Abstract
This study aims to explore the effect of FMT on regulations of dysbacteriosis of pulmonary and intestinal flora in rats with 16S rDNA sequencing technology. A total of 27 SPF rats (3–4 weeks old) were randomly divided into three groups: normal control group (K), model control group (MX), and fecal microbiota transplantation group (FMT); each group contained nine rats. The OTU values of the pulmonary and intestinal flora of the MX group decreased significantly compared with the normal control group. After FMT, the OTU value of pulmonary flora increased, while the value of OTU in intestinal flora declined. At the phylum level, FMT down-regulated Proteobacteria, Firmicutes, and Bacteroidetes in the pulmonary flora. At the genus level, FMT down-regulated Pseudomonas, Sphingobium, Lactobacillus, Rhizobium, and Acinetobacter, thus maintaining the balance of the pulmonary flora. Moreover, FMT could change the structure and diversity of the pulmonary and intestinal flora by positively regulating the pulmonary flora and negatively regulating intestinal flora. This study may provide a scientific basis for FMT treatment of respiratory diseases.
Keywords: Fecal microbiota transplantation, Pulmonary flora, Intestinal flora, 16S rDNA
Introduction
Fecal microbiota transplantation (FMT) is a kind of treatment method for transferring the functional bacteria from normal feces to the gastrointestinal tract, reconstructing the new intestinal flora, and restoring the host function. ZhouHouBeiJiFang in the Chinese Yellow Dragon Soup might be the first record of fecal transplant therapy, suggesting that FMT could treat intestinal and pulmonary diseases. In 1958, for the first time, Eiseman et al. reported that FMT was used to treat patients with severe pseudomembranous colitis, who were not treated with conventional antibiotics, and the satisfactory treatment effects in all the patients (Eiseman et al. 1958). Khoruts et al. treated the refractory Clostridium difficile infection diarrhea patients by FMT and finally found that FMT improved the symptoms of diarrhea and promoted the recovery of intestinal flora function (Khoruts et al. 2010). In 1983, for the first time, Schwan et al. reported that FMT was effective in the treatment of CDI by enema (Schwan et al. 1983). At present, FMT has been widely applied in the treatment of recurrent or refractory CDI enteritis and more mature treatment norms and guidelines have been developed. In addition, in recent years, with the extensive application of sequencing technology and the further studies between flora and diseases, FMT has achieved good treatment effects in many other diseases, such as IBD, IBS, and metabolic syndrome (Colman and Rubin 2014; Zoller et al. 2015; Marotz and Zarrinpar 2016).
The human intestinal tract has the largest surface area as well as the largest contact area with the external environment. There are abundant bacteria in the intestinal tract. Intestinal microflora and its metabolites (short chain fatty acids, etc.) and intestinal mucosal immunity form the intestinal microenvironment, which is generally in a dynamic balance. The damaged balance may lead to various diseases. Intestinal flora is an important part of the intestinal microenvironment, so it is important to balance the intestinal flora (Mcknite et al. 2012; Van den Elsen et al. 2017). In the treatment of chronic respiratory diseases, antibiotics are often used (Suresh et al. 2013). Although antibiotics can relieve diseases, they cannot avoid the repeated occurrence of these diseases. The extensive application of antibiotics and hormone could induce dysbacteriosis and cause diseases. Therefore, the mechanisms of the repeated occurrence of chronic respiratory diseases might involve the regulation of the flora balance (Chakhava et al. 1985; Tsuei et al. 2014; Herbert 2015).
In this study, with 16S rDNA sequencing technology, we explored the influences of FMT on the pulmonary and intestinal flora which could provide a scientific basis for FMT treatment of respiratory diseases.
Materials and instruments
Animals
A total of 27 SPF wistar rats (100 ± 10 g), 3–4 weeks old male, were obtained from Chengdu Dashuo Experimental Animal Co. Ltd. All rats were kept under standard environmental conditions with free access to rodent diet and water.
Experimental drugs
Antibiotics
Cefradine capsules were purchased from Shijiazhuang Pharmaceutical Group Ouyi Pharma Co.Ltd., and gentamicin sulfate was obtained from Shanghai Shen Guang Company.
Hormone
Dexamethasone sodium phosphate was purchased from Shanxi, Ruicheng Kelon Veterinary Medicine Co. Ltd.
Methods
Grouping
The animals were randomly divided into normal control group (K), model control group (MX), and fecal transplantation group (FMT), with nine rats in each group.
