Abstract
IMPORTANCE
Chemotherapy-induced alopecia is a common and distressing adverse effect. In previous studies of scalp cooling to prevent chemotherapy-induced alopecia, conclusions have been limited.
OBJECTIVES
To evaluate whether use of a scalp cooling system is associated with a lower amount of hair loss among women receiving specific chemotherapy regimens for early-stage breast cancer and to assess related changes in quality of life.
DESIGN, SETTING, AND PARTICIPANTS
A prospective cohort study conducted at 5 US medical centers of women with stage I or II breast cancer receiving adjuvant or neoadjuvant chemotherapy regimens excluding sequential or combination anthracycline and taxane (106 patients in the scalp cooling group and 16 in the control group; 14 matched by both age and chemotherapy regimen). The study was conducted between August 2013 and October 2014 with ongoing annual follow-up for 5 years.
EXPOSURES
Use of a scalp cooling system. Scalp cooling was initiated 30 minutes prior to each chemotherapy cycle, with scalp temperature maintained at 3°C (37°F) throughout chemotherapy and for 90 minutes to 120 minutes afterward.
MAIN OUTCOMES AND MEASURES
Self-estimated hair loss using the Dean scale was assessed 4 weeks after the last dose of chemotherapy by unblinded patient review of 5 photographs. A Dean scale score of 0 to 2 (≤50% hair loss) was defined as treatment success. A positive association between scalp cooling and reduced risk of hair loss would be demonstrated if 50% or more of patients in the scalp cooling group achieved treatment success, with the lower bound of the 95% CI greater than 40% of the success proportion. Quality of life was assessed at baseline, at the start of the last chemotherapy cycle, and 1 month later. Median follow-up was 29.5 months.
RESULTS
Among the 122 patients in the study, the mean age was 53 years (range, 28–77 years); 77.0% were white, 9.0% were black, and 10.7% were Asian; and the mean duration of chemotherapy was 2.3 months (median, 2.1 months). No participants in the scalp cooling group received anthracyclines. Hair loss of 50% or less (Dean score of 0–2) was seen in 67 of 101 patients (66.3%; 95% CI, 56.2%–75.4%) evaluable for alopecia in the scalp cooling group vs 0 of 16 patients (0%) in the control group (P < .001). Three of 5 quality-of-life measures were significantly better 1 month after the end of chemotherapy in the scalp cooling group. Of patients who underwent scalp cooling, 27.3% (95% CI, 18.0%–36.6%) reported feeling less physically attractive compared with 56.3% (95% CI, 31.9%–80.6%) of patients in the control group (P = .02). Of the 106 patients in the scalp cooling group, 4 (3.8%) experienced the adverse event of mild headache and 3 (2.8%) discontinued scalp cooling due to feeling cold.
CONCLUSIONS AND RELEVANCE
Among women undergoing non–anthracycline-based adjuvant chemotherapy for early-stage breast cancer, the use of scalp cooling vs no scalp cooling was associated with less hair loss at 4 weeks after the last dose of chemotherapy. Further research is needed to assess outcomes after patients receive anthracycline regimens, longer-term measures of alopecia, and adverse effects.
Breast cancer is the most common malignancy among women worldwide,1 and treatment often includes adjuvant chemotherapy. Improvements in supportive care have reduced toxic effects; however, chemotherapy-induced alopecia remains almost universal and is rated by patients with breast cancer as one of the most distressing treatment-related adverse effects.2,3
Scalp cooling has been used in more than 30 countries as a potential mechanism to prevent chemotherapy-induced alopecia.4 One review of more than 6000 patients suggested that scalp cooling was effective, but not for all patients.5 Efficacy results vary significantly and studies include heterogeneous chemotherapy regimens, patients with multiple types of cancer, nonvalidated outcome measures (such as use of wigs), and subjective hair loss assessment.4
Possible mechanisms for efficacy of scalp cooling include vasoconstriction with reduced delivery of chemotherapy to the scalp, reduced cellular drug uptake, and decreased follicular metabolic rate.6–8 Cooling techniques include frozen caps replaced every 30 minutes and scalp cooling systems that circulate coolant into a cap. Scalp cooling systems have several advantages over cooling caps, including the lack of needing to freeze caps and coordinate cap changes.9
Although scalp cooling has been available for several decades in Europe, use has been limited in the United States due to insufficient prospective efficacy data with current chemotherapy regimens, lack of US Food and Drug Administration (FDA) clearance, underestimation of the psychological effect of hair loss, and concerns about the theoretical risk of scalp metastases. Recent studies showed no association between scalp cooling and scalp metastases or decreased survival among patients with breast cancer,10–13 reducing these concerns.
This prospective cohort study was designed to explore the association between scalp cooling and hair loss among women receiving adjuvant chemotherapy for breast cancer, excluding combination chemotherapy with anthracyclines.
Methods
Study Design and Oversight
This multicenter, prospective cohort study was conducted at 5 medical centers (6 sites) to evaluate scalp hypothermia using a scalp cooling device (DigniCap, Dignitana AB) under an investigational device exemption from the FDA. A concurrent age- and chemotherapy treatment–matched control group was included to demonstrate that the included chemotherapy regimens cause severe hair loss. The study protocol (appears in Supplement 1) and consent process were approved by the institutional review board at each center. All patients signed written informed consent. To encourage return visits, patients at some sites were offered gift cards (valued between $40 and $50) at the 4-week visit and at each annual follow-up visit.
Patients
Eligibility criteria included (1) females aged 18 years or older with stage I or II breast cancer, (2) planned neoadjuvant cytotoxic therapy including an anthracycline or taxane (anthracyline and taxane could not be used together or in sequence), (3) liver function test results of less than 1.5 times the upper limit of normal, (4) a Karnofsky performance status of 80% or greater (normal activity with effort; some sign or symptoms of disease), and (5) a planned chemotherapy regimen to be completed within 6 months. Patients with female-pattern baldness resembling picture I-3 or higher on the Savin (or Ludvig) scale at baseline were excluded.14 Information on race/ethnicity was collected because fitting of the cap may differ according to hair texture and head shape. Race/ethnicity was self-reported by patients using fixed categories.
