].Mitsikostas DD, Mantonakis LI, Chalarakis NG. Nocebo is the enemy, not placebo. A meta-analysis of reported side effects after placebo treatment in headaches. Cephalalgia 2011;31:550–61. [DOI] [PubMed] [Google Scholar]
- [37].Mondaini N, Gontero P, Giubilei G, Lombardi G, Cai T, Gavazzi A, Bartoletti R. Finasteride 5 mg and sexual side effects: how many of these are related to a nocebo phenomenon? J Sex Med 2007;4:1708–12. [DOI] [PubMed] [Google Scholar]
- [38].Mora MS, Nestoriuc Y, Rief W. Lessons learned from placebo groups in antidepressant trials. Philos Trans R Soc Lond B Biol Sci 2011;366:1879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Müller M, Kamping S, Benrath J, Skowronek H, Schmitz J, Klinger R, Flor H. Treatment history and placebo responses to experimental and clinical pain in chronic pain patients. Eur J Pain 2016;20:1530–41. [DOI] [PubMed] [Google Scholar]
- [40].Myers MG, Cairns JA. The consent form as of side effects. Clin Pharmacol Ther 1987;42:250–3. [DOI] [PubMed] [Google Scholar]
- [41].Nestoriuc Y, von Blanckenburg P, Schuricht F, Barsky A, Hadji P, Albert US, Rief W. Is it best to expect the worst? Influence of patients' side-effect expectations on endocrine treatment outcome in a 2-year prospective clinical cohort study. Ann Oncol 2016;27:1909–15. [DOI] [PubMed] [Google Scholar]
- [42].Papadopoulos D, Mitsikostas DD. A meta-analytic approach to estimating nocebo effects in neuropathic pain trials. J Neurol 2012;259:436–47. [DOI] [PubMed] [Google Scholar]
- [43].Peerdeman KJ, van Laarhoven AI, Keij SM, Vase L, Rovers MM, Peters ML, Evers AW. Relieving patients' pain with expectation interventions: a meta-analysis. J Pain 2016;157:1179–91. [DOI] [PubMed] [Google Scholar]
- [44].Petrovic P. Placebo analgesia and nocebo hyperalgesia—two sides of the same coin? PAIN 2008;136:5–6. [DOI] [PubMed] [Google Scholar]
- [45].Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol 2008;59:565–90. [DOI] [PubMed] [Google Scholar]
- [46].Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. PAIN 1999;83:147–56. [DOI] [PubMed] [Google Scholar]
- [47].Rosenzweig P, Brohier S, Zipfel A. The placebo effect in healthy volunteers: influence of experimental conditions on the adverse events profile during phase I studies. Clin Pharmacol Ther 1993;54:578–83. [DOI] [PubMed] [Google Scholar]
- [48].Sakala C. Letter from North America: understanding and minimizing nocebo effects in childbearing women. Birth 2007;34:348–50. [DOI] [PubMed] [Google Scholar]
- [49].Taddio A, Shah V, Gilbert-MacLeod C, Katz J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA 2002;288:857Y61. [DOI] [PubMed] [Google Scholar]
- [50].Varelmann D, Pancaro C, Cappiello EC, Camann WR. Nocebo-induced hyperalgesia during local anesthetic injection. Anesth Analg 2010;110:868–70. [DOI] [PubMed] [Google Scholar]
- [51].Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients: an empirical investigation. PAIN 2003;105:17–25. [DOI] [PubMed] [Google Scholar]
- [52].Wells RE, Kaptchuk TJ. To tell the truth, the whole truth, may do patients harm: the problem of the nocebo effect for informed consent. Am J Bioeth 2012;12:22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