Drug preparation
Preparation of mixed suspension of hormone and antibiotics
Cefradine capsules, gentamicin sulfate, and dexamethasone sodium phosphate injection were combined to a mixture of 22.6 g/L in the proportion of 1:5:6, which was administered intraperitoneally to rats for 2 ml per day.
Preparation of fecal suspension
On the ninth day, 15 g of fresh feces was obtained from the rats of the normal control group, placed in a sterilized beaker containing 150 mL of the saline solution, and mixed fully. After sterile filtration, the filtrate was placed in a 10-mL centrifuge tube and centrifuged at 5000 r/min and 0 °C for 15 min. In addition, the supernatant was then collected and stored at − 4 °C for 3 h. In the seven consecutive days, the supernatant was prepared once a day (Tian et al. 2017).
Induction of dysbacteriosis of pulmonary and intestinal florae in rats and treatment with FMT
Construction of the model of dysbacteriosis
From the ninth day, the mixed suspension of antibiotics and hormone was injected to the model control group and the FMT group once a day for eight consecutive days. Meanwhile, normal saline was injected to the normal control group in the same way.
Treatment with FMT
The rats in the FMT group were fixed on the fixed plate, upside down, and catheters were inserted into the anus up to 5–8 cm. 5 mL of the fecal suspension was injected into the rats by enema and maintained for 3 min.
Index detection
On the 16th day, the rats were killed and the intestinal contents and lung tissues were taken under aseptic conditions and stored at − 80 °C.
High-throughput sequencing
Sample collection
From the K group, MX group, and FMT group, three samples (lung: FK2, FK4, FK6; FMX2, FMX4, FMX6; FFMT2, FMT4, FFMT6; gut: CK2, CK4, CK6; CMX2, CMX4, CMX6; CFMT2, CFMT4, CFMT6) of lung tissues and intestinal contents were randomly selected from each group, to follow up the analysis of flora.
MetaVx™ library preparation and illumina MiSeq sequencing
Next-generation sequencing library preparations and Illumina MiSeq sequencing were conducted at GENEWIZ, Inc. (Suzhou, China). DNA samples were quantified using a Qubit 2.0. Fluorometer (Invitrogen, Carlsbad, CA, USA). 30–50 ng DNA was used to generate amplicons using a MetaVx™ Library Preparation kit (GENEWIZ, Inc., South Plainfield, NJ, USA). V3and V4 hypervariable regions of prokaryotic 16S rDNA were selected for generating amplicons and following the taxonomy analysis. The v3 and v4 regions were amplified using forward primers containing the sequence “CCTACGGRRBGCASCAGKVRVGAAT” and reverse primers containing the sequence “GGACTACNVGGGTWTCTAATCC”. The v4 region was amplified using forward primers containing the sequence “GTGYCAGCMGCCGCGGTAA” and reverse primers containing the sequence “CTTGTGCGGKCCCCCGYCAATTC”. The first round PCR products were used as templates for the second round amplicon enrichment PCR. At the same time, indexed adapters were added to the ends of the 16S rDNA amplicons to generate indexed libraries ready for downstream NGS sequencing on Illumina Miseq. DNA libraries were validated by Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and quantified by Qubit 2.0 Fluorometer. DNA libraries were multiplexed and loaded on an Illumina MiSeq instrument according to the manufacturer’s instructions (Illumina, San Diego, CA, USA). Sequencing was performed using a 2 × 300/250 paired-end (PE) configuration; image analysis and base calling were conducted by the MiSeq Control Software (MCS) embedded in the MiSeq instrument.
Data analysis
The 16S rDNA data analysis was used by the QIIME package data and R programming language. The forward and reverse reads were joined and assigned to samples based on barcode and truncated by cutting off the barcode and primer sequence.
Results
Behavioral changes
The rats in the K group showed no abnormal intake, shiny hair, fecal morphology, loose stools, or other abnormal conditions. In the MX group, the intake decreased, the hair lost luster, the shapes of feces were changed, and sneezing, nasal discharge, and other symptoms occurred. Compared with the MX group, the intakes of rats in the FMT group was increased and hair luster and fecal morphology were slightly improved, but sneezing or nasal secretions were not observed.
16S rDNA gene sequence and operational taxonomic units (OTUs)
The effective sequences were determined by Illumina platform (Table 1). The OTU values of the groups (CK, CMX, CFMT, FK, FMX, and FFMT) were 300, 247, 178, 144, 97, and 121(Table 2).
Table 1.