Eligible chemotherapy regimens included docetaxel and cyclophosphamide, doxorubicin and cyclophosphamide, docetaxel and carboplatin (with human epidermal growth factor receptor 2 [HER2/ERBB2]–targeted therapy), weekly paclitaxel, dose-dense paclitaxel, paclitaxel and carboplatin, and docetaxel with HER2/ERBB2–targeted therapy (eBox in Supplement 2). Dose reductions or delays were allowed for adverse toxic effects per standard guidelines. Patients electing not to undergo scalp cooling were enrolled in the concurrent control group.
Patients in the control group were matched retrospectively to a patient at the same investigative site by age (within 5 years) and chemotherapy regimen. Because complete hair loss occurs in more than 65% of patients receiving the chemotherapy regimens allowed in this study,15 the number of control patients required was minimized after discussion with the FDA (representatives from the FDA, oral communication, February 11, 2013). Thirty control patients were planned; however, if at least 12 of 15 (80%) lost at least 50% of their hair by the time of the preplanned interim analysis, which was assessed by an independent data and safety monitoring board using photographs, enrollment to the control group would be stopped.
Scalp Cooling Procedure
Scalp cooling was initiated 30 minutes prior to each chemotherapy cycle by fitting the silicone cap on the patient’s head, followed by application of an insulating neoprene cap. The silicone cap was then gradually cooled to the target treatment temperature by liquid coolant (monopropylene glycol) circulating through channels within 2 cooling compartments (front and back). Hoses connect the silicone cap to the computerized cooling and control unit to maintain a constant controlled scalp temperature during the treatment period. Scalp temperature was monitored by 2 separate sensors at the front and back of the cap. An additional sensor ensures that the temperature never decreases below freezing. Deviations from the default temperature were automatically adjusted. Scalp temperature was to be maintained at 3°C (37°F) throughout chemotherapy and for 90 minutes to 120 minutes afterward, resulting in a scalp temperature of approximately 15°C.16
Study End Point
The primary end point was prevention of hair loss 4 weeks after the completion of all cycles of chemotherapy. Treatment success was defined as a patient self-assessed maximum Dean score of 2 or less (hair loss of ≤50%; eTable 1 in Supplement 2),12 correlating with grade 1 alopecia as defined using version 4.0 of the Common Terminology Criteria for Adverse Events.
Assessment of Estimated Hair Loss
To assess hair status, photographs of patients’ hair in the treatment group and in the control group were taken by study personnel before the start of each chemotherapy cycle and at 3 to 6 weeks after the last chemotherapy cycle. Patients receiving weekly paclitaxel had photographs taken at weeks 1, 2, 4, 6, 8, 10, and 12, and 3 to 6 weeks after the last dose. Photographs captured hair from the front (bangs held back), back, both sides, and the top with hair divided in the midline with hands. Patients assessed and estimated the percentage of hair loss using the Dean scale (score range: 0, 0% hair loss to 4, hair loss >75%) without blinding and in real time by comparing baseline photographs with the photographs taken during the current chemotherapy cycle and displayed side by side on a tablet device. Preplanned analyses of 4-week follow-up photographs were scored by an independent panel consisting of 3 raters to further validate patient assessment results. The independent panel also used the Dean scale and standardized photographs for the assessments and were blinded to treatment group.
Secondary End Points
Secondary objectives included patient-reported toxic effects and tolerability. Toxic effects were determined by patient-reported adverse events and by scalp examination. Annual follow-up is ongoing and will last for 5 years to determine any incidence of scalp metastases.
Tolerability was defined as the percentage of patients who completed all planned cycles of chemotherapy while using the scalp cooling system. Feelings of chilliness, headaches, and scalp pain were recorded using the Patient Symptom Survey (eFigure in Supplement 2) along with any use of head coverings. Satisfaction with the scalp cooling system was recorded 1 month after completion of chemotherapy. In addition, cap device malfunction (such as sensor or cooling cable issues, cooling fluid leakage) were captured.
Quality of life was measured using the European Organization for Research and Treatment of Cancer Breast Cancer-Specific Quality of Life Questionnaire administered at baseline, at the time of the last chemotherapy cycle, and 1 month after completion of chemotherapy.17 Four response categories were collapsed to (1) not at all or a little bit and (2) quite a bit or very much. Quality of life was compared in a prespecified analysis for (1) patients who underwent scalp cooling vs those who did not and (2) patients with hair loss of 50% or less (Dean score of 0–2) vs those with hair loss greater than 50% (Dean score of 3–4) 1 month after completing chemotherapy.
The assessment of the association of hair loss with breast cancer treatment decisions at 6 months after completion of chemotherapy is ongoing.
Statistical Considerations
According to the predetermined statistical analysis plan, a positive association between scalp cooling and reduced risk of hair loss would be demonstrated if the following criteria were met: (1) the proportion was 50% or greater for patients enrolled in the scalp cooling group having hair loss of less than 50% (Dean score of 0–2); (2) the lower bound of the 95% CI of the success proportion was greater than 40%; and (3) the statistical superiority of the scalp cooling group over the control group was confirmed by the Fisher exact test at a significance level of .05.
A sample size of 110 patients was planned for the scalp cooling group with an expected dropout rate of 10% to achieve 100 patients for the primary analysis and 15 to 30 control patients. Using the Fisher exact test to compare the groups with a type I error rate of 5% (2-sided test), there was 90% power to detect the between-group proportion differences of 20% or less when the sample size was 15 for the control group and 66% or greater when the sample size was 100 for the scalp cooling group. To ensure relative balance across sites, a minimum of 15 patients per site were enrolled; however, the analysis was not stratified by study center.
