ABSTRACT
Heparin has been used clinically as an anti-coagulant for more than 100 y and the major source of this therapeutic is still animal tissues. Contamination issues in some batches of heparin over 10 y ago have highlighted the need to develop alternative methods of production of this essential drug.1 Bioengineering heparin by expressing serglycin in mammalian cells is a promising approach that was recently reported by the authors.2 This addendum explores the approaches that the authors are taking to increase the yield of recombinantly expressed serglycin decorated with heparin/heparan sulfate focusing on cell culture and bioreactor conditions and proposes that the cell microenvironment is a key modulator of heparin biosynthesis.
KEYWORDS: Heparin, heparan sulfate, mast cells, microenvironment, recombinant expression, serglycin
Bioengineering is used to manufacture a range of therapeutics including immunotherapies, insulin and vaccines while other drugs, such as heparin, continue to be made the way they have been for more than 100 y. Heparin used clinically is isolated from animal tissues including porcine intestinal mucosa and bovine lungs3 and is used as an anticoagulant to prevent thrombosis peri-operatively and to treat deep vein thrombosis. In 2007, the death of patients from an anaphylactic-like response who were receiving heparin was found to be due to the presence of contaminating over-sulfated chondroitin sulfate in some of the batches of heparin. This has led to a world-wide effort to produce heparin that is safe by the introduction of quality control measures for the presence of chondroitin sulfate by detecting the presence of sulfated galactosaminoglycan structures. The bioengineering approach to produce heparin from non-animal sources reported by the authors uses a process that is less susceptible to contamination.2
Despite the recent contamination crisis, the main stay of anticoagulant therapy remains animal-derived heparin.4 Fondaparinux is a clinical alternative to animal-derived heparin that is a pentasaccharide synthesized chemically from D-glucose and cellobiose that contains the anti-thrombin binding site. Since launching, it has been not been able to take over a significant proportion of market share due to the cost of production and the limited clinical indications for use.5 Other methods of heparin production that are being developed include chemical synthesis, chemo-enzymatic synthesis, sulfation of unsulfated polysaccharides and metabolic engineering of cells to overexpress enzymes involved in the biosynthesis of heparin.6-8 The authors of this current article have reported a bioengineering approach to produce heparin involving the recombinant expression of the proteoglycan serglycin in mammalian cells.2 This method produced crude heparin with one-seventh the activity of clinical-grade unfractionated heparin. It is speculated that the level of activity of the bioengineered heparin would increase once it is purified using methods similar those currently used to manufacture clinical-grade heparin from crude extracts.
The production of bioengineered heparin was achieved through the expression of human serglycin in human cells. This form of heparin can therefore be considered human heparin as it is produced by human cells that may have distinct advantages over porcine and bovine heparins that are structurally different and require different dosing to achieve therapeutic anticoagulant activity.9 Additionally, there is some evidence to suggest a higher propensity to form heparin-platelet factor 4 complexes with bovine heparin than porcine heparin, which is a precursor to heparin-induced thromobocytopenia.10 The expressed serglycin was shown to bind platelet factor 4 via its pendent glycosaminoglycan chains11 however, the propensity of the isolated bioengineered human heparin chains to form complexes with platelet factor 4 and the immunogenicity of these complexes remains unknown and is under investigation.
Optimization of the production of heparin/heparan sulfate was explored using the HEK-293 cells that expressed the recombinant serglycin and exposing them to different levels of glucose in the culture medium. High levels of glucose in the culture medium can increase glycosaminoglycan synthesis.12 The level of glucose in the culture medium was found to affect the yield of recombinant serglycin protein core as well as its level of decoration with heparin/heparan sulfate chains.2 Further analysis of the yield of serglycin from HEK-293 cells exposed to different levels of glucose in the culture medium indicated that the production of the protein core was lowest when cells were cultured in 25 mM glucose and highest when the cells were cultured in 50 mM glucose (Fig. 1). The level of post-translational modification of the serglycin protein core with heparin/heparan sulfate chains was highest when the cells were cultured in medium containing 25 mM glucose (Fig. 1). These data support the notion that the speed with which proteins transit through the Golgi is a factor in determining the type of glycosaminoglycan chains that are post-translationally added to proteoglycans.13 Culturing the cells in medium containing 25 mM glucose provided the highest level of heparin/heparan sulfate substitution of the protein core, however this level did not reach the maximal theoretical heparin/heparan sulfate production. Thus it is speculated that additional factors may enhance post-translational modification of serglycin with heparin/heparan sulfate chains and thus increase the yield of bioengineered human heparin.