Filtered data quality statistics
| Samples | #PE_reads | #Nochimera | AvgLen (nt) | GC (%) | Effective (%) |
|---|---|---|---|---|---|
| CFMT2 | 43138 | 32848 | 461.21 | 51.63 | 76.15 |
| CFMT4 | 252584 | 186310 | 460.46 | 52.34 | 73.76 |
| CFMT6 | 69040 | 55359 | 461.06 | 52.93 | 80.18 |
| CK2 | 67902 | 49032 | 453.63 | 53.13 | 72.21 |
| CK4 | 70416 | 49807 | 454.83 | 52.6 | 70.73 |
| CK6 | 67081 | 48314 | 455.06 | 52.98 | 72.02 |
| CMX2 | 64579 | 44820 | 456.67 | 52.84 | 69.4 |
| CMX4 | 80856 | 58467 | 454.47 | 53.45 | 72.31 |
| CMX6 | 67245 | 47065 | 458.68 | 52.81 | 69.99 |
| FFMT2 | 78609 | 58976 | 451.85 | 52.72 | 75.02 |
| FFMT4 | 70965 | 53345 | 451.35 | 52.7 | 75.17 |
| FFMT6 | 74921 | 56016 | 451.9 | 52.65 | 74.77 |
| FK2 | 63854 | 47940 | 452.59 | 52.67 | 75.08 |
| FK4 | 73497 | 55780 | 450.57 | 53.15 | 75.89 |
| FK6 | 77270 | 58904 | 451.93 | 52.75 | 76.23 |
| FMX2 | 67107 | 49658 | 452.29 | 52.68 | 74 |
| FMX4 | 48050 | 34673 | 451.23 | 52.77 | 72.16 |
| FMX6 | 52788 | 38156 | 452.02 | 52.73 | 72.28 |
Table 2.
OTU values of all the groups
| Groups | OTU |
|---|---|
| CFMT | 178 |
| CK | 300 |
| CMX | 247 |
| FFMT | 121 |
| FK | 144 |
| FMX | 97 |
Changes of Chao1 and Shannon indices
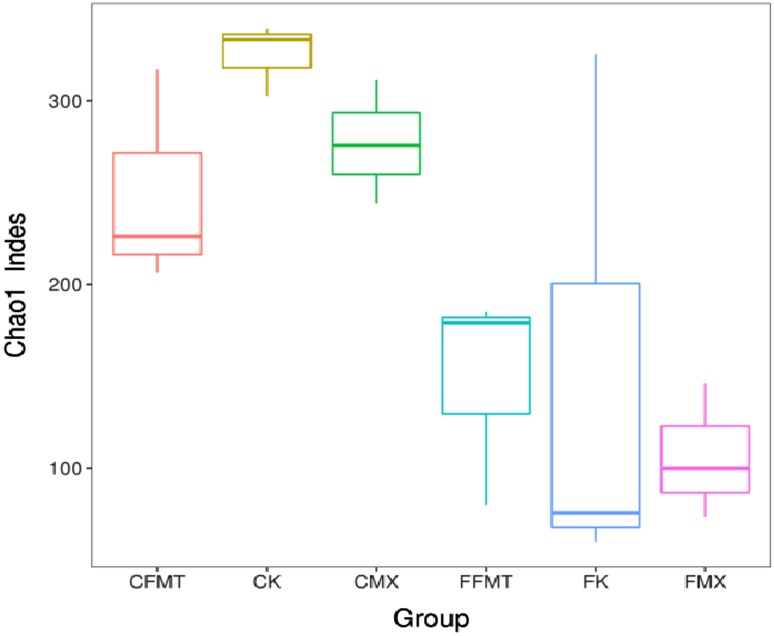
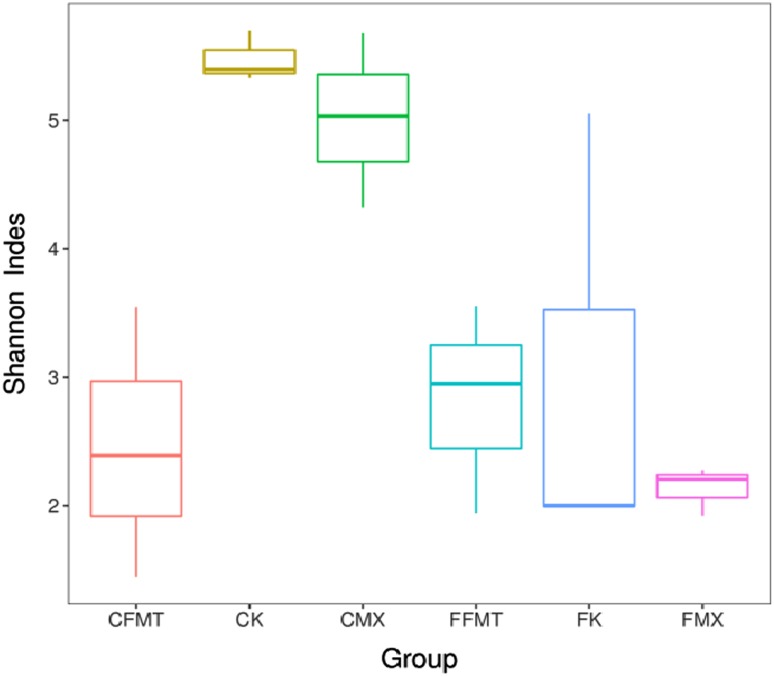
Chao1 and Shannon are important components of alpha diversity index. Chao1 index is used to estimate the total number of OTUs contained in the sample. Shannon index is used to estimate the diversity of microbial communities in the samples. Compared with Chao1 and Shannon indices of the CK and FK group, the two indices in the groups of CMX, FMX, and CFMT were decreased, but the two indices in the FFMT group increased (Figs. 1, 2).