To analyze the primary end point, results were recorded as treatment failures (>50% hair loss) for patients with missing end point assessment (ie, without Dean scores) or study dropouts for any reason other than chemotherapy adverse effects. The results from patients who dropped out due to chemotherapy adverse effects without assessment were not imputed. Descriptive statistics using sample size, mean, standard deviation, median, and range were used for continuous variables and count and percentage were used for categorical variables. Between-group comparisons of the Dean score for hair loss was analyzed using the Fisher exact test. The 95% CI of the success proportion for patients using the scalp cooling system was estimated using an exact method based on binomial distribution. For the quality-of-life analyses, the 95% CI of the proportion responding “quite a bit or very much” for each question was generated using an asymptotic approach.
For patients in the scalp cooling group, data are reported only for those answering quality-of-life questions 1 month after the end of chemotherapy. The last observation carried forward was used for missing quality-of-life data for the control group. The P value for the comparison between success and failure was determined using a 2-sided χ2 test and a significance level of .05.
In addition to the patients included in the primary analysis, a sensitivity analysis was performed to examine 2 different scenarios for the maximum Dean score. The first scenario of the safety population was an efficacy analysis in which all patients who dropped out of the study for any reason were considered treatment failures. The second scenario was a per-protocol analysis in which only patients who completed the full series of measurements and adhered to the protocol were included. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc).
Results
Patients and Chemotherapy
There were 122 patients enrolled in the study (106 in the scalp cooling group and 16 in the control group; Figure 1) and included in the tolerability assessment. The study was conducted between August 2013 and October 2014 with ongoing annual follow-up for 5 years. A total of 117 patients were included in the primary analysis (101 in the scalp cooling group and 16 in the control group) and completed their prescribed chemotherapy regimen or dropped out for any reason other than chemotherapy adverse effects.
Figure 1. Flowchart of Patients in Scalp Cooling Trial.
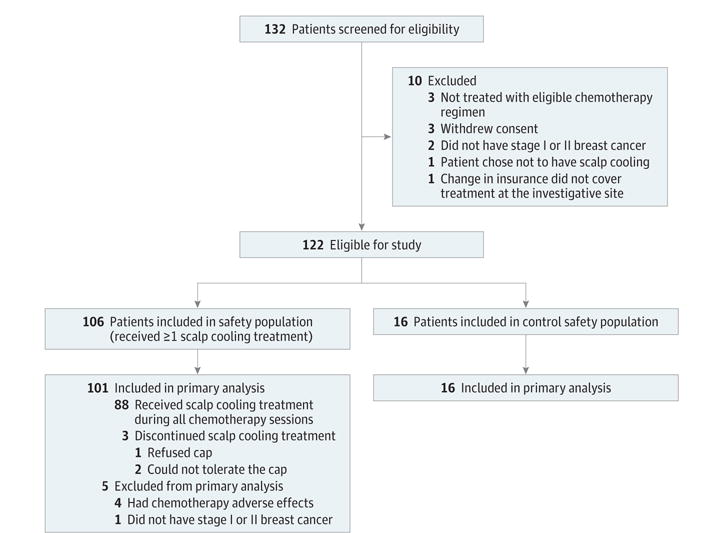
The primary analysis estimate of hair loss included all eligible patients who received at least 1 cycle of chemotherapy and who did not discontinue scalp cooling due to adverse effects of chemotherapy or a major protocol violation (nonadherence). Patients electing not to undergo scalp cooling were enrolled in the concurrent control group. Patients in the control group were matched retrospectively to a patient at the same investigative site by age (within 5 years) and the same chemotherapy regimen.
The mean age of treated patients was 53 years (range, 28–77 years); 77.0% were white, 9.0% were black, and 10.7% were Asian. The demographic profile of the patients in the scalp cooling group and the control group was closely matched (Table 1). Baseline medical characteristics for medical history, physical examination, vital signs, clinical laboratory results, prior cancer therapy, scalp surgery, and concomitant medications were similar between groups.
Table 1.
Demographics and Baseline Information
| No. (%) of Patientsa | ||
|---|---|---|
| Scalp Cooling Group (n = 106) | Control Group (n = 16) | |
| Age, mean (SD), y | 53 (11.2) | 55 (8.6) |
| Body mass index, mean (SD)b | 26.1 (0.5) | 28.0 (1.2) |
| Postmenopausal | 51 (48.1) | 9 (56.3) |
| Type of thyroid problem | ||
| Hypothyroid | 11 (10.4) | 1 (6.3) |
| Hyperthyroid | 2 (1.9) | 0 |
| Prior chemotherapy | 4 (3.8) | 0 |
| Prior hormone therapy | 19 (17.9) | 2 (12.5) |
| Ethnicity | ||
| Non-Hispanic | 88 (83.0) | 15 (93.8) |
| Hispanic | 17 (16.0) | 1 (6.3) |
| Missing | 1 (0.9) | 0 |
| Race | ||
| White | 82 (77.4) | 12 (75.0) |
| Black | 11 (10.4) | 0 |
| Asian | 10 (9.4) | 3 (18.8) |
| Multiracial | 1 (0.9) | 1 (6.3) |
| Missing | 2 (1.9) | 0 |
Unless otherwise indicated.
Calculated as weight in kilograms divided by height in meters squared.
The most common chemotherapy regimen was docetaxel and cyclophosphamide for 4 to 6 cycles (75%; 76 of 89 for 4 cycles); other regimens included docetaxel and carboplatin (12.8%), weekly paclitaxel (11.9%), and docetaxel (1%) (Table 2). The last 3 chemotherapy regimens were given with HER2/ERBB2–targeted therapy. The mean duration of chemotherapy was 2.3 months (median, 2.1 months).
Table 2.
Chemotherapy Regimens in Treatment and Control Groups
| Chemotherapy Regimen | No. (%) of Patients | |
|---|---|---|
| Scalp Cooling Group (n = 101)a | Control Group (n = 16) | |
| Docetaxel (75 mg/m2) and cyclophosphamide (600 mg/m2) for 4–6 cycles every 3 wk | 76 (75.2) | 10 (62.5) |
| Paclitaxel (80 mg/m2) weekly for 12 cycles | 12 (11.9) | 2 (12.5) |
| Docetaxel (75 mg/m2), carboplatin (area under the concentration time curve: 6) for 6 cycles every 3 wk, and trastuzumab weekly or every 3 wk with or without pertuzumab every 3 wk | 12 (11.9) | 3 (18.8) |
| Docetaxel (75 mg/m2), trastuzumab, and pertuzumab every 3 wk for 6 cycles | 1 (1.0) | 0 |
| Doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every 3 wk for 4 cycles | 0 | 1 (6.3) |
There were 5 patients who were not included in the primary analysis.