Figure 1.
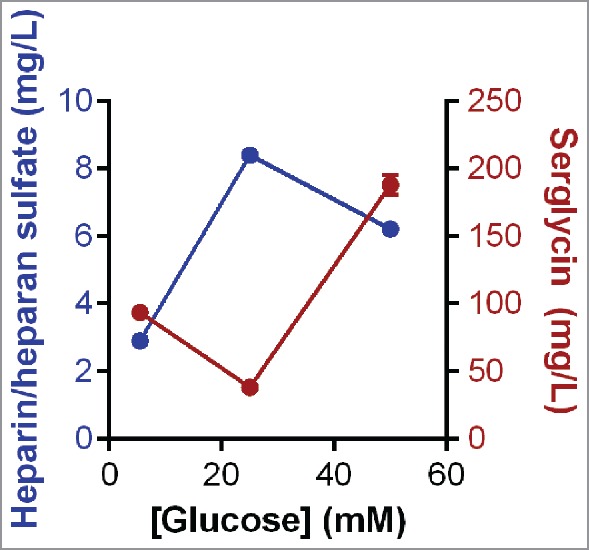
The yield of serglycin and heparin/heparan sulfate from HEK-293 cells expressing serglycin cultured in different glucose concentrations and purified by anion exchange chromatography using methods described by Lord et al.2
The challenge in optimizing the production of glycosaminoglycan chains is that they are synthesized by over 40 enzymes in the Golgi of cells for chain elongation, epimerization and sulfate modification.14 It is not known how to precisely control the expression and timed activity of these enzymes, although a review of recombinantly expressed proteoglycans indicates they are predominantly decorated with chondroitin sulfate.13 Detailed imaging of the enzymes involved in glycosaminoglycan biosynthesis revealed their spatial orientation in the Golgi and provides some evidence for the speed with which a protein transits the Golgi may be a factor in the type and extent of glycosaminoglycan decoration.15 There is also evidence to suggest that the amino acid sequences upstream of the glycosaminoglycan attachment site play a role in dictating the type of glycosaminoglycan attachment.16
There is substantial evidence in the literature that serglycin produced by different cell types is decorated with different glycosaminoglycan chains. Neutrophils, macrophages, platelets, smooth muscle and endothelial cells produce serglycin with chondroitin/dermatan and heparan sulfate chains.2 Both circulating and connective tissue-resident mast cells produce serglycin while only the connective tissue-resident mast cells are known to decorate serglycin with heparin.17 Thus, it is possible to speculate that the connective tissue microenvironment provides the appropriate cues for mast cells to produce heparin. To explore this phenomenon, the human mast cell line, HMC-1, was used because it expressed both heparin and chondroitin sulfate when it was either cultured alone (Fig. 2A) or co-cultured with the human fetal lung fibroblast cell line, MRC-5 (Fig. 2D). MRC-5 cells themselves do not express detectable levels of either glycosaminoglycan when cultured alone (Fig. 2B). Phase contrast images of the MRC-5 cells indicated that a confluent monolayer of cells had formed after 7 d in culture (Fig. 2C (i)). Co-cultures of MRC-5 and HMC-1 cells were prepared by culturing MRC-5 cells for 7 d and confluent followed by the addition of HMC-1 cells for a further 7 d. Phase contrast microscopy indicated that the MRC-5 cells maintained a confluent monolayer on the tissue culture surface with the HMC-1 cells adhering to the surface of the MRC-5 cells (Fig. 2C (ii)). The presence of the HMC-1 cells in the co-cultures was confirmed by Toluidine blue staining that indicated the presence of their acidic granules by a purple/pink color (Fig. 2C (ii) inset) that were localized intracellularly. HMC-1 cells maintained their expression of heparin in the co-cultures, however they reduced their expression of chondroitin sulfate (Fig. 2D). These data demonstrate for the first time that the microenvironment plays a role in directing glycosaminoglycan biosynthesis. This is most likely due to affecting intracellular processes such as protein production rates. These data make it possible to speculate that the microenvironment of cells has a deterministic role in the glycosaminoglycan decoration of proteoglycans. Indeed, there is already evidence for this in the way different cell types, both in vivo and in vitro, differentially decorate the protein core of proteoglycans with glycosaminoglycan chains. These data raise the possibility of regulating the type of glycosaminoglycan chains that decorate proteoglycans by providing the appropriate microenvironment. This will be particularly useful to optimize the production of bioengineered human heparin.