Fig. 1.
Chao1 Index of all the six groups
Fig. 2.
Shannon Index of all the six groups
Compositions and changes at the levels of phylum and genus
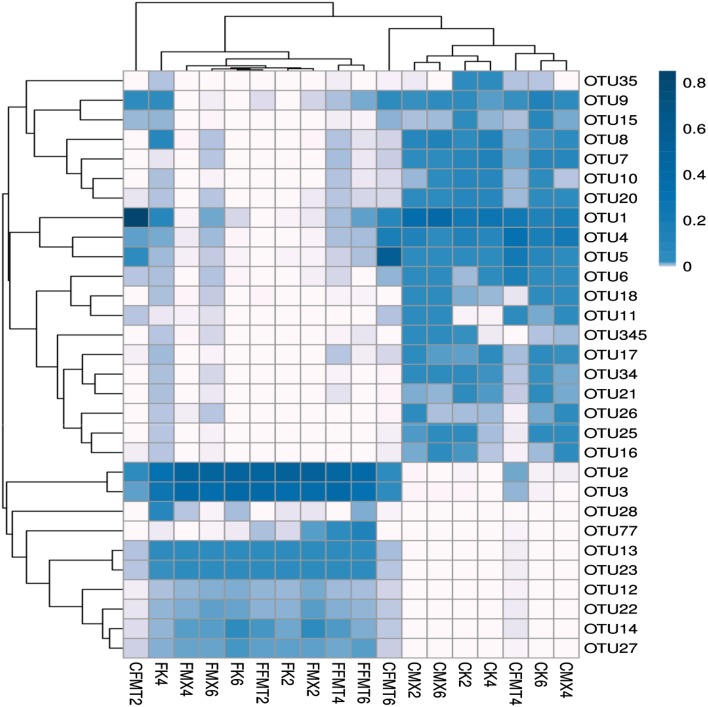
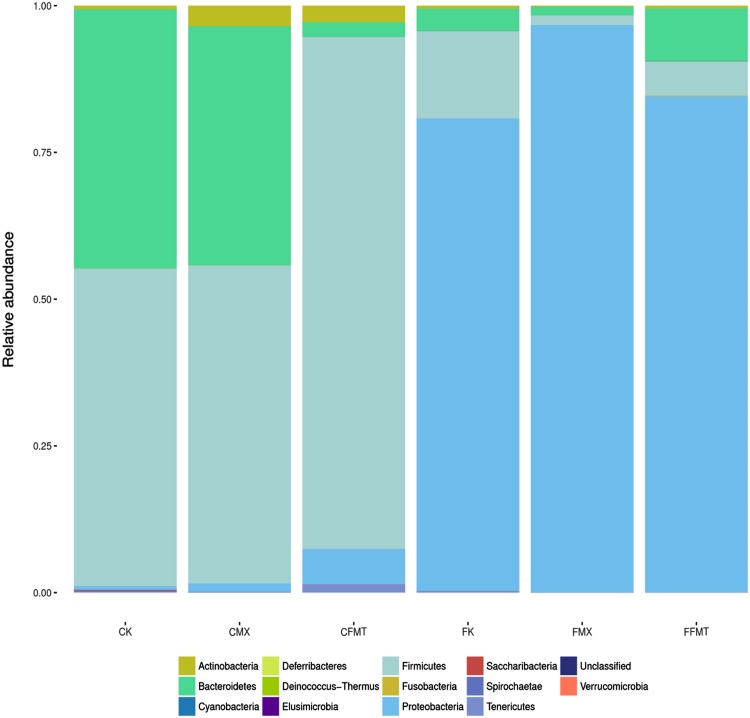
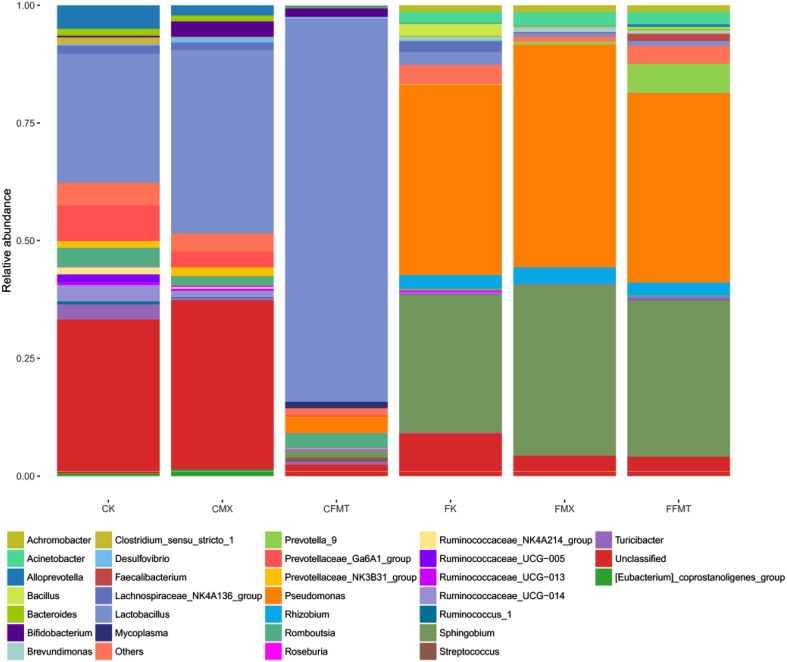
According to the sequencing results, the composition of each sample could be obtained (Fig. 3, Table 3). At the phylum level, the groups of CK, CMX, CFMT, FK, FMX, and FFMT were mainly composed of Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, Tenericutes, Spirochaetae, and Saccharibacteria (Fig. 4, Table 4). At the genus level, the groups of CK, CMX, and CFMT were mainly composed of Lactobacillus, Prevotellaceae_Ga6A1_group, and Allrevotella. The groups FK, FMX, and FFMT were mainly composed of Pseudomonas, Sphingobium, Lactobacillus, Rhizobium, and Acinetobacter (Fig. 5 and Tables 5 and 6).
Fig. 3.
OTU heatmap of all the samples. Thermal analysis provides abundant information of the selected OTU, further demonstrating the similarity and differences among these data, including the 30 OTUs with the highest default abundances. The left names are OTU ID, and the color value of each square for each row indicates the relative abundance of OTU