Scalp Cooling and Hair Loss
Of the 101 patients in the scalp cooling group included in the primary analysis, 67 (66.3%; 95% CI, 56.2%–75.4%) demonstrated hair loss of 50% or less (Dean score of 0–2) compared with 0 of 16 (0%) in the control group (P < .001; Table 3). Not all patients had completed the 1-month evaluation before the post hoc analysis was performed by the independent panel. The independent panel analysis reported hair loss of 50% or less (Dean score of 0–2) in 74 of the 88 patients (84.1%) who were evaluated (eTable 2 in Supplement 2).
Table 3.
Alopecia Self-Report
| Maximum Dean Scorea | Hair Loss, % | Scalp Cooling Group (n = 101)b | Control Group (n = 16) | P Valuec | ||
|---|---|---|---|---|---|---|
| No. of Patients | % (95% CI) | No. of Patients | % (95% CI) | |||
| 0 | 0 | 5 | 5.0 (1.6–11.2) | 0 | <.001 | |
| 1 | >0–≤25 | 31 | 30.7 (21.9–40.7) | 0 | ||
| 2 | >25–≤50 | 31 | 30.7 (21.9–40.7) | 0 | ||
| 3 | >50–≤75 | 19 | 18.8 (11.7–27.8) | 1 | 6.3 (0–30.2) | |
| 4 | >75 | 15 | 14.9 (8.6–23.3) | 15 | 93.8 (69.8–99.8) | |
| 0–2 | 0–≤50 | 67 | 66.3 (56.2–75.4) | 0 | <.001 | |
| ≥3 | >50 | 34 | 33.7 (24.6–43.8) | 16 | 100.0 (79.4–100.0) | |
Indicates highest score measured during the study up to 4 weeks after last chemotherapy treatment. A score of less than 3 indicates that the treatment was a success; a score of equal to or greater than 3 indicates that the treatment was a failure.
There were 5 patients who were not included in the primary analysis.
Calculated using the Fisher exact test.
Photographs of a patient with a Dean score of 1 appear in Figure 2A and of a patient with a Dean score of 4 appear in Figure 2B. Two patients in the scalp cooling group discontinued treatment after 1 chemotherapy cycle (1 discontinued for nonadherence and 1 refused further scalp cooling). For these 2 patients, a Dean score of 4 was recorded. The sensitivity analysis performed on the control safety population showed similar results for the self-reported maximum Dean score compared with the patients included in the primary analysis with alopecia self-report at 1 month after the last cycle of chemotherapy (eTable 3 in Supplement 2).
Figure 2. Photographic Results of 2 Patients Treated With Scalp Cooling.
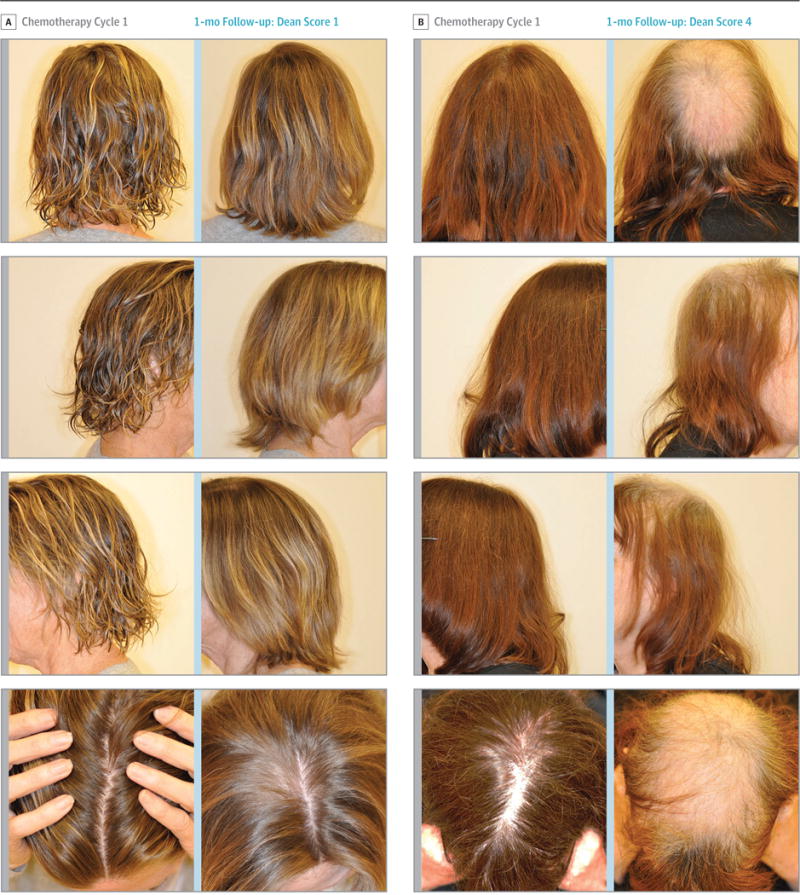
A, Patient photographs from 4 different angles before the start of chemotherapy cycle 1 (Dean score of 0; hair loss of 0%) and 1 month after completion of 4 cycles of chemotherapy with docetaxel (60 mg/m2) and cyclophosphamide (600 mg/m2) (Dean score of 1; hair loss >0%-≤25%).8
B, Patient photographs from 4 different angles before the start of chemotherapy cycle 1 (Dean score of 0) and 1 month after completion of 4 cycles of chemotherapy with docetaxel (60 mg/m2) and cyclophosphamide (600 mg/m2) (Dean score of 4; hair loss >75%).8
Patients who had a self-reported alopecia Dean score of 0 to 2 were analyzed by chemotherapy regimen: (1) docetaxel and carboplatin (10 of 12 patients in the scalp cooling group [83.3%; 95% CI, 51.6%–97.9%] vs 0 of 3 in the control group; P = .02), (2) docetaxel and cyclophosphamide (46 of 76 patients in the scalp cooling group [60.5%; 95% CI, 48.6%–71.6%] vs 0 of 10 in the control group; P < .001), (3) paclitaxel (10 of 12 patients in the scalp cooling group [83.3%; 95% CI, 51.6%–97.9%] vs 0 of 2 in the control group; P = .07), and (4) docetaxel with monoclonal antibodies (1 patient in the scalp cooling group vs 0 of 0 in the control group; eTable 4 in Supplement 2). One patient in the control group received doxorubicin and cyclophosphamide and was not included in the analysis.