Figure 2.
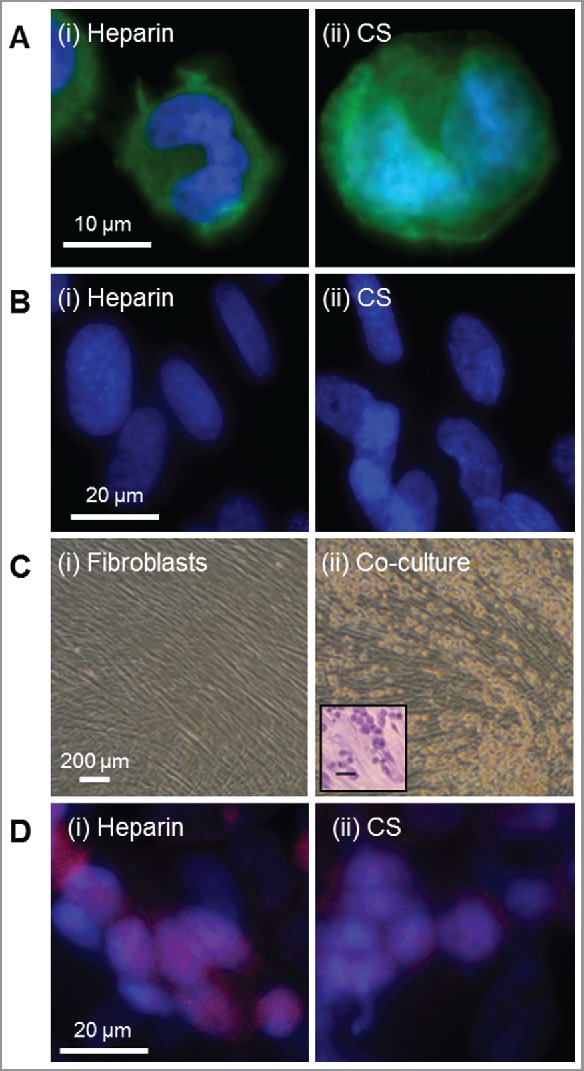
The human mast cell line, HMC-1, expressed both heparin and chondroitin sulfate however, when co-cultured with human fetal lung fibroblast cell line, MRC-5 increased the relative expression of heparin. Expression of (i) heparin (clone 2Q546) and (ii) chondroitin sulfate (clone CS-56) by (A) HMC-1, (B) MRC-5 and (D) co-cultures of HMC-1 and MRC-5 cells as determined by immunocytochemistry using methods described by Jung et al.18 Heparin and chondroitin sulfate are depicted in green in panels A and B and depicted in red in panel D. Cell nuclei were stained with DAPI, shown in blue in panels A, B and D. (C) Phase contrast microscope images of (i) MRC-5 cells and (ii) co-cultures of HMC-1 and MRC-5 cells. The inset in panel C (ii) indicates the presence of HMC-1 cells on top of the confluent MRC-5 cell layer as determined by Toluidine blue that stained the acidic granules a purple/pink color using methods described by Kirshenbaum et al.19 Scale bar in panel C (ii) inset represents 200 µm.
Disclosure of potential conflicts of interest
No potential conflict of interest was disclosed.
Funding
The authors gratefully acknowledge funding from the Australian Research Council Linkage Project scheme.
References
- [1].World Health Organization WHO model list of essential medicines, http://www.who.int/medicines/publications/essentialmedicines/en/index.html (2015) Accessed 8February2017.