Table 3.
The numbers at the phylum and genus levels of all the groups
| Taxon | Phylum | Genus |
|---|---|---|
| CK | 8 | 77 |
| CMX | 8 | 70 |
| CFMT | 7 | 85 |
| FK | 10 | 93 |
| FMX | 7 | 74 |
| FFMT | 11 | 94 |
Fig. 4.
Phylum composition of all the six groups. At the phylum level, the groups of CK, CMX, CFMT, FK, FMX, and FFMT were mainly composed of Bacteroidetes, Firmicutes, Proteobacteria, Actinobacteria, Tenericutes, Spirochaetae, and Saccharibacteria
Table 4.
The top seven percentages at the phylum level of all groups (%)
| Taxon | CK | CMX | CFMT | FK | FMX | FFMT |
|---|---|---|---|---|---|---|
| Firmicutes | 54.09 | 54.17 | 87.20 | 14.87 | 1.61 | 5.82 |
| Bacteroidetes | 44.14 | 40.75 | 2.50 | 3.81 | 1.47 | 8.99 |
| Proteobacteria | 0.65 | 1.45 | 6.05 | 80.51 | 96.71 | 84.56 |
| Actinobacteria | 0.61 | 3.49 | 2.81 | 0.49 | 0.18 | 0.44 |
| Tenericutes | 0.17 | 0.02 | 1.38 | 0.22 | 0.00 | 0.00 |
| Spirochaetae | 0.15 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 |
| Saccharibacteria | 0.17 | 0.00 | 0.03 | 0.02 | 0.00 | 0.01 |
Fig. 5.