The proportion of patients with hair loss of 50% or less (Dean score of 0–2) did not differ when analyzed by hair thickness and quality (self-assessed by the patient), history of previous chemotherapy, median age, median body mass index, and use of prior hormone therapy (eTable 5 in Supplement 2).
In the interim analysis, of 16 patients in the control group, 15 were classified as having a Dean score 4 and 1 had a Dean score of 3; therefore, recruitment to the control group was discontinued as planned.
Quality of Life
Each quality-of-life measure at study entry was comparable for patients in the scalp cooling group with subsequent hair loss of 50% or less (Dean score of 0–2) and those with hair loss of greater than 50% (Dean score of 3 or 4; eTable 6 in Supplement 2). Among all patients, there were significant between-group differences 1 month after chemotherapy for 3 of 5 quality-of-life measures (Table 4). For example, 27.3% (95% CI, 18.0%–36.6%) of patients in the scalp cooling group reported feeling less physically attractive compared with 56.3% (95% CI, 31.9%–80.6%) of patients in the control group (P = .02). The results were similar for patients with hair loss of 50% or less in the scalp cooling group compared with the control group (eTable 7 in Supplement 2).
Table 4.
Quality-of-Life Responses to the EORTC Questionnaire
| EORTC Breast Cancer-Specific Quality of Life Questionnaire Item | Response of “Quite a Bit” or “Very Much”a | Absolute Difference, % (95% CI) | P Valuec | |||
|---|---|---|---|---|---|---|
| Scalp Cooling Group | Control Groupb | |||||
| Analytic Sample Size | Response Rate, % (95% CI) | Analytic Sample Size | Response Rate, % (95% CI) | |||
| Have you lost any hair? | 88 | 33.0 (23.1 to 42.8) | 16 | 68.8 (46.0 to 91.5) | −35.8 (−60.5 to −11.1) | .007 |
| Were you upset about your loss of hair? | 74 | 32.4 (21.8 to 43.1) | 15 | 60.0 (35.2 to 84.8) | −27.6 (−54.6 to −0.58) | .04 |
| Have you felt physically less attractive as a result of your disease or treatment? | 88 | 27.3 (18.0 to 36.6) | 16 | 56.3 (31.9 to 80.6) | −29.0 (−55.0 to −3.0) | .02 |
| Have you been feeling less feminine as a result of your disease or treatment? | 88 | 21.6 (13.0 to 30.2) | 16 | 31.3 (8.5 to 54.0) | −9.7 (−33.9 to −14.6) | .40 |
| Did you find it difficult to look at yourself naked? | 88 | 15.9 (8.3 to 23.6) | 16 | 18.8 (0 to 37.9) | −2.8 (−23.4 to −17.8) | .78 |
| Have you been dissatisfied with your body? | 88 | 15.9 (8.3 to 23.6) | 16 | 37.5 (13.8 to 61.2) | −21.6 (−46.5 to −3.3) | .04 |
Abbreviation: EORTC, European Organization for Research and Treatment of Cancer.
Responses collected 1 month after the end of chemotherapy.
The last observation carried forward was used.
Calculated using the χ2 test.
Adverse Events
Six of the 106 patients in the safety population experienced 7 adverse events that were considered related to the scalp cooling system treatment, including headache (4 patients), pruritus (1 patient), skin pain (1 patient), and head discomfort (1 patient); none of these events were rated severe and 1 patient had a headache that was rated as moderate using version 4.0 of the Common Terminology Criteria for Adverse Events. No patient has developed scalp metastases with a median follow-up from last chemotherapy administration of 29.5 months (range, 24.4–34.8 months). Patient follow-up will continue for 5 years.
Tolerability
Of 106 patients in the scalp cooling group, 88 (83%) completed all planned cycles of chemotherapy. Three patients (2.8%) discontinued scalp cooling because of feeling cold. Eleven patients (10%) did not complete all chemotherapy cycles due to hair loss (n = 7) or chemotherapy adverse effects (n = 4).
In the Patient Symptom Survey (on a scale of 0 to 100 with a score of 100 indicating the worst), a feeling of chilliness was reported by 104 of 106 (98%) with a mean score of 49.0 (range, 7.5–97.5; n = 102) during the cooling down period and a mean score of 49.5 (range, 2.5–92.5; n = 104) during overall cooling. Forty-three of 106 patients (41%) reported having headaches (mean, 1 cycle of headaches; range, 0–10 cycles of headaches) triggered or exacerbated by the scalp cooling treatment and reported a mean pain level of 39.3 (range, 10–95).
Scalp pain associated with the scalp cooling treatment was reported by 75 of 106 patients (71%) with a mean pain level of 24.2 (range, 1.7–85.0). Patients reported use of a pain medication in 101 of 517 chemotherapy cycles. Of 86 patients, head coverings were used at least some of the time in 47 (55%) and never in 39 (45%) (eTable 8 in Supplement 2). Patients had a mean satisfaction score of 90.3 (range, 10–100 with a score of 100 indicating complete satisfaction) regarding their decision to use scalp cooling.
A device incident was reported during 22 of 519 scalp cooling chemotherapy sessions in 21 patients. The majority of incidents were related to cap or sensor malfunction, which were resolved by replacing the cap. There were no cases of scalp cooling treatment failure due to a device incident.