- [2].Lord MS, Cheng B, Tang F, Lyons JG, Rnjak-Kovacina J, Whitelock JM. Bioengineered human heparin with anticoagulant activity. Metab Eng 2016; 38:105-14; PMID:27445159; http://dx.doi.org/ 10.1016/j.ymben.2016.07.006 [DOI] [PubMed] [Google Scholar]
- [3].Linhardt RJ. Heparin: An important drug enters its seventh decade. Chem Ind 1991; 2:2-5. [Google Scholar]
- [4].Mintz CS, Liu J. China's heparin revisited: What went wrong and has anything changed? J Commerc Biotechnol 2013; 19:33-9; http://dx.doi.org/ 10.5912/jcb579 [DOI] [Google Scholar]
- [5].Lin F, Lian G, Zhou Y. Synthesis of Fondaparinux: modular synthesis investigation for heparin synthesis. Carbohydr Res 2013; 371:32-9; PMID:23474455; http://dx.doi.org/ 10.1016/j.carres.2013.01.003 [DOI] [PubMed] [Google Scholar]
- [6].Choay J, Petitou M, Lormeau JC, Sinaÿ P, Casu B, Gatti G. Structure-activity relationship in heparin: A synthetic pentasaccharide with high affinity for antithrombin III and eliciting high anti-factor Xa activity. Biochem Biophys Res Comm 1983; 116:492-9; PMID:6651824; http://dx.doi.org/ 10.1016/0006-291X(83)90550-8 [DOI] [PubMed] [Google Scholar]
- [7].DeAngelis PL, Liu J, Linhardt RJ. Chemoenzymatic synthesis of glycosaminoglycans: Re-creating, re-modeling and re-designing nature's longest or most complex carbohydrate chains. Glycobiol 2013; 23:764-77; http://dx.doi.org/ 10.1093/glycob/cwt016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baik JY, Gasimli L, Yang B, Datta P, Zhang F, Glass CA, Esko JD, Linhardt RJ, Sharfstein ST. Metabolic engineering of Chinese hamster ovary cells: Towards a bioengineered heparin. Metab Eng 2012; 14:81-90; PMID:22326251; http://dx.doi.org/ 10.1016/j.ymben.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Linhardt RJ, Rice KG, Kim YS, Lohse DL, Wang HM, Loganathan D. Mapping and quantification of the major oligosaccharide components of heparin. Biochem J 1988; 243:781-7; http://dx.doi.org/ 10.1042/bj2540781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Francis JL, Palmer GJ, Moroose RDA. Comparison of bovine and porcine heparin in heparin antibody formation after cardiac surgery. Ann Thorac Surg 2003; 75:17-22; PMID:12537186; http://dx.doi.org/ 10.1016/S0003-4975(02)04349-7 [DOI] [PubMed] [Google Scholar]
- [11].Lord MS, Cheng B, Farrugia BL, McCarthy S, Whitelock JM. Platelet factor 4 binds to vascular proteoglycans and controls both growth factor activities and platelet activation. J Biol Chem 2017; PMID:28115521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang A, Midura RJ, Vasanji A, Wang AJ, Hascall VC. Hyperglycemia diverts dividing osteoblastic precursor cells to an adipogenic pathway and induces synthesis of a hyaluronan matrix that is adhesive for monocytes. J Biol Chem 2014; 289:11410-20; PMID:24569987; http://dx.doi.org/ 10.1074/jbc.M113.541458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lord MS, Whitelock JM. Recombinant production of proteoglycans and their boactive domains. FEBS J 2013; 280:2490-510; PMID:23419186; http://dx.doi.org/ 10.1111/febs.12197 [DOI] [PubMed] [Google Scholar]
- [14].Salmivirta M, Lidholt K, Linhadl U. Heparan sulfate: A piece of information. FASEB J 1996; 10:1270-9; PMID:8836040 [DOI] [PubMed] [Google Scholar]
- [15].Multhaupt HAB, Couchman JR. Heparan sulfate biosynthesis: Methods for investigation of the heparanosome. J Histochem Cytochem 2012; 60:908-15; PMID:22899865; http://dx.doi.org/ 10.1369/0022155412460056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Costell M, Mann K, Yamada Y, Timpl R. Characteriszation of recombinant perlecan domain I by glycosaminoglycans and oligosaccharides. Eur J Biochem 1997; 243:115-21; PMID:9030729; http://dx.doi.org/ 10.1111/j.1432-1033.1997.t01-1-00115.x [DOI] [PubMed] [Google Scholar]
- [17].Kjellen L, Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem 1991; 60:443-75; PMID:1883201; http://dx.doi.org/ 10.1146/annurev.bi.60.070191.002303 [DOI] [PubMed] [Google Scholar]
- [18].Jung M, Lord MS, Cheng B, Lyons JG, Alkhouri H, Hughes JM, McCarthy SJ, Iozzo RV, Whitelock JM. Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: a role in angiogenesis and wound healing. J Biol Chem 2013; 288:3289-304; PMID:23235151; http://dx.doi.org/ 10.1074/jbc.M112.387811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol 1991; 146:1410-5; PMID:1704394 [PubMed] [Google Scholar]