Genus composition of all the six groups. At the genus level, the groups CK, CMX, and CFMT were mainly composed of Lactobacillus, Prevotellaceae_Ga6A1_group, and Alloprevotella; the groups FK, FMX and FFMT were mainly composed of Pseudomonas, Sphingobium, Lactobacillus, Rhizobium, and Acinetobacter
Table 5.
The top three percentages at the genus level in the intestinal flora (%)
| Taxon | CK | CMX | CFMT |
|---|---|---|---|
| Lactobacillus | 27.4 | 38.95 | 81.24 |
| Prevotellaceae_Ga6A1_group | 7.57 | 3.38 | 0.42 |
| Alloprevotella | 5 | 2.17 | 0.25 |
Table 6.
The top five percentages at the genus level in the pulmonary flora (%)
| Taxon | FK | FMX | FFMT | |
|---|---|---|---|---|
| Pseudomonas | 40.4 | 47.29 | 40.04 | |
| Sphingobium | 29.27 | 36.3 | 33.2 | |
| Rhizobium | 2.79 | 3.53 | 2.69 | |
| Acinetobacter | 2.45 | 2.91 | 2.71 | |
| Lactobacillus | 2.71 | 0.8 | 1 | |
Principal component analysis (PCA)
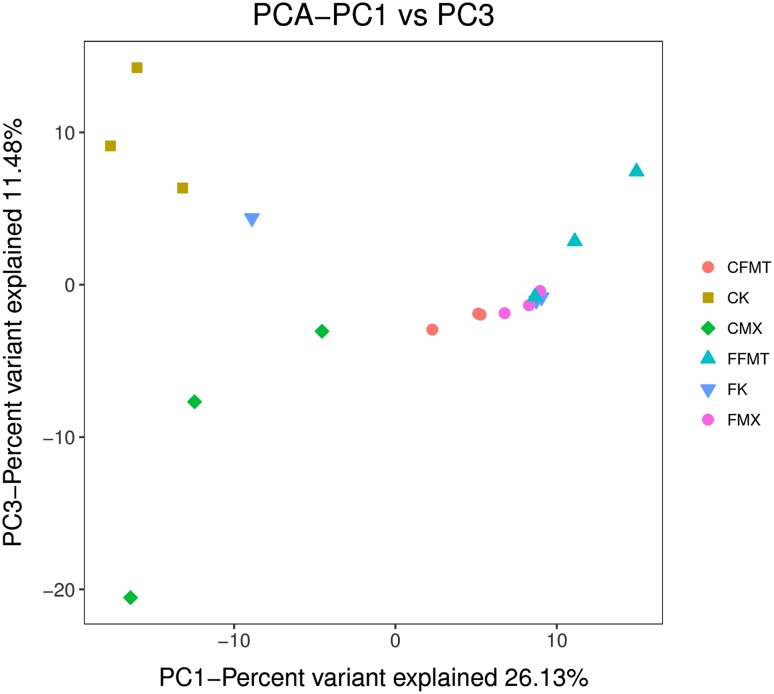
In the coordinate system, the closer the distance between two points, the higher was the similarity. The analysis results showed that the relative dispersion between different groups was relatively concentrated in the same group. According to the results of PCA analysis, there were significant differences between the pulmonary flora and the intestinal flora (Fig. 6).
Fig. 6.
Principal component analysis results of all the six groups. Principal component analysis (PCA) is a technique to simplify data analysis and can effectively identify the most important elements and structures in the data. PC1, PC2, and PC3 represent the first, second, and third principal components, respectively. The percentage of the principal component represents the contribution rate of this component to the sample difference, and it measures how much the principal component extracts from the original information. The distance between the sample points represents the similarity of the function classification distribution in the samples. The closer the distance, the higher is the similarity
Discussion
Through the analysis of the combined influence of antibiotics and hormone on the behaviors of rats, we found that in the MX group, the rats showed some changes including mental fatigue and irregular stool shape. In this study, the pulmonary and intestinal florae were sequenced. The OTU values of the CK and FK groups were relatively large and the pulmonary and intestinal florae in the normal rat group showed the highest diversity. The OTU values of the pulmonary and intestinal florae in the MX group decreased significantly compared with the normal control group. After FMT, the OTU value of pulmonary flora increased, while the value of OTU in intestinal flora decreased. Chao1 is often used to estimate the total number of species in ecology, and Shannon index is used to estimate the diversity of microbial communities. The larger Chao1 and Shannon values mean a larger total number of species and higher diversity (Valverde and Mellado 2013; Michelle et al. 2016).