Discussion
Among women undergoing non–anthracycline-based chemotherapy for stage I or II breast cancer, the use of a scalp cooling system during chemotherapy cycles was associated with less hair loss after 4 weeks of completing all planned cycles of chemotherapy. All patients in the control group experienced severe hair loss. Three of 5 quality-of-life measures were significantly better for women who underwent scalp cooling while receiving chemotherapy.
The scalp cooling system was well tolerated and 83% of patients completed all planned cycles of chemotherapy. The majority of the device incidents were related to cap malfunction, but none resulted in scalp cooling treatment failure. Estimated hair loss was not related to patient-assessed characteristics, including hair quality or thickness.
Multiple studies have documented the importance of hair loss to patients, and the effect of chemotherapy-induced alopecia on quality of life. In this study, patients in the scalp cooling group felt less upset about losing their hair compared with patients in the control group and were less dissatisfied with their body. Among those in the scalp cooling group, patients who had less hair loss were less upset about the overall loss of their hair, experienced less of a negative effect from their disease or treatment regarding feelings of physically attractiveness, and experienced less of a negative effect regarding feelings of femininity compared with those who experienced more hair loss in the control group. These data suggest that when scalp cooling is successful at decreasing hair loss, it could improve the treatment experience for women undergoing adjuvant chemotherapy for early-stage breast cancer.
In the present study, no patients have developed scalp metastases during a median follow-up of more than 2 years; however, long-term follow-up is ongoing. A review of all published studies found no evidence of increased scalp metastases in patients who received scalp cooling,12 and a recent retrospective cohort study demonstrated no association of scalp cooling with survival in patients with early-stage breast cancer receiving adjuvant or neoadjuvant chemotherapy.13 Based on these and other data, the risk of scalp metastases after receipt of scalp cooling treatment appears to be very small.10–13
The efficacy of scalp cooling has been reported to depend on the type of chemotherapy regimen, dose and schedule, infusion duration, patient performance status, drug metabolism, concomitant comorbidities, scalp cooling temperature, postinfusion cooling time, and the type of scalp cooling system.18,19 The optimal duration of postinfusion cooling time is not clear, with one study suggesting similar efficacy with shorter durations.20 Our study included commonly used regimens for stage I or II breast cancer in the United States21 and excluded patients receiving sequential or concordant anthracycline and taxane regimens, but we plan to study more intensive regimens in subsequent studies. A postcooling duration of 90 minutes to 120 minutes was used.
Previous studies have evaluated patients with various non-validated methods and few matched controls, or obtained data retrospectively.8,9,22–26 One larger trial (N = 238 patients) allocated patients with a variety of malignancies and who were receiving a range of doses and cycles of docetaxel to 2 different cooling systems (Paxman system or Penguin cold caps) or to no cooling; efficacy was determined by physician assessment or wig use.27 A Dutch registry prospectively evaluated scalp cooling by use of a head covering in 1411 patients, and reported high success rates in most patients except for those receiving the most intense anthracycline- and taxane-based regimens.28
This study differs from the majority of reports in several ways. It was a multicenter study of a self-contained scalp cooling system that did not rely on freezing or changing caps during treatment and used standardized photographs to grade hair loss. The study enrolled patients with early-stage breast cancer treated only with specific chemotherapy regimens to standardize the analysis of the results. The primary end point was based on patient self-assessment, a relevant method for determining relative usefulness or worth of the device to patients themselves.3,29–32 Age- and chemotherapy treatment–matched controls were enrolled to demonstrate that the chemotherapy regimens in this study caused severe hair loss.
There are a number of limitations to this study. First is the use of a cohort study design without randomization, and a relatively small sample size. Given that the chemotherapy regimens used in this study are known to cause marked alopecia, it was determined that a relatively small, single-group study, with a limited number of matched controls and a carefully assessed end point would be likely to provide adequate data. In this study, all patients in the control group were graded as treatment failures (>50% hair loss). Second, patients included in this study did not receive anthracycline-based chemotherapy regimens. Scalp cooling has been studied in a randomized clinical trial that included patients with breast cancer receiving combination anthracycline and taxane regimens.33 Third, the choice of a patient-assessed end point rather than by independent raters masked to treatment group is another limitation; however, this end point was carefully considered. Compared with a blinded panel, patient scores were generally lower in a 20-patient pilot study using the same scalp cooling system34 as used in the current study. Because hair preservation by scalp cooling is only important if assessed as successful by patients, the FDA requested the use of patient assessment based on 5 photographs compared with baseline as the primary end point (representatives from the FDA, oral communication, February 13, 2013). The similarity in success rates found by the preplanned assessment by an independent panel further supports this approach. However, the necessary lack of patient blinding for the primary outcome of hair loss remains a limitation. Fourth, the follow-up for risk of scalp metastases is short at 2.5 years; however, follow-up is ongoing.
Conclusions
Among women undergoing non–anthracycline-based adjuvant chemotherapy for early-stage breast cancer, the use of scalp cooling vs no scalp cooling was associated with less hair loss at 4 weeks after the last dose of chemotherapy. Further research is needed to assess outcomes after patients receive anthracycline regimens, longer-term measures of alopecia, and adverse effects.
Supplementary Material
Key Points.
Question
Is scalp cooling associated with a lower risk of hair loss when used by women receiving common adjuvant chemotherapy regimens for early-stage breast cancer?
Findings
In this multicenter study, hair loss of 50% or less (Dean score of 0–2) was seen in 66.3% of patients in the scalp cooling group vs 0% of patients in the control group at 4 weeks after completing non–anthracycline-based adjuvant chemotherapy. Three of 5 quality-of-life measurements, including feeling less physically attractive, showed benefit for women who received scalp cooling.
Meaning
This self-contained cooling system was associated with a lower risk of hair loss among women receiving non–anthracycline-based chemotherapy for early-stage breast cancer.
Acknowledgments
Funding/Support: The study was funded partially by Dignitana AB, the Lazlo Tauber Family Foundation (awarded to the University of California, San Francisco), the Anne Moore Breast Cancer Research Fund (awarded to the Weil Cornell Medical College), and the Friedman Family Foundation (awarded to Mount Sinai Beth Israel).
Dr Hurvitz reported receiving grant funding Amgen, Bayer, Boehringer Ingelheim, Dignitana, Genentech, GlaxoSmithKline, Lilly, Medivation, Novartis, Pfizer, Roche, Biomarin, Merrimack, OBI Pharma, and Puma; and travel funding from Lilly, Novartis, and OBI Pharma. Dr Melisko reported receiving institutional grant funding from the Tauber Foundation. Dr Park reported receiving compensation from Dignitana for the conduct and analysis of this study. Dr Mitchel reported receiving compensation from Dignitana for managing this trial and interacting with the US Food and Drug Administration. Dr Bågeman reported being an employee of Dignitana AB, the sponsor of study. Dr D’Agostino reported receiving fees for serving as a consultant to Sanofi, Cardioxyl, Edwards Lifesciences, Sandoz, and GlaxoSmithKline.
Role of the Funder/Sponsor: Dignitana AB supported the design and conduct of the study; including collection, management, analysis, and interpretation of the data. The investigators were free to decide about publication of the study results in oral presentations at meetings, posters, or full journal articles but were expected to give Dignitana AB a minimum of 2 weeks to comment on drafts.
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01831024
Author Contributions: Dr Rugo had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Rugo, Melin, Melisko, Moore, Mitchel, Bågeman, D’Agostino, Esserman.
Acquisition, analysis, or interpretation of data: Rugo, Klein, Melin, Hurvitz, Melisko, Moore, Park, Mitchel, Ver Hoeve, Cigler.
Drafting of the manuscript: Rugo, Moore, Park, Mitchel, Bågeman, D’Agostino, Ver Hoeve, Esserman.
Critical revision of the manuscript for important intellectual content: Rugo, Klein, Melin, Hurvitz, Melisko, Park, Mitchel, D’Agostino, Esserman, Cigler.
Statistical analysis: Park, Mitchel, D’Agostino. Obtained funding: Rugo.
Administrative, technical, or material support: Rugo, Melin, Melisko, Moore, Park, Bågeman, Ver Hoeve, Esserman, Cigler.
Supervision: Rugo, Mitchel.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
Meeting Presentation: The data were presented in part at the annual American Society of Clinical Oncology meeting; May 29–June 2, 2015; Chicago, Illinois.
Additional Contributions: We thank the patients for granting permission to publish this information.
Contributor Information
Hope S. Rugo, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco.
Paula Klein, Icahn School of Medicine at Mount Sinai, New York, New York.
Susan Anitra Melin, Wake Forest Baptist Health Medical Center, Wake Forest School of Medicine, Winston-Salem, North Carolina.
Sara A. Hurvitz, Jonsson Comprehensive Cancer Center, University of California, Los Angeles.
Michelle E. Melisko, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco.
Anne Moore, Weill Cornell Medical College, New York, New York.
Glen Park, Target Health Inc, New York, New York.
Jules Mitchel, Target Health Inc, New York, New York.
Erika Bågeman, Dignitana AB, Lund, Sweden.
Ralph B. D’Agostino, Jr, Wake Forest School of Medicine, Winston-Salem, North Carolina.
Elizabeth S. Ver Hoeve, Columbia University, New York, New York.
Laura Esserman, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco.
Tessa Cigler, Weill Cornell Medical College, New York, New York.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Batchelor D, Golant M, Lyman GH, Rhodes N, Yardley D. Chemotherapy-induced alopecia: psychosocial impact and therapeutic approaches. Support Care Cancer. 2004;12(8):543–549. doi: 10.1007/s00520-003-0562-5. [DOI] [PubMed] [Google Scholar]
- 3.van den Hurk CJ, Mols F, Vingerhoets AJ, Breed WP. Impact of alopecia and scalp cooling on the well-being of breast cancer patients. Psychooncology. 2010;19(7):701–709. doi: 10.1002/pon.1615. [DOI] [PubMed] [Google Scholar]
- 4.Shin H, Jo SJ, Kim DH, Kwon O, Myung SK. Efficacy of interventions for prevention of chemotherapy-induced alopecia: a systematic review and meta-analysis. Int J Cancer. 2015;136(5):E442–E454. doi: 10.1002/ijc.29115. [DOI] [PubMed] [Google Scholar]
- 5.Breed W, van den Hurk C, Peerbooms M. Presentation, impact and prevention of chemotherapy-induced hair loss: scalp cooling potentials and limitations. Expert Rev Dermatol. 2011;6:109–125. [Google Scholar]
- 6.Bülow J, Friberg L, Gaardsting O, Hansen M. Frontal subcutaneous blood flow, and epi- and subcutaneous temperatures during scalp cooling in normal man. Scand J Clin Lab Invest. 1985;45(6):505–508. doi: 10.3109/00365518509155250. [DOI] [PubMed] [Google Scholar]
- 7.Janssen FP, Rajan V, Steenbergen W, van Leeuwen GM, van Steenhoven AA. The relationship between local scalp skin temperature and cutaneous perfusion during scalp cooling. Physiol Meas. 2007;28(8):829–839. doi: 10.1088/0967-3334/28/8/006. [DOI] [PubMed] [Google Scholar]
- 8.Dean JC, Salmon SE, Griffith KS. Prevention of doxorubicin-induced hair loss with scalp hypothermia. N Engl J Med. 1979;301(26):1427–1429. doi: 10.1056/NEJM197912273012605. [DOI] [PubMed] [Google Scholar]
- 9.Ridderheim M, Bjurberg M, Gustavsson A. Scalp hypothermia to prevent chemotherapy-induced alopecia is effective and safe: a pilot study of a new digitized scalp-cooling system used in 74 patients. Support Care Cancer. 2003;11(6):371–377. doi: 10.1007/s00520-003-0451-y. [DOI] [PubMed] [Google Scholar]
- 10.van den Hurk CJ, van de Poll-Franse LV, Breed WP, Coebergh JW, Nortier JW. Scalp cooling to prevent alopecia after chemotherapy can be considered safe in patients with breast cancer. Breast. 2013;22(5):1001–1004. doi: 10.1016/j.breast.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 11.Lemieux J, Amireault C, Provencher L, Maunsell E. Incidence of scalp metastases in breast cancer: a retrospective cohort study in women who were offered scalp cooling. Breast Cancer Res Treat. 2009;118(3):547–552. doi: 10.1007/s10549-009-0342-0. [DOI] [PubMed] [Google Scholar]
- 12.Rugo H, Melin A. Expert statement on scalp cooling with adjuvant/neoadjuvant chemotherapy for breast cancer and the risk of scalp metastases; Presented at: St Gallen 13th International Breast Cancer Conference; March 13ߝ16, 2013; St Gallen, Switzerland. [Google Scholar]
- 13.Lemieux J, Provencher L, Perron L, et al. No effect of scalp cooling on survival among women with breast cancer. Breast Cancer Res Treat. 2015;149(1):263–268. doi: 10.1007/s10549-014-3231-0. [DOI] [PubMed] [Google Scholar]
- 14.McAndrews PJ. Degree of hair loss. http://www.americanhairloss.org/women_hair_loss/degree_of_hair_loss.asp. Accessed January 2, 2016.