The results of OTU, Chao1, Shannon indices, and the distribution and abundance of flora showed that FMT positively regulates the pulmonary flora and negatively regulates the intestinal flora. This change in the intestinal flora may be interpreted as follows. The variation of intestinal microflora could lead to the resistance phenomenon and self-reaction due to the direct effect of FMT, but FMT had no direct effect on the respiratory tract flora. The changes in the main compositions at the phylum and genus level indicated that the changes in the structure and abundance of the pulmonary and intestinal flora in rats were induced by antibiotics and hormone. At the phylum level, FMT down-regulated the levels of Proteobacteria, Firmicutes, and Bacteroidetes, which were the dominant phyla in the pulmonary flora and could maintain the balance of the bacterial flora (Pragman et al. 2012; Poroyko et al. 2015). At the genus level, FMT down-regulated Pseudomonas, Sphingobium, Lactobacillus, Rhizobium, and Acinetobacter, which might cause lung infection, inflammation, and metabolic disorders, and maintained the balance of the pulmonary flora (Dickson et al. 2014; Hu et al. 2015; Lo et al. 2015; Souto et al. 2014; Datta et al. 2017). After FMT, the pulmonary flora in the levels of phylum and genus was restored, suggesting that FMT might be one of the effective ways to prevent and treat chronic respiratory diseases.
In addition, FMT-related adverse reactions have been widely reported (Li et al. 2015; Patel et al. 2013; De Leon et al. 2013), including abdominal distension, intestinal peristalsis, and other symptoms, which may be related to the changes in the intestinal flora in this experiment. FMT is a new non-standardized treatment, and its adverse effects, potential risks, or long-term safety are unknown (Kelly et al. 2015; Paramsothy et al. 2015). Therefore, the treatment of respiratory tract infections by FMT needs to be further studied.
In this study, FMT regulated the intestinal bacterial flora imbalance effectively in rats, which could provide a scientific basis for clinical prevention and treatment of chronic respiratory diseases by FMT. Chakradhar also reported the same treatment method (Chakradhar 2017; Tamburini and Clemente 2017). Respiratory tract flora and intestinal flora play an important role in maintaining the ecological balance in the human body. For the first time in this study, we found that the intestinal flora could regulate the respiratory tract flora. These 16S rDNA analysis results verified the theory “pulmonary lung diseases could be treated via the intestinal regulation” recorded in Chinese ancient books “HuangDiNeiJing” and provided a scientific basis for FMT treatment of respiratory diseases.
Acknowledgements
We appreciate the help provided by GENEWIZ, Inc. (Suzhou, China). At the same time, we thank the scholars who have provided relevant guidance for the study.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was supported by grants from the National Natural Science Foundation of China (81460684,81660765) and Yunnan Science and Technology Plan Project (2015FB194).
References
- Chakhava OV, Ruban SZ, Shustrova NM. beta-Aspartylglycine during antibiotic induced dysbacteriosis of intestinal microflora in the rat. Prog Clin Biol Res. 1985;181:159–161. [PubMed] [Google Scholar]
- Chakradhar S. A curious connection: teasing apart the link between gut microbes and lung disease. Nat Med. 2017;23:402–404. doi: 10.1038/nm0417-402. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohn’s Colitis. 2014;8:1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P, Gupta V, Mohi G K, Chander J (2017) Lactobacillus coryniformis Causing Pulmonary Infection in a Patient with Metastatic Small Cell Carcinoma: Case Report and Review of Literature on Lactobacillus Pleuro-Pulmonary Infections. Journal of Clinical & Diagnostic Research Jcdr 11:DE01–DE05 [DOI] [PMC free article] [PubMed]
- De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2013;11:1036–1038. doi: 10.1016/j.cgh.2013.04.045. [DOI] [PubMed] [Google Scholar]
- Dickson RP, Erbdownward JR, Freeman CM, Walker N. Changes in the lung microbiome following lung transplantation include the emergence of two distinct pseudomonas species with distinct clinical associations. PLoS ONE. 2014;9:e97214. doi: 10.1371/journal.pone.0097214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiseman B, Silen W, Bascom GS, Kauvar AJ. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. 1958;44:854–859. [PubMed] [Google Scholar]
- Herbert KM. The gastrointestinal microbiome and diet-induced dysbiosis: influence on obesity and chronic disease risk. Dissertations Theses Gradworks. 2015;18:515–520. [Google Scholar]