- 15.Trüeb RM. Chemotherapy-induced alopecia. Semin Cutan Med Surg. 2009;28(1):11–14. doi: 10.1016/j.sder.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Ekwall EM, Nygren LM, Gustafsson AO, Sorbe BG. Determination of the most effective cooling temperature for the prevention of chemotherapy-induced alopecia. Mol Clin Oncol. 2013;1(6):1065–1071. doi: 10.3892/mco.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 18.Komen MM, Smorenburg CH, van den Hurk CJ, Nortier JW. Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. Oncologist. 2013;18(7):885–891. doi: 10.1634/theoncologist.2012-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grevelman EG, Breed WP. Prevention of chemotherapy-induced hair loss by scalp cooling. Ann Oncol. 2005;16(3):352–358. doi: 10.1093/annonc/mdi088. [DOI] [PubMed] [Google Scholar]
- 20.van den Hurk CJ, Breed WP, Nortier JW. Short post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer. 2012;20(12):3255–3260. doi: 10.1007/s00520-012-1465-0. [DOI] [PubMed] [Google Scholar]
- 21.Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30(18):2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cigler T, Isseroff D, Fiederlein B, et al. Efficacy of scalp cooling in preventing chemotherapy-induced alopecia in breast cancer patients receiving adjuvant docetaxel and cyclophosphamide chemotherapy. Clin Breast Cancer. 2015;15(5):332–334. doi: 10.1016/j.clbc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Friedrichs K, Carstensen MH. Successful reduction of alopecia induced by anthracycline and taxane containing adjuvant chemotherapy in breast cancer—clinical evaluation of sensor-controlled scalp cooling. Springerplus. 2014;3:500. doi: 10.1186/2193-1801-3-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van den Hurk CJ, van den Akker-van Marle ME, Breed WP, van de Poll-Franse LV, Nortier JW, Coebergh JW. Impact of scalp cooling on chemotherapy-induced alopecia, wig use and hair growth of patients with cancer. Eur J Oncol Nurs. 2013;17(5):536–540. doi: 10.1016/j.ejon.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Kargar M, Sarvestani RS, Khojasteh HN, Heidari MT. Efficacy of penguin cap as scalp cooling system for prevention of alopecia in patients undergoing chemotherapy. J Adv Nurs. 2011;67(11):2473–2477. doi: 10.1111/j.1365-2648.2011.05668.x. [DOI] [PubMed] [Google Scholar]
- 26.Protière C, Evans K, Camerlo J, et al. Efficacy and tolerance of a scalp-cooling system for prevention of hair loss and the experience of breast cancer patients treated by adjuvant chemotherapy. Support Care Cancer. 2002;10(7):529–537. doi: 10.1007/s00520-002-0375-y. [DOI] [PubMed] [Google Scholar]
- 27.Betticher DC, Delmore G, Breitenstein U, et al. Efficacy and tolerability of two scalp cooling systems for the prevention of alopecia associated with docetaxel treatment. Support Care Cancer. 2013;21(9):2565–2573. doi: 10.1007/s00520-013-1804-9. [DOI] [PubMed] [Google Scholar]
- 28.van den Hurk CJ, Peerbooms M, van de Poll-Franse LV, Nortier JW, Coebergh JW, Breed WP. Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients—results of the Dutch Scalp Cooling Registry. Acta Oncol. 2012;51(4):497–504. doi: 10.3109/0284186X.2012.658966. [DOI] [PubMed] [Google Scholar]
- 29.Roe H. Chemotherapy-induced alopecia: advice and support for hair loss. Br J Nurs. 2011;20(10):S4–S11. doi: 10.12968/bjon.2011.20.Sup5.S4. [DOI] [PubMed] [Google Scholar]
- 30.Batchelor D. Hair and cancer chemotherapy: consequences and nursing care—a literature study. Eur J Cancer Care (Engl) 2001;10(3):147–163. doi: 10.1046/j.1365-2354.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 31.Lemieux J, Maunsell E, Provencher L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: a literature review. Psychooncology. 2008;17(4):317–328. doi: 10.1002/pon.1245. [DOI] [PubMed] [Google Scholar]
- 32.van den Hurk CJ, Winstanley J, Young A, Boyle F. Measurement of chemotherapy-induced alopecia-time to change. Support Care Cancer. 2015;23(5):1197–1199. doi: 10.1007/s00520-015-2647-3. [DOI] [PubMed] [Google Scholar]
- 33.Nangia J, Wang T, Osborne C, et al. Effect of a scalp cooling device on alopecia in women undergoing chemotherapy for breast cancer: the SCALP randomized clinical trial. JAMA. doi: 10.1001/jama.2016.20939. [DOI] [PubMed] [Google Scholar]
- 34.Rugo HS, Serrurier KM, Melisko A, et al. Use of the DigniCap™ system to prevent hair loss in women receiving chemotherapy (CTX) for stage I breast cancer (BC) Cancer Res. 2012;72(24 suppl):A2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