- Hu J, Qian M, Zhang Q, Cui J. Sphingobium fuliginis HC3: a novel and robust isolated biphenyl- and polychlorinated biphenyls-degrading bacterium without dead-end intermediates accumulation. PLoS ONE. 2015;10:e0122740. doi: 10.1371/journal.pone.0122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CR, Kahn S, Kashyap P, Laine L. Update on FMT 2015: indications, methodologies, mechanisms and outlook. Gastroenterology. 2015;149:223–237. doi: 10.1053/j.gastro.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A, Dicksved J, Jansson JK, Sadowsky MJ. Changes in the composition of the human fecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J Clin Gastroenterol. 2010;44:354–360. doi: 10.1097/MCG.0b013e3181c87e02. [DOI] [PubMed] [Google Scholar]
- Li M, Liang P, Li Z, Wang Y. Fecal microbiota transplantation and bacterial consortium transplantation have comparable effects on the re-establishment of mucosal barrier function in mice with intestinal dysbiosis. Front Microbiol. 2015;6:692. doi: 10.3389/fmicb.2015.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SC, Hung GC, Li B, Lei H. Mixed group of Rhizobiales microbes in lung and blood of a patient with fatal pulmonary illness. Int J Clin Exp Pathol. 2015;8:13834–13852. [PMC free article] [PubMed] [Google Scholar]
- Marotz CA, Zarrinpar A. Treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J Biol Med. 2016;89:383–388. [PMC free article] [PubMed] [Google Scholar]
- Mcknite AM, Perez-Munoz ME, Lu L, Williams EG. Murine gut microbiota is defined by host genetics and modulates variation of metabolic traits. PLoS ONE. 2012;7:e39191. doi: 10.1371/journal.pone.0039191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelle B, Goodrich JK, Jackson MA, Idil Y. Heritable components of the human fecal microbiome are associated with visceral fat. Genome Biol. 2016;17:189. doi: 10.1186/s13059-016-1052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramsothy S, Walsh AJ, Borody T, Samuel D. Gastroenterologist perceptions of faecal microbiota transplantation. World J Gastroenterol. 2015;21:10907–10914. doi: 10.3748/wjg.v21.i38.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NC, Griesbach CL, Dibaise JK, Orenstein R. Fecal microbiota transplant for recurrent Clostridium difficile infection: Mayo Clinic in Arizona experience. Mayo Clin Proc. 2013;88:799–805. doi: 10.1016/j.mayocp.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Poroyko V, Meng F, Meliton A, Afonyushkin T. Alterations of lung microbiota in a mouse model of LPS-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2015;309:L76–L83. doi: 10.1152/ajplung.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragman AA, Kim HB, Reilly CS, Wendt C. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS ONE. 2012;7:e47305. doi: 10.1371/journal.pone.0047305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan A, Sjolin S, Trottestam U, Bo A. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2:845. doi: 10.1016/S0140-6736(83)90753-5. [DOI] [PubMed] [Google Scholar]
- Souto R, Silvaboghossian CM, Colombo AP. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz J Microbiol. 2014;45:495–501. doi: 10.1590/S1517-83822014000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh BK, Kastelik J, Morjaria JB. Role of long term antibiotics in chronic respiratory diseases. Respir Med. 2013;107:800–815. doi: 10.1016/j.rmed.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Tamburini S, Clemente JC. Gut microbiota: neonatal gut microbiota induces lung immunity against pneumonia. Nat Rev Gastroenterol Hepatol. 2017;14:263–264. doi: 10.1038/nrgastro.2017.34. [DOI] [PubMed] [Google Scholar]
- Tian H, Ge X, Nie Y, Yang L. Fecal microbiota transplantation in patients with slow-transit constipation: a randomized. clinical trial. PLoS ONE. 2017;12:e0171308. doi: 10.1371/journal.pone.0171308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuei J, Chau T, Mills D, Wan Y-JY. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med (Maywood, NJ) 2014;239:1489–1504. doi: 10.1177/1535370214538743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde JR, Mellado RP. Analysis of metagenomic data containing high biodiversity levels. PLoS ONE. 2013;8:e58118. doi: 10.1371/journal.pone.0058118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Elsen LW, Poyntz HC, Weyrich LS, Young W, Forbes-Blom EE. Embracing the gut microbiota: the new frontier for inflammatory and infectious diseases. Clin Transl Immunol. 2017;6(1):e125. doi: 10.1038/cti.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller V, Laguna AL, Prazeres DCO, Buch T. Fecal microbiota transfer (FMT) in a patient with refractory irritable bowel syndrome. Dtsch Med Wochenschr. 2015;140:1232–1236. doi: 10.1055/s-0041-103798. [DOI] [PubMed] [Google Scholar]